Abstract
As populations decline to levels where reproduction among close genetic relatives becomes more probable, subsequent increases in homozygous recessive deleterious expression and/or loss of heterozygote advantage can lead to inbreeding depression. Here, we measure how inbreeding across replicate lines of the flour beetle Tribolium castaneum impacts on male reproductive fitness in the absence or presence of male–male competition. Effects on male evolution from mating pattern were removed by enforcing monogamous mating throughout. After inbreeding across eight generations, we found that male fertility in the absence of competition was unaffected. However, we found significant inbreeding depression of sperm competitiveness: non-inbred males won 57 per cent of fertilizations in competition, while inbred equivalents only sired 42 per cent. We also found that the P2 ‘offence’ role in sperm competition was significantly more depressed under inbreeding than sperm ‘defence’ (P1). Mating behaviour did not explain these differences, and there was no difference in the viability of offspring sired by inbred or non-inbred males. Sperm length variation was significantly greater in the ejaculates of inbred males. Our results show that male ability to achieve normal fertilization success was not depressed under strong inbreeding, but that inbreeding depression in these traits occurred when conditions of sperm competition were generated.
Keywords: inbreeding depression, polyandry, sperm competition, mating strategies, flour beetles
1. Introduction
Inbreeding occurs when genetically related individuals that carry identical alleles reproduce (Waser 1993). Inbreeding inevitably leads to an increase in homozygosity, frequently resulting in a fitness reduction known as inbreeding depression (Wright 1921; Charlesworth & Charlesworth 1987). Increasing homozygosity can lead to fitness depression either via partial dominance through an increased expression of deleterious homozygous recessive alleles (Charlesworth & Charlesworth 1987), or via overdominance through a reduction in heterozygote advantage (Crow 1952). Either way, biologists now recognize inbreeding depression across a range of fitness traits, in both captive and wild situations (Keller & Waller 2002). With unprecedented levels of habitat depletion and fragmentation currently occurring in the natural environment, reduction in population size and/or genetic diversity will be an increasing phenomenon that can lead to inbreeding (Frankham et al. 2002). There is therefore a need to understand in more detail how inbreeding might impact upon the fitness of important phenotypic traits.
Traits that are more directly associated with reproduction appear vulnerable to inbreeding depression, with fertilization being especially sensitive. Directional selection on reproductive traits, with more direct fitness consequences, creates higher directional dominance that could predispose such traits to more severe depression under inbreeding (e.g. DeRose & Roff 1999). In their review, Keller & Waller (2002) report that for more than half of the studies where inbreeding depression in the wild was reported, it was manifested specifically through compromised fertility. Several studies have reported inbreeding depression of pre- and post-copulatory male traits that are known to be important for fertilization, including mating success (e.g. Sharp 1984; Höglund et al. 2002; Ilmonen et al. 2009) and sperm production (e.g. Roldan et al. 1998; Margulis & Walsh 2002; Gage et al. 2006; Asa et al. 2007; Fitzpatrick & Evans 2009; Weeks et al. 2009), potentially leading to reduced offspring siring success (e.g. Slate et al. 2000; Charpentier et al. 2005; Asa et al. 2007).
Another important and increasingly recognized phenomenon is that inbreeding depression may only become apparent, or exacerbated, in the naturally relevant context of competition and environmental stress (Hoffmann & Parsons 1991; Joron & Brakefield 2003; Marr et al. 2006; Szulkin & Sheldon 2007). Experimental studies reveal that abiotic stress can amplify inbreeding depression, for example, under variable humidity in the red flour beetle Tribolium castaneum (Pray et al. 1994) or under thermal and ethanol intolerance in the fruitfly Drosophila melanogaster (Bijlsma et al. 1999, 2000). Also, competition can exacerbate inbreeding depression: for example, inbreeding amplifies the inability of male mice to win dominance hierarchies and maintain territories, both of which are important pre-copulatory traits for reproductive success (Meagher et al. 2000). In the context of post-copulatory competition for fertilization, recent experimental evidence shows that inbred male mites, hamsters and guppies have reduced ability to win sperm competitions (Konior et al. 2005; Fritzsche et al. 2006; Zajitschek et al. 2009). Since multiple mating and sperm competition are widespread phenomena across diverse taxa (Birkhead & Møller 1998), it is important to understand the consequences of inbreeding for reproductive success under pre- and post-copulatory sexual selection.
To address this issue, we examine how inbreeding influences male fertilization success through time, and compare that with effects upon sperm competitive ability, measuring both sperm ‘defence’ (P1) and ‘offence’ (P2) in two-male competitions. We assess both these two mechanisms of sperm competition as they can select for different sperm competitive functions (Clark et al. 1999; Bjork et al. 2007). Alongside the fertilization and sperm competition trials, we also assay male mating behaviour for inbreeding effects, examine how sperm morphometry changes under inbreeding and check that differential offspring viability is not confounded by inbreeding.
Our experimental model is T. castaneum (Coleoptera), which is a global pest of stored products with a promiscuous mating pattern (Fedina & Lewis 2008) that goes through cyclic changes in population size (inter alia via pest control) and spreads via repeated founder effects (Sokoloff 1972). This pest species sustains high levels of sperm competition, while passing through repeated population bottlenecking episodes, and our T. castaneum ancestral laboratory stock is likely to carry relatively reduced genetic diversity, comparable to wild populations that also show reduced genetic diversity (e.g. New Zealand black robins, Petroica traversi, Ardern & Lambert 1997; cheetahs, Acinonyx jubatus, Menotti-Raymond & O'Brien 1993). In contrast to such vulnerable wild populations, T. castaneum allows a controlled experimental approach with high replication. Using multiple inbred lines, we measure the effects of inbreeding on pre- and post-mating success, in the absence and presence of male–male competition. Importantly, our experiment was designed to make comparisons against equivalent non-inbred control lines that have been selected under an identical mating pattern regime. Recent experimental evolution studies have revealed that changes in mating pattern can be a potent force shaping a range of male reproductive adaptations (e.g. Hosken et al. 2001; Martin & Hosken 2003; Simmons & García-González 2008; Crudgington et al. 2009). To generate inbred lines via controlled sib × sib crosses, it is necessary to maintain a monogamous mating regime, which accordingly relaxes selection from male–male competition. Any reduction in male reproductive competence after inbreeding could hence be the result of selection owing to the absence of male–male competition and/or female choice by enforcing a monogamous adult mating pattern, and not because of reduced genetic variability. To control for this, we have created equivalent non-inbred lines from the same founder stock, mating all adults under identical monogamous regimes, but with random genetic mixing of families between each adult mating episode to prevent inbreeding in these lines.
2. Material and Methods
(a). Beetle and line management
Beetles were maintained under 16 L : 8 D photoperiod at 30°C and 65 per cent relative humidity, in food containing plain white organic flour and powdered organic brewer's yeast (9 : 1) with some large organic rolled oats to aid traction and food aeration. Pairs were formed by placing single virgin females with single virgin males for 7 days in an 8 ml vial filled with 3 g of food topped with rolled oats and secured with a perforated cap. Under these conditions beetles were able to mate freely and the females were able to lay eggs. Subsequent to egg laying, adults were removed and food with eggs and larvae was transferred to a 5 cm diameter Petri dish (35 ml). After pupae developed, they were sexed and isolated. At approximately 14 days after eclosion into adults, new pairs were created.
Inbred and non-inbred lines were created using male–female pairs of randomly selected virgin beetles from the ‘Georgia 1’ (GA1) strain (originally derived from stored corn in 1980, cultured since in the Beeman Laboratory, US Department of Agriculture, Biological Research Unit, Grain Marketing and Production Research Center, 1515 College Avenue, Manhattan, KS, USA). To create inbred lines, we first generated 50 families from random pairings of single virgin male and female GA1 adults. Inbreeding was then initiated in each family through reproducing offspring from a single male and female virgin adult sibling. The resulting offspring were then grown up to adult emergence, and again a randomly selected male × female sibling pair in each family was used to produce the next generation. These sib × sib crosses were repeated for four generations of inbreeding. We anticipated extinctions throughout the inbreeding protocol, and almost half of the original families went extinct over these first four generations of inbreeding. Beyond generation 4, we initiated the generation of separate inbreeding lines by splitting the surviving families. Again, monogamous sib × sib pairings were used to generate each new inbred line, and again some of these lines were terminated through extinction. Thus, each remaining family potentially gave origin to two lines in the fifth generation, four lines in the sixth generation, eight lines in the seventh generation and potentially 16 lines in the eighth generation. Because of extinctions, the eighth generation gave rise to 77 lines from 16 of the original surviving families, and in the ninth and final generation, we split only those lines with five or less lines from the original families (to avoid the over-representation of individual original families across the different final lines). After eight generations of these sib × sib pairings, and some families being split into separate lines and other families going extinct, we finally retained 98 extant lines producing inbred males for experimentation. Only one inbred male per line was used and replicated within any experimental treatment to minimize pseudo-replication within statistical comparisons.
Such relatively intense inbreeding was conducted because (i) pilot tests after one generation of full sib × sib inbreeding did not show depression of reproductive output compared with non-inbred crosses (n = 29 inbred and 27 non-inbred crosses, t54 = −0.851, r = 0.12, p = 0.398) and (ii) we expected our GA1 laboratory stock to have already passed through previous population genetic bottlenecks, potentially purging the population of some deleterious recessive alleles (e.g. Barrett & Charlesworth 1991; Hedrick 1994).
Alongside the inbred lines, we created non-inbred controls that were maintained identically and mated in monogamous pairings as for the inbred lines, except that we randomly mixed mating individuals between familial lines, so that chances for sib × sib pairings were very low. Three lines were established, each perpetuated by 20 monogamous pairings of single male and female virgin adults (maximal effective population size in each line Ne = 40), as for the inbred lines. Beetles were able to mate and lay eggs for a week and then adults were removed. All eggs and larvae within each of the three groups were then pooled and provided with ad libitum food as for the inbred lines. At pupation, male and female virgins were randomly paired (thus maintaining random genetic mixing) to create offspring for the next generation. As a consequence, the degree of inbreeding was (statistically) up to 20 times lower than in the inbred lines. Throughout, we present arithmetic means ± standard errors.
(b). Male fertility
To compare male fertility in detail over time between inbred and non-inbred males, we crossed control virgin females (from the GA1 stock) with either inbred (n = 30, each individual from a different line) or non-inbred control males (n = 11, chosen randomly from the GA1 stock). After 10 days of mating interaction, males were removed and females moved to new Petri dishes with fresh food for another 10 days, and then repeatedly transferred to new dishes with new food every 10 days across 140 days of oviposition. When all females had stopped producing offspring, they were placed with new virgin control males for an additional 10 days in order to establish whether the decrease in female fertility was a female or a male effect. The number of adults emerging within each Petri dish gave a measure of fertility for each cross, and by comparing offspring productivity between inbreeding treatments under sperm competition, were able to confirm that differential offspring viability was not a confound (see §3). A mixed repeated-measures ANOVA was used to analyse male fertility through time: male origin (inbred or non-inbred) was entered as a fixed independent factor, 10-day time period as a repeated-measures variable and number of offspring as the dependent variable. A separate, Gosset's paired t-test for females mated with inbred and control males was run to compare offspring production in the first 10-day block and the 15th 10-day block when females were mated with new control males. The proportion of females that survived to the 15th 10-day block was compared between the treatments using a χ2-test.
(c). Sperm competitiveness
Sperm competition experiments were conducted between the experimental males (inbred or non-inbred) and control males carrying the Reindeer (Rd) phenotypic marker, using control GA1 females. Reindeer males are dominant homozygotes at the Rd allele, so all offspring sired by an Rd male express the Rd phenotype with antler-shaped antennae. All individuals were virgins and ca 14 days since emergence. A female was provided with the first male for 24 h, and then this male was replaced with the second male for 24 h. After this 48 h mating period, females were allowed to oviposit into fresh medium for one week and then removed. Although females can oviposit fertile eggs for up to 20 weeks after such mating treatments (e.g. figure 2), and high (greater than 70%) last-male sperm precedence persists in T. castaneum for at least 30 days of oviposition without re-mating (Arnaud et al. 2001a,b), under normal circumstances, the females will re-mate repeatedly during adulthood with new males (Fedina & Lewis 2008). Therefore, the most informative representation of sperm competition success for an individual male exists over the initial period after any bout of multiple mating, and before the female re-mates with a new male who will then go on to achieve high last-male precedence.
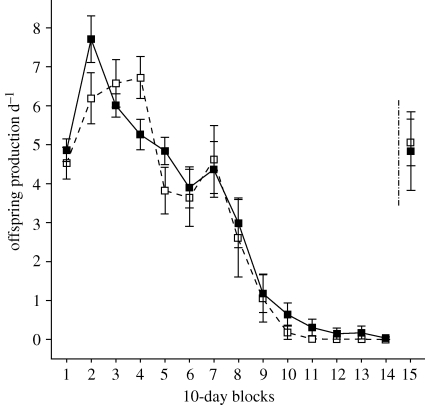
Figure 2.
Offspring production by control GA1 females mated to either inbred (closed squares and solid lines) or control non-inbred (open squares and dashed lines) males shows no difference over 140 days of oviposition (in 10-day blocks). The initial numbers of females mated with inbred and control males were 30 and 11, respectively. Re-mating females after inbred or non-inbred male treatments restores female fertility (means at block 15), thus proving that the offspring production decline is due to male or sperm effects, not female senescence.
After 40 days when all adults had emerged, sperm competition success was scored as the relative number of wild-type (GA1) and Rd phenotypes (employing a balanced experimental design to control for potential differences between marker and GA1 offspring).
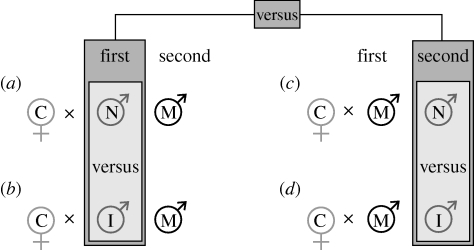
We assessed sperm precedence for both first-mated (P1) and second-mated (P2) experimental males, comparing the fertilization success of inbred males with non-inbred males, when they were in competition with Rd marker males for GA1 control females (figure 1). Seventy-four to 90 sperm competition replicates were performed across the four possible treatments (=either inbred or non-inbred, and either P1 or P2; see figure 1), generating a total of 330 separate sperm competition trials. Within these trials, an average of 47 offspring per female/cross were produced and scored. Only two males from each inbred line were used for each set of matings, i.e. one male as the first to mate and the other one as the second to mate (figure 1a,c). Non-inbred males were chosen randomly from all available lines.
Figure 1.
Crossing design for the two-male sperm competition experiments to measure influence of inbreeding on relative fertilization success of the first- and second-male-to-mate. Paternity can be assigned for the phenotypic marker male (M) using the Reindeer strain, and therefore sperm competition success for the inbred (I) versus non-inbred (N) experimental males, and P1 versus P2; females are non-inbred virgin controls (C) from the GA1 stock (see §2c for details, (a)–(d) correspond with the same denotations in figure 3).
The sperm competition data were distributed binomially. Thus, in order to obtain statistical information equivalent to a full factorial two-way ANOVA with two fixed independent variables, we used the generalized linear model with a quasi-binomial error distribution (the quasi-extension was used to account for overdispersion of the data, dispersion parameter = 26.72) and a logit-link function (Crawley 2005). The analysis was performed in R v. 2.6.2 (The R Development Core Team 2005). The significance of variables was assessed through the analysis of deviance, where a variable was removed from the full model and the reduced model was compared with the full model. The deviance (G2) is defined as −2 × the difference between the log likelihood of the reduced model, and the log likelihood of the full model and the resulting statistic was compared with the F-distribution (Crawley 2005).
It is important to note that any differences in male reproductive success under competition may theoretically be due to sperm competitiveness as well as to the viability of offspring. If the latter explains some of the variance, we would expect differences in total offspring number produced between trials, especially when the inbred and non-inbred males were the second male to mate (because of the high second-male precedence). To check for this potential effect, we ran a two-way ANOVA with the origin of the experimental male (inbred or non-inbred) and the order in which he was mated relative to the marker male (first or second) entered as independent fixed factors and the total number of offspring (=offspring viability) as the dependent variable. If inbred males fertilized the same number of eggs as non-inbred males, but offspring of inbred males had lower survival, there should be a significant effect of the origin of the experimental male on the total number of offspring produced by females. If, however, offspring sired by inbred males are not less viable than offspring sired by non-inbred males, we should expect no significant differences in female total offspring production.
(d). Male mating behaviour
To compare effects of inbreeding on male mating behaviour, and to estimate the potential effect of the number of matings on the results of the sperm competition experiments, we assayed male mating competence. Thirty-six control virgin GA1 females were randomly assigned to two groups in which they were maintained with either an inbred or a non-inbred male for 1 h. Within this time, the number of mounts (a male attempting to mate with a female or pairs in copula for less than 45 s), copulation latency and duration (copulation = a pair remaining in copula for 45 s or more; see also Attia & Tregenza 2004) were recorded. In the second hour, females that had previously been given inbred males were provided with non-inbred males (and vice versa). Again, the same behaviours were recorded. These mating behaviour assays followed the protocols of the sperm competition experiments (see above), thus allowing us a measure of the potential contribution of behavioural variability to sperm competition success. Moreover, the relative number of mounts and latency to mate will represent the male's mating success under inter-sexual competition from female choice (Fedina & Lewis 2008). Given that the χ2-test expected counts in the proportions of females that mated with inbred and non-inbred males were less than 5, we conducted a two-tailed Fisher's exact test instead. All other variables (number of mounts, copulation latency and duration) were analysed with separate independent samples two-tailed Mann–Whitney's U-tests. This was because for some variables the distribution was not normal, whereas for others the number of observations was insufficient to assess the distribution for normality. One male from a given inbred line was used in the experiment and non-inbred males were chosen randomly from all available lines.
(e). Sperm morphometry
Mature spermatozoa were recovered from the vesicula seminalis of 15 inbred and 15 non-inbred males (each from a different family line) and suspended in a drop of water on a microscope slide under a square coverslip. Previous work established that mature spermatozoa from the vesicula seminalis do not differ in length from those ejaculated and stored in female spermathecae (Michalczyk 2008). The lengths of five spermatozoa per male were measured under phase contrast light microscope at ×40 using Synoptics image analysis exact to 0.01 µm. Differences in both the total sperm lengths and their coefficients of variation (CV) were analysed with separate Gosset's independent samples t-tests.
3. Results
(a). Male fertility
The number of offspring produced decreased significantly over time, but there were no differences between inbred and non-inbred males (figure 2 and table 1). Results therefore show that (i) sperm from inbred males are equally fertile and have similar longevity to non-inbred males and/or (ii) that there are no differences in the viability of offspring sired by inbred and non-inbred fathers.
Table 1.
Results of an RM-ANOVA for the differences in fitness (=offspring number) of control females mated to either inbred or control males. Given that the assumption of sphericity was violated in the model (p < 0.001), the Greenhouse–Geisser correction was applied to the number of degrees of freedom. See also figure 2 for the illustration of changes in the female offspring output over time. Statistically significant factors (p < 0.05) are marked in italic.
| source | d.f. | MS | F | p |
|---|---|---|---|---|
| time | 4.264 | 391.429 | 54.469 | <0.001 |
| time×male (inbred/control) | 4.264 | 10.089 | 1.404 | 0.237 |
| error | 85.279 | 7.186 | ||
| male (inbred/control) | 1 | 4.064 | 0.428 | 0.520 |
| error | 20 | 9.493 |
When females were re-mated with a new (non-inbred) male after they had ceased offspring production (beyond 140 days in the 15th 10-day block), full female fertility was restored. Additionally, there were no differences in the number of offspring produced in the first versus the 15th 10-day block for females mated originally to inbred (p = 0.571, r = 0.15, t14 = 0.579) or non-inbred (p = 0.831, r = 0.10, t5 = 0.225) males. Thus, female fertility decreased and then ceased as a result of a decline in male-derived fertility, not female senescence; females from both treatments were as fertile in the 15th 10-day block as they were in the first 10 days of the experiment.
Approximately the same proportion of females from the two treatments survived to the 15th 10-day block: six of 11 (55%) females mated with non-inbred males and 15 of 30 (50%) females mated with inbred males (p = 1.000, 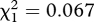 ). Male treatment did not influence female longevity either: females mated to non-inbred males survived on average 130.2 ± 7 days, and females mated to inbred males survived on average 141 ± 6 days (p = 0.329, r = 0.19, t27 = 0.993).
). Male treatment did not influence female longevity either: females mated to non-inbred males survived on average 130.2 ± 7 days, and females mated to inbred males survived on average 141 ± 6 days (p = 0.329, r = 0.19, t27 = 0.993).
(b). Sperm competition
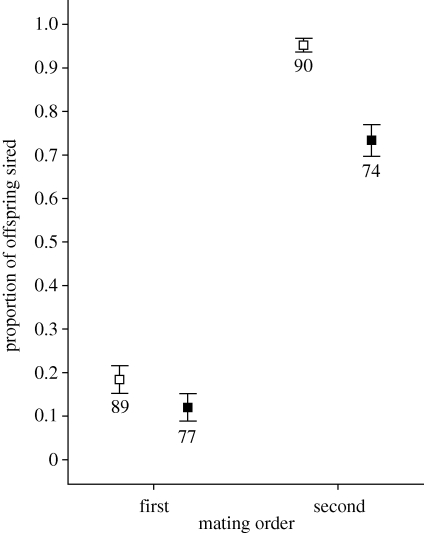
Overall, and as widely reported for insects (e.g. Simmons 2001), there was evidence of strong last-male sperm precedence (table 2 and figure 3); first males sired 15.3 ± 0.2 per cent versus second males siring 85.4 ± 0.2 per cent of offspring (n = 166 first-male and 164 second-male trials). Sperm precedence was significantly reduced for inbred males regardless of the mating order. Non-inbred males sired 57.0 ± 0.3 per cent of offspring (n = 179 trials), and inbred males only 42.0 ± 0.3 per cent of offspring (n = 151 trials). Non-inbred males were superior in both sperm defence (18.3 ± 0.3 versus 11.9 (±0.3) per cent, inbreeding load δ = 0.18) and sperm offence ability (95.2 ± 0.2 versus 73.3 ± 0.4 per cent, inbreeding load δ = 0.23), and there was a significant interaction such that inbred male sperm competitiveness was even more depressed for P2, i.e. in the sperm offence situation (table 2 and figure 3).
Table 2.
Results of the generalized linear model analysis for the sperm competition assay. There was strong second-male precedence overall, as well as superiority of non-inbred males in both first- and second-male sperm competition treatments. The male type × mating order interaction was also highly significant, as the difference between inbred and non-inbred males was greater in the second-male (P2) treatment. Statistically significant factors (p < 0.05) are marked in italic. See also figure 1 for the illustration of the experimental design and figure 3 for means and standard errors.
| source | d.f. | G2 | F | p |
|---|---|---|---|---|
| male (inbred/non-inbred) | 1 | 721.7 | 25.5 | <0.001 |
| mating order (first/second) | 1 | 8656.2 | 306.6 | <0.001 |
| male × mating order | 1 | 204.2 | 7.6 | 0.006 |
Figure 3.
Relative fertilization success for inbred (closed squares) and non-inbred (open squares) as either first- or second-to-mate males in two-male sperm competitions. Inbreeding significantly depresses both sperm defence and offence competitiveness (see figure 1 for the experimental design). Means presented ± standard error bars, numbers below error bars are sample sizes for each treatment. There are significant differences between inbred and non-inbred male sperm competition successes (p < 0.001), and a significant interaction with mating order (p = 0.006) (see table 2 for details).
We found no evidence for differences in total offspring number produced after sperm competitions involving either inbred or non-inbred males (p = 0.180, F1,326 = 1.804). Similarly, offspring number did not vary according to inbred or non-inbred males mating first or second in the sperm competitions (p = 0.161, F1,326 = 1.970), and there was no interaction between mating order and inbred/non-inbred male type (p = 0.570, F1,326 = 0.324). Therefore, we found no evidence for an effect of differential offspring viability on our sperm competition or male fertility results.
Although a failure to mate or sterility is an important contributor to male reproductive fitness, we were able to establish that the patterns are the result of competition between both males' sperm in the majority of trials. The second male to mate achieved at least some paternity in 310 of 330 trials; the rate of zero P2 was shared equally between inbred and non-inbred males (n = 10 and 10, respectively). These paternity data, combined with the fertility and mating behaviour results, suggest that the complete failure of a male to achieve insemination was very rare in our sperm competition trials. To further check that insemination failure was not an explanation for the sperm competition results, we conducted an analogous analysis as presented in table 2, but only for those trials where both first and second males achieved some paternity. The patterns were identical to the full dataset, with significant effects of treatment (p < 0.001, F1,155 = 14.086), mating order (p < 0.001, F1,155 = 469.418) and an interaction between these two (p = 0.037, F1,155 = 4.441).
(c). Male behaviour
Overall, inbred males were no less persistent or successful in mating than non-inbred males. Out of 18 females in each group, five females mated with the first male when he was inbred, and five when he was non-inbred (28% each). Similarly, during the second hour of the experiment (when females were given new males), three out of 18 females mated with inbred males and four of 18 females mated with non-inbred males (17 and 22%, respectively, Fisher's exact test: p = 1.000). Inbred males mounted as frequently as non-inbred males (inbred number of mounts = 3.28 (±0.48, first hour, n = 18) and 4.11 (±0.44, second hour, n = 18) versus non-inbred = 3.67 (±0.49, first hour, n = 18) and 3.56 (±0.44, second hour, n = 18)). Mounting rates did not differ statistically between inbred and non-inbred males (first hour: p = 0.479, r = −0.12, U = 139; second hour: p = 0.414, r = −0.14, U = 136).
Inbred males were as fast to achieve copula as non-inbred males. Copulation latency (seconds) for inbred males = 573 (±260, first hour, n = 5) and 310 (±31, second hour, n = 3) versus non-inbred males = 1012 (±421, first hour, n = 5) and 200 (±53, second hour, n = 4) did not differ statistically between inbred and non-inbred males (first hour: p = 0.690, r = −0.09, U = 10; second hour: p = 0.095, r = −0.30, U = 4). Inbred and non-inbred males did not differ in the length of mating either: copula duration (seconds) for inbred males = 310 (±31, first hour, n = 5) and 208 (±22, second hour, n = 3) versus non-inbred males = 200 (±53, first hour, n = 5) and 179 (±28, second hour, n = 4); first hour: p = 0.095, r = −0.30, U = 4; second hour: p = 0.629, r = −0.12, U = 4.
(d). Sperm morphometry
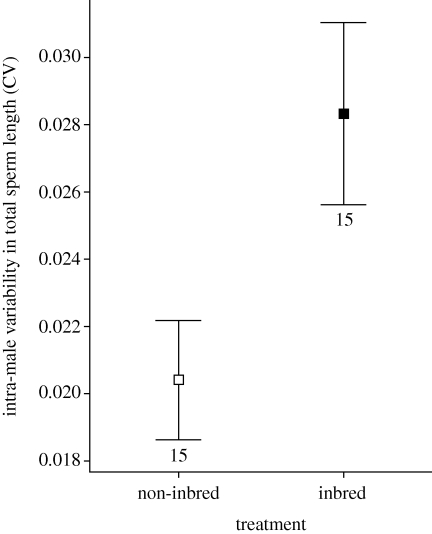
There was no difference in sperm length between inbred and non-inbred males (p = 0.617, r = 0.23, t28 = 1.248, nI = 15 and nN = 15; inbred mean sperm length = 92.5 ± 0.6 µm, non-inbred sperm length = 93.5 ± 0.6 µm). However, inbred males exhibited a 40 per cent higher intra-male variability in sperm length than non-inbred males (CV: 0.028 ± 0.003 versus 0.020 ± 0.002; inbreeding load δ = −0.40, figure 4); the difference was statistically significant at p = 0.020 (r = 0.42, t28 = 2.458, nI = 15 and nN = 15).
Figure 4.
Sperm length coefficients of variation are greater for inbred versus non-inbred males (N = 15 males in each, five sperm measured per male, p = 0.020, r = 0.42, t28 = 2.458). Means presented ± standard error bars, numbers below error bars are sample sizes for each treatment.
4. Discussion
After eight consecutive generations of inbreeding across replicate lines of T. castaneum, we find that male mating competence, male fertility and offspring viability show no inbreeding depression, but that inbred males suffer significantly reduced sperm competitiveness. Combining sperm competition results for both first- and second-male fertilization success (P1 and P2), inbred males sired an average of 15 per cent fewer offspring overall across 330 sperm competition comparisons. We also found a significant interaction, such that sperm offence (P2) suffered a greater degree of inbreeding depression (in absolute measures) than sperm defence (P1). The effect size in sperm offence was as large as the effect size of the overall high second-male precedence (i.e. r = −0.71). There are three non-mutually exclusive explanations for this interaction. It could be explained simply by the strength of the second-male precedence: because P2 is high in this species, any differences between males will be exaggerated compared with lower P1 values. It is also possible that inferior sperm will not be as disadvantaged in the P1 situation, because there is no competition for sperm from the first male to mate to enter and occupy storage, while in the P2 situation, sperm must be of sufficient quality to usurp established sperm from storage. A third potential explanation is cryptic female choice (Eberhard 1996): females that have been already mated and ensured fertility can afford to be more choosy, preferring sperm of males carrying greater genetic diversity, and there is some evidence for such sperm selection mechanisms (e.g. Stockley 1999; Tregenza & Wedell 2002; Firman & Simmons 2008).
Our sperm competition results are in contrast to the unexpected lack of inbreeding effect upon male fertility or mating competence. Detailed measures of fertility across 140 days of oviposition, followed by controlled re-mating to check that female fecundity/fertility was not affected by age, showed no effect of inbreeding upon sperm longevity or viability. Male T. castaneum transfer on average 100 000 spermatozoa during a single mating (Bloch Qazi et al. 1996; Arnaud et al. 2001a,b), and our fertility assay measured offspring production after 10 days of male–female interaction, which would have probably translated into multiple copulations (i.e. greater than 100 000 spermatozoa transferred to females) (Fedina & Lewis 2008). Females from both inbred and non-inbred treatments that survived until the end of the experiment produced on average 357 ± 23 total offspring during the whole ovipositing period, amounting to less than 0.5 per cent of a single average ejaculate. Thus, any fertility declines through time are likely to have occurred via sperm loss or death in the spermatheca, and not as the result of limited sperm numbers initially being transferred.
Inbred males did not differ from non-inbred males in mating latency, mating duration or number of mounts, and we found evidence of insemination success for the overwhelming majority of second-mating males. Furthermore, females from treatments where experimental males were inbred produced the same numbers of offspring as females from non-inbred male treatments, indicating that offspring viability could not account for the decline in sperm competition ability. Finally, because we controlled for the lack of sexual selection in the inbred lines by enforcing monogamous selection through the control non-inbred lines, the inevitably relaxed selection on male reproductive traits from monogamy under inbreeding (e.g. Hosken et al. 2001) cannot explain the inbreeding depression in sperm competitiveness. Thus, it seems most likely that inbreeding depression in sperm competitiveness was caused by a decrease in either sperm quantity or quality that is critical for relative competitiveness, but still allows full male fertilization success to be achieved under benign, competition-free conditions.
Inbreeding depression of sperm competitive ability has been recently identified in three other systems. Male mites subjected to a single sib × sib generation of inbreeding (F1 = 0.25) showed reduced sperm competitiveness against non-inbred equivalents, with a reduction in average relative fertilization success from 44 per cent for non-inbred males to 21 per cent for inbred males in two-male sperm competitions (Konior et al. 2005). Wild, outbred golden hamster males (Mesocricetus auratus) also showed significantly increased fertilization success (87%) in competition with inbred, laboratory hamsters (Fritzsche et al. 2006). Measures of mating behaviour and sperm quality and quantity showed no differences between the inbred laboratory strains and the outbred wild strains, and laboratory males show full effective fertility (Fritzsche et al. 2006). This hamster study therefore parallels our own findings that inbreeding depression of male reproductive fitness is only exposed under competition. While it is not possible to exclude an effect of wild selection on this increased sperm competition success in hamsters, it is notable that laboratory hamster heterozygosity was effectively zero, compared with H = 0.71 for wild-derived males. Finally, using artificial insemination to control sperm number and male mating behaviour effects, Zajitschek et al. (2009) showed that sperm from highly inbred (F4 = 0.59) male guppies was significantly inferior in competition for fertilizations with sperm from non-inbred males; moderately inbred males (F1 = 0.25) did not show inbreeding depression in sperm competitiveness (Zajitschek et al. 2009).
There are three explanations for the different responses of male fertility and sperm competitiveness to inbreeding. First, it is possible that genes for male fertility traits have been purged of deleterious recessive alleles through one or more genetic bottlenecks, so do not suffer from inbreeding depression (Barrett & Charlesworth 1991; Hedrick 1994), while genes for traits that govern sperm competitiveness retain deleterious recessives. It is possible that there may be spermatozoal trait optima within sperm competition that do not match those within fertility; for example, there are differences between trait optima that govern sperm offence (P1) and sperm defence (P2) within sperm competition (e.g. Clark et al. 1999; Bjork et al. 2007). However, purging of male fertility genes alone seems an unlikely single explanation for our results because, in this highly promiscuous species, fertilization will almost always be achieved in the face of sperm competition. Genes that control relative fertility will therefore mostly also control sperm competitiveness, and the promiscuous mating pattern will force selection on them in tandem.
Second, it is possible that the relaxed selection on sperm competition traits under monogamy has led to more rapid ‘experimental evolution’ of the inbred lines away from the ancestral state driven by polyandry. Experimental evolution of sperm competitiveness has been demonstrated after selection from enforced monogamy (e.g. in dung flies, Hosken et al. 2001), and although it is possible that resource allocation away from sperm competition traits might logically occur under challenges arising from inbreeding, we might predict that the non-inbred lines would respond to experimental evolution more rapidly because of greater standing genetic variation from the start of selection. It is possible that any decline in sperm quality under inbreeding is only detectable when sperm competition is invoked.
The third, and more likely, explanation for the clear differences between the fertility and competitiveness we find is that inbreeding depression can be revealed or exacerbated under selection from competition (e.g. Bijlsma et al. 1999, 2000; Joron & Brakefield 2003). Studies under benign laboratory or captive conditions may fail to detect inbreeding depression that would otherwise be relevant for inherent selection pressures of the natural environment. Under our own laboratory conditions, we have shown that male fertility and mating competence are not affected by inbreeding. However, when the relevant and widespread condition of post-copulatory male–male competition for fertilization is generated, significant inbreeding depression is exposed. This more subtle constraint on male reproductive fitness could translate into influences on individual male gene flow within animal mating systems in the natural environment that generate sperm competition. Tribolium castaneum is likely to undergo frequent population genetic bottlenecking as a sporadic invader of stored food products; the same could be said of any taxon that is an opportunistic invader of unpredictable resources, where reproducing populations can be created from the invasion of single adult females. Given that our ancestral GA1 population is a laboratory stock, it seems reasonable to assume that some previous population bottlenecking has reduced genetic diversity prior to our experiments. Thus, in spite of the relatively high coefficient of inbreeding of Tribolium males in our study (F8 = 0.83, calculated according to Wright 1921), our findings could be relevant to wild populations that have already experienced such genetic bottlenecks, such as New Zealand black robins (Ardern & Lambert 1997) or cheetahs (Menotti-Raymond & O'Brien 1993).
It has long been suspected that spermatozoal form and function is sensitive to inbreeding, with highly inbred big cat species showing inferior sperm quality (e.g. Wildt et al. 1983; Roelke et al. 1993) and inbreeding under domestication, leading to impaired sperm quality (e.g. Diarra et al. 1997). More replicated comparisons across inbreeding coefficients in the wild (Gage et al. 2006) and captivity (Gomendio et al. 2000) show that increasing sperm abnormality is correlated with decreasing measures of heterozygosity. A recent comparative analysis across 20 mammal species with varying levels of inbreeding shows a significant interspecific relationship between genetic variability and sperm abnormality for endangered taxa (Fitzpatrick & Evans 2009). Spermatozoa may be more prone to inbreeding depression because they are the most specialized of eukaryotic cells and are under complex developmental control within the diploid male (e.g. White-Cooper et al. 2009), in which disruption of gene regulation can lead to infertility (e.g. Hargreave 2000). Insect sperm do not generally manifest cellular abnormalities, akin to those commonly found in more complex mammalian sperm. However, we did examine variability in sperm morphometry and found that inbred males had a 40 per cent higher CV in sperm length compared with non-inbred males (figure 4). This finding may suggest that the production of less uniform sperm could be an expression of developmental errors, analogous to those found across mammals (e.g. Fitzpatrick & Evans 2009).
Acknowledgements
We are grateful to Richard Beeman and his laboratory at the US Department of Agriculture in Kansas who kindly provided us with the strains of flour beetles, and Claudia Fricke (UEA) for her help with analyses of binomial distributions. Paula Stockley and Jon Evans improved the presentation and interpretation of the study. This work was funded by the Natural Environment Research Council and the University of East Anglia. O.Y.M. is supported by the Swiss National Science Foundation.
References
- Ardern S. L., Lambert D. M.1997Is the black robin in genetic peril? Mol. Ecol. 6, 21–28 (doi:10.1046/j.1365-294X.1997.00147.x) [Google Scholar]
- Arnaud L., Haubruge E., Gage M. J. G.2001aThe dynamics of second- and third-male fertilization precedence in Tribolium castaneum. Ent. Exp. Appl. 99, 55–64 (doi:10.1023/A:1018902307721) [Google Scholar]
- Arnaud L., Haubruge E., Gage M. J. G.2001bSperm size and number variation in the red flour beetle. Zool. J. Linn. Soc. 133, 369–375 (doi:10.1111/j.1096-3642.2001.tb00631.x) [Google Scholar]
- Asa C., Miller P., Agnew M., Rebolledo J. A. R., Lindsey S. L., Callahan M., Bauman K.2007Relationship of inbreeding with sperm quality and reproductive success in Mexican gray wolves. Anim. Conserv. 10, 326–331 (doi:10.1111/j.1469-1795.2007.00116.x) [Google Scholar]
- Attia F. A., Tregenza T.2004Divergence revealed by population crosses in the red flour beetle Tribolium castaneum. Evol. Ecol. Res. 6, 927–935 [Google Scholar]
- Barrett S. C. H., Charlesworth D.1991Effects of a change in the level of inbreeding on the genetic load. Nature 352, 522–524 (doi:10.1038/352522a0) [DOI] [PubMed] [Google Scholar]
- Bijlsma R., Bundgaard J., Van Putten W. F.1999Environmental dependence of inbreeding depression and purging in Drosophila melanogaster. J. Evol. Biol. 12, 1125–1137 (doi:10.1046/j.1420-9101.1999.00113.x) [Google Scholar]
- Bijlsma R., Bundgaard J., Boerema A. C.2000Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. J. Evol. Biol. 13, 502–514 (doi:10.1046/j.1420-9101.2000.00177.x) [Google Scholar]
- Birkhead T. R., Møller A. P.1998Sperm competition and sexual selection. London, UK: Academic Press [Google Scholar]
- Bjork A., Starmer W. T., Higginson D. M., Rhodes C. J., Pitnick S.2007Complex interactions with females and rival males limit the evolution of sperm offence and defence. Proc. R. Soc. B 274, 1779–1788 (doi:10.1098/rspb.2007.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi M. C., Herbeck J. T., Lewis S. M.1996Mechanisms of sperm transfer and storage in the red flour beetle (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 89, 892–897 [Google Scholar]
- Charlesworth D., Charlesworth B.1987Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 18, 237–268 (doi:10.1146/annurev.es.18.110187.001321) [Google Scholar]
- Charpentier M., Setchell J. M., Prugnolle F., Knapp L. A., Wickings E. J., Peignot P., Hossaert-McKey M.2005Genetic diversity and reproductive success in mandrills (Mandrillus sphinx). Proc. Natl. Acad Sci. USA 102, 16 723–16 728 (doi:10.1073/pnas.0507205102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Begun D. J., Prout T.1999Female × male interactions in Drosophila sperm competition. Science 283, 217–220 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- Crawley M. J.2005Statistics: an introduction using R. New York, NY: John Wiley & Sons [Google Scholar]
- Crow J. F.1952Dominance and overdominance. In Heterosis (ed. Gowen J. W.), pp. 282–297 Ames, IA: Iowa State College Press [Google Scholar]
- Crudgington H. S., Fellows S., Badcock N. S., Snook R. R.2009Experimental manipulation of sexual selection promotes greater male mating capacity but does not alter sperm investment. Evolution 63, 926–938 (doi:10.1111/j.1558-5646.2008.00601.x) [DOI] [PubMed] [Google Scholar]
- DeRose M. A., Roff D. A.1999A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution 53, 1288–1292 (doi:10.2307/2640831) [DOI] [PubMed] [Google Scholar]
- Diarra M. S., Paré J. P., Roy G.1997Genetic and environmental factors affecting semen quality of young Holstein bulls. Can. J. Anim. Sci. 77, 77–85 (doi:10.4141/A95-084) [Google Scholar]
- Eberhard W. G.1996Female control: sexual selection by female cryptic choice. Monographs in Behavior and Ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- Fedina T. Y., Lewis S. M.2008An integrative view of sexual selection in Tribolium flour beetles. Biol. Rev. 83, 151–171 (doi:10.1111/j.1469-185X.2008.00037.x) [DOI] [PubMed] [Google Scholar]
- Firman R. C., Simmons L. W.2008Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution 62, 603–611 (doi:10.1111/j.1558-5646.2007.00307.x) [DOI] [PubMed] [Google Scholar]
- Fitzpatrick J. L., Evans J. P.2009Reduced heterozygosity impairs sperm quality in endangered mammals. Biol. Lett. 5, 320–323 (doi:10.1098/rsbl.2008.0734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankham R., Ballou J. D., Briscoe D. A.2002Introduction to conservation genetics. Cambridge, UK: Cambridge University Press [Google Scholar]
- Fritzsche P., Neumann K., Nasdal K., Gattermann R.2006Differences in reproductive success between laboratory and wild-derived golden hamsters (Mesocricetus auratus) as a consequence of inbreeding. Behav. Ecol. Sociobiol. 60, 220–226 (doi:10.1007/s00265-006-0159-3) [Google Scholar]
- Gage M. J. G., Surridge A. K., Tomkins J. L., Green E., Wiskin L., Bell D. J., Hewitt G. M.2006Reduced heterozygosity depresses sperm quality in wild rabbits, Oryctolagus cuniculus. Curr. Biol. 16, 612–617 (doi:10.1016/j.cub.2006.02.059) [DOI] [PubMed] [Google Scholar]
- Gomendio M., Cassinello J., Roldan E. R. S.2000A comparative study of ejaculate traits in three endangered ungulates with different levels of inbreeding: fluctuating asymmetry as an indicator of reproductive and genetic stress. Proc. R. Soc. Lond. B 267, 875–882 (doi:10.1098/rspb.2000.1084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreave T. B.2000Genetic basis of male fertility. Brit. Med. Bull. 56, 650–671 (doi:10.1258/0007142001903454) [DOI] [PubMed] [Google Scholar]
- Hedrick P. W.1994Purging inbreeding depression. Heredity 73, 363–372 (doi:10.1038/hdy.1994.183) [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Parsons P. A.1991Evolutionary genetics and environmental stress. Oxford, UK: Oxford University Press [Google Scholar]
- Höglund J., Piertney S. B., Alatalo R. V., Lindell J., Lundberg A., Rintamäki P. T.2002Inbreeding depression and male fitness in black grouse. Proc. R. Soc. Lond. B 269, 711–715 (doi:10.1098/rspb.2001.1937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D. J., Garner T. W. J., Ward P. I.2001Sexual conflict selects for male and female reproductive characters. Curr. Biol. 11, 489–493 [DOI] [PubMed] [Google Scholar]
- Ilmonen P., Stundner G., Thoß M., Penn D. J.2009Females prefer the scent of outbred males: good-genes-as-heterozygosity? BMC Evol. Biol. 9, 104 (doi:10.1186/1471-2148-9-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M., Brakefield P. M.2003Captivity masks inbreeding effects on male mating success in butterflies. Nature 424, 191–194 (doi:10.1038/nature01713) [DOI] [PubMed] [Google Scholar]
- Keller L. F., Waller D. M.2002Inbreeding effects in wild populations. Trends. Ecol. Evol. 17, 230–241 (doi:10.1016/S169_5347(02)02489-8) [Google Scholar]
- Konior M., Keller L., Radwan J.2005Effect of inbreeding and heritability of sperm competition success in the bulb mite Rhizoglyphus robini. Heredity 94, 577–581 (doi:10.1038/sj.hdy.6800649) [DOI] [PubMed] [Google Scholar]
- Margulis S. W., Walsh A.2002The effects of inbreeding on testicular sperm concentration in Peromyscus polionotus. Reprod. Fertil. Dev. 14, 63–67 (doi:10.1071/RD01120) [DOI] [PubMed] [Google Scholar]
- Marr A. B., Arcese P., Hochachka W. M., Reid J. M., Keller L. F.2006Interactive effects of environmental stress and inbreeding on reproductive traits in a wild bird population. J. Anim. Ecol. 75, 1406–1415 (doi:10.1111/j.1365-2656.2006.01165.x) [DOI] [PubMed] [Google Scholar]
- Martin O. Y., Hosken D. J.2003Costs and benefits of evolving under experimentally enforced polygamy or monogamy. Evolution 57, 2765–2772 [DOI] [PubMed] [Google Scholar]
- Meagher S., Penn D. J., Potts W. K.2000Male–male competition magnifies inbreeding depression in wild house mice. Proc. Natl. Acad Sci. USA 97, 3324–3329 (doi:10.1073/pnas.060284797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti-Raymond M., O'Brien S. J.1993Dating the genetic bottleneck of the African cheetah. Proc. Natl. Acad Sci. USA 90, 3172–3176 (doi:10.1073/pnas.90.8.3172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk Ł.2008Sexual selection and reproductive compatibility in Tribolium castaneum. PhD thesis, University of East Anglia, Norwich [Google Scholar]
- Pray L. A., Schwartz J. M., Goodnight C. J., Stevens L.1994Environmental dependency of inbreeding depression: implications for conservation biology. Conserv. Biol. 8, 562–568 (doi:10.1046/j.1523-1739.1994.08020562.x) [Google Scholar]
- Roelke M. E., Martenson J. S., O'Brien S. J.1993The consequences of demographic reduction and genetic depletion in the endangered Florida panther. Curr. Biol. 3, 340–350 (doi:10.1016/0960-9822(93)90197-V) [DOI] [PubMed] [Google Scholar]
- Roldan E. R. S., Cassinello J., Abaigar T., Gomendio M.1998Inbreeding, fluctuating asymmetry and ejaculate quality in an endangered ungulate. Proc. R. Soc. Lond. B 265, 243–248 (doi:10.1098/rspb.1998.0288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M.1984The effect of inbreeding on competitive male-mating ability in Drosophila melanogaster. Genetics 106, 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W.2001Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- Simmons L. W., García-González F.2008Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591 (doi:10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- Slate J., Kruuk L. E. B., Marshall T. C., Pemberton J. M., Clutton-Brock T. H.2000Inbreeding depression influences lifetime breeding success in a wild population of red deer (Cervus elaphus). Proc. R. Soc. Lond. B 267, 1657–1662 (doi:10.1098/rspb.2000.1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff A.1972The biology of Tribolium with special emphasis on genetic aspects. Oxford, UK: Clarendon Press [Google Scholar]
- Stockley P.1999Sperm selection and genetic incompatibility: does relatedness of mates affect male success in sperm competition? Proc. R. Soc. Lond. B 266, 1663–1669 (doi:10.1098/rspb.1999.0829) [Google Scholar]
- Szulkin M., Sheldon B. C.2007The environmental dependence of inbreeding depression in a wild bird population. PLoS ONE 2, e1027 (doi:10.1371/journal.pone.0001027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- The R Development Core Team 2005R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Tregenza T., Wedell N.2002Polyandrous females avoid costs of inbreeding. Nature 415, 71–73 (doi:10.1038/415071a) [DOI] [PubMed] [Google Scholar]
- Waser N. M.1993Sex, mating systems, inbreeding, and outbreeding. In The natural history of inbreeding and outbreeding—theoretical and empirical perspectives (ed. Wilmsen-Thornhill N.), pp. 1–13 Chicago, IL: The University of Chicago [Google Scholar]
- Weeks S. C., Reed S. K., Ott D. W., Scanabissi F.2009Inbreeding effects on sperm production in clam shrimp (Eulimnadia texana). Evol. Ecol. Res. 11, 125–134 [Google Scholar]
- White-Cooper H., Doggett K., Ellis R. E.2009The evolution of spermatogenesis. In Sperm biology: an evolutionary perspective (eds Birkhead T. R., Hosken D. J., Pitnick S.), pp. 151–183 Burlington, VT: Academic Press [Google Scholar]
- Wildt D. E., Bush M., Howard J. G., O'Brien S. J., Meltzer D., Dyk A. V., Ebedes H., Brand D. J.1983Unique seminal quality in the South African cheetah and a comparative evaluation in the domestic cat. Biol. Reprod. 29, 1019–1025 (doi:10.1095/biolreprod29.4.1019) [DOI] [PubMed] [Google Scholar]
- Wright S.1921Systems of mating (I–V). Genetics 6, 111–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek S. R. K., Lindholm A. K., Evans J. P., Brooks R. C.2009Experimental evidence that high levels of inbreeding depress sperm competitiveness. J. Evol. Biol. 22, 1338–1345 (doi:10.1111/j.1420-9101.2009.01738.x) [DOI] [PubMed] [Google Scholar]