Abstract
Interactions within and between species complicate quantification of climate effects, by causing indirect, often delayed, effects of climate fluctuations and compensation of mortality. Here we identify direct and indirect climate effects by analysing unique Russian time-series data from the Norwegian Sea–Barents Sea ecosystem on the first life stages of cod, capelin, herring and haddock, their predators, competitors and zooplanktonic prey. By analysing growth and survival from one life stage to the next (eggs–larvae–juveniles–recruits), we find evidence for both bottom-up, direct and top-down effects of climate. Ambient zooplankton biomass predicts survival of all species, whereas ambient temperature mainly affects survival through effects on growth. In warm years, all species experienced improved growth and feeding conditions. Cohorts born following a warm year will, however, experience increased predation and competition because of increased densities of subadult cod and herring, leading to delayed climate effects. While climate thus affects early growth and survival through several mechanisms, only some of the identified mechanisms were found to be significant predictors of population growth. In particular, our findings exemplify that climate impacts are barely propagated to later life stages when density dependence is strong.
Keywords: ecological climate effects, ecosystem dynamics, density dependence, multiple imputation, population models, zooplankton
1. Introduction
Understanding the ecological effects of past and present climate variability is important for predicting the effects of the anticipated future climate change (Stenseth et al. 2002). However, our comprehension of how climate affects populations is superficial and fragmented. Field and experimental studies may, for example, shed light on the mechanisms through which climate act (e.g. Rijnsdorp et al. 2009), but often cover only part of the food web (often only one species), part of the life cycle and/or relatively short time scales. Climate factors that affect survival of one species may also indirectly affect its prey, competitors and predators, often with time lags (Hjermann et al. 2004b). Furthermore, climate effects on early survival may be modulated by density dependence of survival later in life, causing compensation or depensation. For these reasons, many studies are inadequate when it comes to assessing which mechanisms are quantitatively important in influencing interannual fluctuations in the populations. Improved understanding of how climate affects populations—directly or indirectly, linearly or nonlinearly, and additively or non-additively—may lead to improved predictions of how climate will affect the ecosystem in the future (Lima & Berryman 2006).
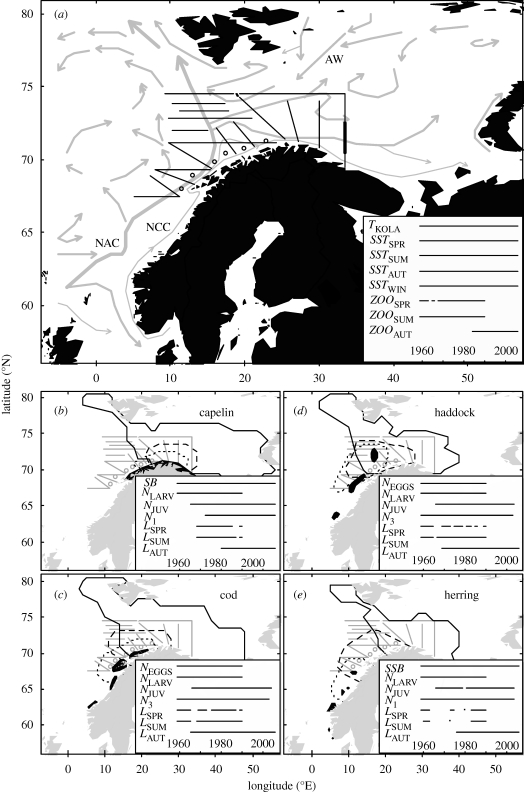
Here we analyse climate effects on four fish species in the Barents and Norwegian Seas of the Northeast Atlantic (figure 1). The high productivity of this region sustains rich fish resources, including the currently largest stocks of cod, herring and capelin in the world. Partly because of these rich, but fluctuating, biological resources, the region has been a focus of primarily Russian and Norwegian scientific investigations for more than a century (Matyshov et al. 1998). After the foundation of the International Council for the Exploration of the Sea (ICES) in 1902, the region became one of the model regions from which marine ecology in general and fisheries oceanography in particular emerged. During the last century, large amounts of data on hydrography, plankton, larval and adult fish were collected using the most advanced methods of the era. This ecosystem is thus a powerful model system and is well studied through statistical analysis of both climate effects on single species, especially cod (Ottersen & Loeng 2000) and herring (Ottersen & Loeng 2000; Sætre et al. 2002), and recently, multi-species dynamics (Dingsør et al. 2007; Hjermann et al. 2007). However, some of the most exceptional Russian data series on zooplankton, fish eggs and fish larvae, which were consistently collected biannually for more than 30 years (figure 1), remain underused, in particular for analysing climate effects.
Figure 1.
Multi-species system analysed in the Norwegian and Barents Seas. (a) Simplified current system (arrows, NAC: North Atlantic Current, NCC: Norwegian Coastal Current, AW: Arctic Water) and coverage of spring and summer zoo- and ichthyo-plankton-surveys (lines: transects, circles: coastal banks). Autumn surveys generally covered ice-free parts of the Barents Sea. Bold line: part of Kola transect used to calculate TKOLA. Superimposed: temporal coverage of direct and indirect climate series analysed (table 1). (b–e) Spawning areas (black; capelin: March–April, cod: February–April, haddock: March–June, herring: February–March) and typical larval/juvenile distribution areas (stippled lines: spring, broken lines: summer, unbroken lines: autumn; see electronic supplementary material, appendix S1 for information sources). Superimposed: temporal coverage of fish population time- series analysed (table 1). Focal period: 1959–2006. Some series (recruitment, spawning stock, temperature) start earlier than 1959 (§2).
The fish stocks analysed are Northeast Arctic cod (Gadus morhua), Norwegian spring spawning herring (Clupea harengus), Northeast Arctic haddock (Melanogrammus aeglefinus) and Barents Sea capelin (Mallotus villosus), hereafter only referred to as cod, herring, haddock and capelin. All four populations spawn in partly overlapping areas off the western and northern coasts of Norway and Russia in late winter or early spring (figure 1). Eggs and larvae drift northwards and eastwards with the North Atlantic and Norwegian coastal currents, and by autumn, juveniles are widely distributed in the Barents Sea, which is the main nursery area for all populations. All species feed on zooplankton during their early life stages (Huse & Toresen 1996; Orlova et al. 2008; Dalpadado et al. 2009).
Russian survey data and other biological and climatologic data (figure 1) are used here to disentangle direct and indirect climate effects on the four species. We ask: (i) How are growth and survival through early life stages influenced by temperature, prey, competitors and predators? (ii) How do these factors influence population growth? (iii) How do climate fluctuations in sum (through both direct and indirect effects) affect the populations? Results shed new light on how climate affects the dynamics of marine fish populations through interplay between effects on individual growth and feeding conditions, density dependence and species interactions.
2. Material and methods
(a). Data
Fish eggs and larvae were sampled in April–May and June–July 1959–1990 by the Knipovich Polar Research Institute of Marine Fisheries and Oceanography (PINRO), Murmansk (Mukhina 1992; Mukhina et al. 2003). Fish juveniles were sampled by international zero-group surveys in August–September 1965–2006 (ICES 2007a). From these data sources, we obtained relative indices of egg, larva and juvenile abundances (see electronic supplementary material, appendix S1). Recruitment (age 1 year) and biomass of capelin were estimated from September–October acoustic surveys (ICES 2007a, 1973–2006 data) and from the frequency of capelin in cod stomach samples (Marshall et al. 2000, 1946–1972 biomass estimates). Recruitment (age 3 years) and biomass of cod and haddock were estimated by virtual population analysis based on catch data (Hylen 2002, 1913–1946 estimates for cod; ICES 2007a, 1946 (haddock: 1950)–2006 data). Recruitment (age 1 year) and biomass of herring were estimated using ‘SeaStar’ analysis based on data from several sources, including catch and survey data (1950–2006 data, obtained from ICES 2007b) and virtual population analysis (1907–1950 data, obtained from Toresen & Østvedt 2000).
Zooplankton biomass in April–May and June–July 1959–1990 (biannual average values for each transect and coastal bank; figure 1) was sampled from 50 m to surface using Juday plankton nets (37 cm diameter, 180 μm mesh) by PINRO, Murmansk (Nesterova 1990; Stige et al. 2009). Zooplankton biomass in August–October 1981–2006 was sampled from bottom to surface using WP2 and MOCNESS plankton nets (1984–1990: 333 μm mesh, 1991–: 180 μm) by the Institute of Marine Research, Bergen (Dalpadado et al. 2003). Interannual differences in sampling methods were not found to bias biomass estimates (analysis not shown).
The zooplankton sampling gears capture mesozooplankton most efficiently, such as the dominant Calanus finmarchicus. As the diets of fish larvae and juveniles also contain smaller and larger items, respectively, food effects may be underestimated. Especially in spring, fish larvae mainly eat copepod nauplii, while zooplankton samples mainly represent the parental generation of copepods. In addition, the typical spring distribution of herring larvae is only partly covered by the surveys (figure 1), which reduces the accuracy of the estimation of spring feeding conditions for herring. Mesozooplankton remain important prey for capelin and herring throughout life, but for cod and haddock the autumn sampling period represents a transition stage, as after about 0.5 years age they feed mainly on larger prey (Dalpadado et al. 2009).
Monthly mean sea surface temperature data (ICOADS two-degree enhanced dataset; Worley et al. 2005) for 1906–2006 were provided by the NOAA/OAR/ESRL PSD, Boulder, CO, USA (http://www.cdc.noaa.gov/). Annual temperature data from the Kola section (Tereschenko 1996) for 1921–2006 were provided by PINRO (http://www.pinro.ru/).
(b). Statistical analysis
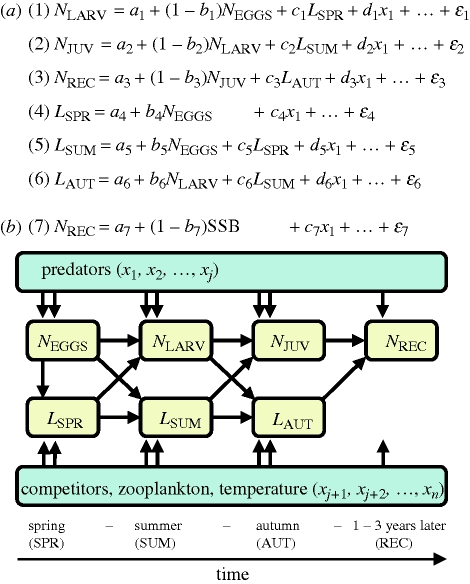
The analysis was divided into two main steps, as outlined in figure 2. In step 1, we developed models to predict growth and survival of each species as a function of temperature, prey, predators and competitors. Akaike's information criterion corrected for small sample size (AICC; Hurvich & Tsai 1991) was used to choose the best models. In step 2, we addressed how important each of the mechanisms identified in step 1 are in influencing population growth rates of the four species, and how climate fluctuations, in sum, affect populations. The focal period was 1959–2006 in step 1 and 1906–2006 in step 2 (but data availability restricted most analyses to shorter periods within these time ranges). The program R (R Development Core Team 2006) was used for all analyses.
Figure 2.
Approach for analysing climate effects. (a) Step 1: For each species, growth and survival can be partitioned into changes in abundance and mean length (N[STAGE] and L[SEASON], respectively) through successive life stages, which are interrelated and affected by predators, competitors, zooplanktonic prey and temperature. Environmental effects on each population are quantified by a series of regression models, in which predictor variables are selected based on AICC. These models identify the key mechanisms through which climate directly or indirectly affects cohort survival. (b) Step 2: all thus selected environmental variables are included in one spawning stock–recruitment model, quantifying their effects on population growth.
(i). Estimation of stage-wise growth and survival models
The first step was to develop stage-wise growth and survival models, quantifying effects of environmental variables on growth and survival through successive early life stages of each species (figure 2a). To do so, six time-series regression models were fitted for each species: (i) survival from egg (alternatively, egg production) to larval stage, (ii) survival from larval to juvenile stage, (iii) survival from juvenile to recruitment stage, and growth of larvae until (iv) spring, (v) summer, and (vi) autumn.
In survival models (models 1–3, figure 2a), we assumed a log-linear relationship between past and present cohort size (the ‘Gompertz model’, Hjermann et al. 2004a):
| 2.1 |
Here, ln(nt) denotes the natural logarithm of cohort size at stage t, and the parameters ‘a’ and ‘b’ represent density-independent and density-dependent mortality, respectively. By adding predictor variables into this model, environmental effects on survival were estimated. The problem of an upward bias in b in equation (2.1) as a result of measurement errors in ln(nt−1) (Carrol et al. 1995) is expected to be quite small owing to the large variance in ln(nt−1). In growth models (models 4–6, figure 2a), response variables were mean length-at-age in different seasons. Previous-season length-at-age was optionally included as a covariate, but omitted when non-significant in order to include more years in the analysis. The models were estimated using least-squares linear regression, or in the presence of significant residual serial autocorrelation, by order-1 auto-regressive-moving-average (ARMA) generalized least-squares regression as implemented in the nlme library of R.
(ii). Selection of predictor variables
Potential predictor variables included intra-cohort variables (previous-stage cohort size and length-at-age as shown in figure 2), indices of growth and feeding conditions (ambient zooplankton biomass, ambient sea surface temperature and annual temperature in Atlantic water at the Kola section in the southern Barents Sea), competitors and predators (table 1). See electronic supplementary material, table S1.1 in appendix S1 to see which variables were considered in each model. All environmental variables refer to conditions during the first year of life of the fish. Alternative seasonal formulations of the zooplankton and sea surface temperature indices were initially considered, and for each model (1–6) we chose the formulation providing the overall best explanatory power across the four species (see electronic supplementary material, table S1.2 in appendix S1).
Table 1.
Variables used in the analysisa.
| N[STAGE] | cohort size (ln-scale) at subsequent life stages: (i) relative abundance of eggs in springb (NEGGS), or alternatively, for species with demersal eggs, stock biomass the preceding autumn (SB; capelin) or spawning stock biomass (SSB; herring). (ii) Relative abundance of larvaec in spring and summer (NLARV). (iii) Relative abundance of juveniles (0.5 year olds) in autumn (NJUV). (iv) Abundance of recruits at age 1 years (N1; capelin, herring) or 3 years (N3; cod, haddock) back-calculated to the year of spawning |
| L[SEASON] | mean lengths of larvaec or juveniles in springb (LSPR), summer (LSUM) and autumn (LAUT) |
| SST[SEASON] | seasonal ambient sea surface temperature indices calculated as mean sea surface temperature in the typical distribution areas (figure 1) of each species in different seasons during their first living-yearb (SSTSPR, SSTSUM, SSTAUT, SSTWIN) or integrated across seasons (SSTSPR-SUM, SSTAUT-WIN) |
| ZOO[SEASON] | seasonal ambient zooplankton indices calculated as ln of mean zooplankton biomass in the typical distribution areas (figure 1) of each species in different seasons during their first living-yearb (ZOOSPR, ZOOSUM, ZOOAUT) or integrated across seasons (ZOOSPR-SUM) |
| TKOLA | annual (January–December) mean sea temperature (0–200 m depth) in the Kola section (70°30′ N to 72°30′ N, along 33°30′ E; figure 1)—an indicator of the climate of the southern, Atlantic-influenced, part of the Barents Sea (Ottersen & Sundby 1995; Ottersen & Loeng 2000) |
| CAP[AGE] | potential competitors (see electronic supplementary material, table S1.1 in appendix S1): ln(abundance) of capelin larvae (CAPLARV) or juveniles (CAP0) or ln(stock biomass) (CAPSB) |
| COD[AGE] | potential competitors and/or predators (see electronic supplementary material, table S1.1): ln(abundance) of cod eggs (CODEGGS), larvae (CODLARV), juveniles (COD0) or subadults (age 3–6 years, COD3–6)d |
| HAD[AGE] | potential competitors (see electronic supplementary material, table S1.1): ln(abundance) of haddock eggs (HADEGGS), larvae (HADLARV) or juveniles (HAD0) |
| HER[AGE] | potential competitors and/or predators (see electronic supplementary material, table S1.1): ln(biomass) of herring spawners (HERSSB), ln(abundance) of herring larvae (HERLARV), juveniles (HER0), subadults (age 1–2, HER1–2) or adults (age 3+, HER3+) |
aSee figure 1 or §2a for temporal coverage of data series.
bSeasons: spring (April–May), summer (June–July), autumn (August–October) and winter (November–March).
cLarvae: young-of-the-year fish sampled in both spring and summer, notwithstanding undetermined proportions of the individuals sampled in summer have metamorphosed to post-larval stages.
dBecause predation by subadult cod may depend on the cod/capelin ratio (Hjermann et al. 2007), CAPSB was considered as predictor together with COD3–6. This is equivalent to considering ln[(number of age 3–6 cod)/(capelin biomass)a] as predictor. The effect of CAPSB was not significantly larger than zero in any model with COD3–6. CAPSB was therefore not included (‘a’ = 0).
We selected which predictor variables to include in the models by comparing AICC-values of alternative model formulations, starting with the full additive model and removing non-significant terms (p > 0.05 or 0.01, see below) one by one. Model selection was repeated on extended datasets if potential predictor variables containing missing values were not selected. Because of the large number of models and potential predictor variables, we expected some effects to reach statistical significance just by chance. We also expected that spurious effects could arise from correlations with factors other than the one tested for through a given variable. To find an optimal trade-off between types I (‘false positive’) and II (‘false negative’) error rates, we decided critical levels (p = 0.05 or 0.01) on a variable group by variable group basis by comparing initial results with calculated type I error rates (see electronic supplementary material, appendix S1 for details). Using an initial cut-off value of p = 0.05 (see electronic supplementary material, table S1.3 in appendix S1), we found that numbers of significant effects of variables measuring intra-cohort effects, growth and feeding conditions and predation exceeded numbers expected to arise from chance by factors of around 4 (see electronic supplementary material, table S1.4 in appendix S1). We found this to indicate an acceptable ‘signal : noise’ ratio of reported results and retained a critical level of 0.05 for these variable groups. However, the number of significant effects of competition variables was not higher than expected to arise by chance. Further, effects were more often positive than negative; from direct interactions, negative effects were to be expected. We considered this indicative of a high proportion of spurious effects and chose a stricter critical level of 0.01 for competition variables.
(iii). Non-additive and nonlinear effects
After finding the most parsimonious additive models, we considered models with interactions between the effects of population size and zooplankton, temperature and zooplankton, temperature and predators, zooplankton and predators, and zooplankton and competitors. We explored possible nonlinear effects using generalized additive models (Wood 2006).
(iv). Effects on population growth
In the second step of the analysis (figure 2b), we addressed how environmental factors influence population growth rates. We did this through two complementary approaches, both involving regression models with recruitment back-calculated to spawning year as response, and spawning stock biomass and covariates identified in step 1 as predictor variables.
We first assessed the statistical evidence for the effects of the selected temperature, zooplankton, predation and competition variables on recruitment, using imputation models to fill in missing data. That way all available data were analysed in one model for each species (data coverage: year-classes 1972–2006, 1910–2003, 1947–2003 and 1906–2004, respectively, for capelin, cod, haddock and herring; number of missing/total covariate values: 18/140, 159/564, 59/285 and 172/495). Specifically, data were analysed by using multiple imputation by chained equations (Van Buuren 2007). The core of this method is to randomly generate possible values for missing data M times (we used M = 500), fit the regression model to each of the M-imputed datasets and pool results to obtain estimates of regression coefficients and standard errors that incorporate the uncertainty regarding the missing values. Missing values were imputed on a variable-by-variable basis using predictive mean matching based on the relationship with the other variables in the model and with possible augmenting variables. See electronic supplementary material, appendix S2 for details and comparison with results from ordinary least-squares regression on subsets of the data with complete observations of different combinations of the variables.
Secondly we used least-squares regression to assess the immediate and delayed effects of ocean temperature fluctuations (TKOLA; table 1) on recruitment (data ranges for the four species: 1973–2006, 1927–2003, 1950–2003 and 1921–2004). These models synthesize how climate affects population growth through direct and indirect effects combined.
3. Results
(a). Stage-wise growth and survival models (step 1)
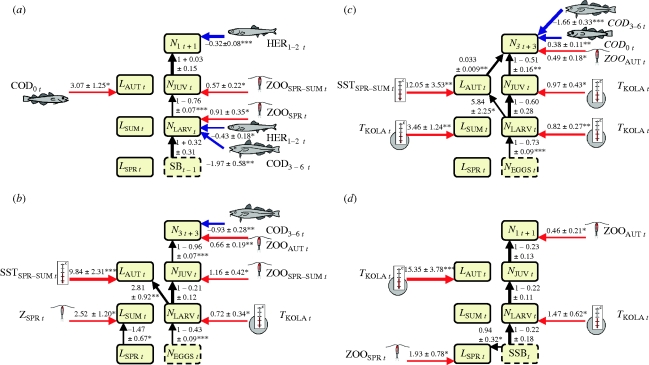
The selected growth and survival models are presented schematically in figure 3 (see electronic supplementary material, table S1.5 in appendix S1 for full model equations). We found significant effects of ambient zooplankton biomass on survival of all species (as well as on growth of cod and herring), and highly significant effects of ambient sea surface temperature on growth of cod and haddock. In addition, TKOLA predicted survival and/or growth of all species except capelin. Effects of all temperature and zooplankton variables were positive. Of potential predation variables, we found negative effects of subadult cod (age 3–6 years) on survival of capelin and herring and younger cohorts of cod, negative effects of subadult herring (age 1–2 years) on capelin survival to the larval stage and positive effects of juvenile cod (age 0.5 years) on growth of capelin. Of potential competition variables, we found negative effects of juvenile cod on survival of haddock and of subadult herring on survival of capelin to recruitment. Of intra-cohort variables, we found positive effect of length-at-age (i.e. of growth) on subsequent survival of haddock, positive effects of cohort size on growth of cod, haddock and herring, and a negative association between spring and summer length-at-age of cod. We found evidence for density dependence (b > 0, equation (2.1)) for all species. Density dependence was found to be strongest for cod, intermediate for haddock and capelin and weakest for herring. No models suggested depensatory effects (i.e. increased mortality at low density, see electronic supplementary material, figures S1.2–S1.4 in appendix S1).
Figure 3.
Climate effects on recruitment dynamics. Whole-lined boxes: response variables of growth and survival models (see electronic supplementary material, table S1.5 in appendix S1). Arrows: selected predictor effects of growth and survival models (red, positive; blue, negative; line widths reflect strengths of associations). Numbers: parameter estimates with standard errors. See table 1 for explanations of variables. Subscripts (t) refer to year. *p < 0.05, **p < 0.01, ***p < 0.001. (a) Capelin; (b) cod; (c) haddock; (d) herring.
(b). Model diagnostics
Diagnostics of the final growth and survival models did not reveal significant autocorrelation of normalized residuals, undue influence of extreme values or any artefacts in the results related to correlations between predictor variables (see electronic supplementary material, appendix S1).
The models generally separated the effects of alternative predictor variables unequivocally (ΔAICC > 2, see electronic supplementary material, table S1.6 in appendix S1). The exceptions were TKOLA and ZOOSPR effects in cod model 1, ZOOSPR, TKOLA and SSTSPR effects in cod model 5, ZOOAUT and SSTWIN effects in herring model 3, and COD0 and HER1–2 predation effects in capelin model 6 (numbers refer to figure 2a). It should be stressed, however, that because of the large number of models and variables tested and the correlational nature of the study, the causal basis for all identified effects should be considered hypotheses for further research.
(c). Non-additive and nonlinear formulations
Results of non-additive and nonlinear models suggested that additive, linear formulations were generally reasonable approximations and adequate in describing the dynamics of the four species in the current analysis (see electronic supplementary material, appendix S1).
(d). Effects on population growth (step 2)
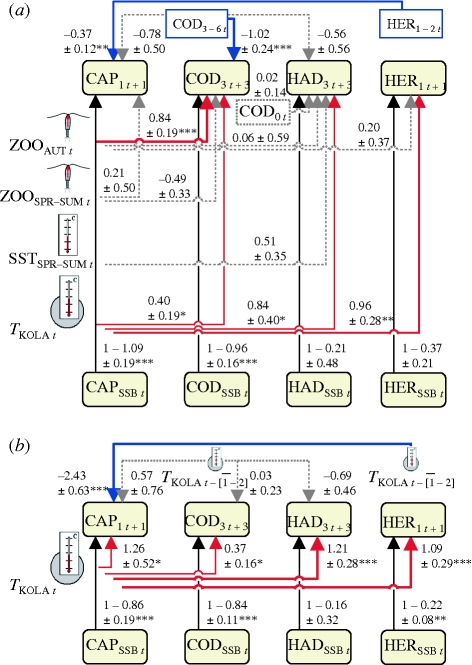
Figure 4a summarizes spawning stock–recruitment models with predictor variables selected in the stage-wise analysis (see electronic supplementary material, tables S2.2–S2.3 in appendix S2 for model summaries). We found that TKOLA significantly predicts recruitment of cod, haddock and herring. In contrast, effects of SSTSPR-SUM and ZOOSPR-SUM were non-significant, and ZOOAUT significantly predicts recruitment of cod, but not haddock and herring. Among the competition and predation variables, only the effects of subadult herring on capelin recruitment and of subadult cod on cod recruitment were significant.
Figure 4.
Effects on population growth, as estimated in spawning stock-recruitment models. (a) Predictor variables selected in stage-wise growth and survival models (figure 3). Regression coefficients with standard errors (s.e.) were estimated using multiple imputation of missing covariate values in order to use all available recruitment data. (b) Climate effects measured by TKOLA in spawning year, or average of 1–2 or 3–6 years preceding spawning, reflecting effects through HER1–2 or COD3–6, respectively. Regression coefficients and s.e. were estimated by least-squares methods. Arrows: red, significantly positive; blue, significantly negative; grey, non-significant effects; line widths and types reflect degrees of statistical support. See electronic supplementary material, appendix S2 for details and table 1 for explanations of variables. Subscript (t) refer to year. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4b shows spawning stock–recruitment models with climate effects quantified as immediate and delayed effects of TKOLA (see electronic supplementary material, table S2.4 in appendix S2 for model equations). The rationale behind the delayed effects is that herring and cod recruitment is positively affected by TKOLA (figure 4a); we therefore expected that high TKOLA with some time lags lead to increased abundances of subadult herring and cod, which in turn might affect recruitment of later cohorts of the four species negatively. Choosing time lags following the age definitions of subadult herring and cod, we first confirmed that HER1–2 and COD3–6 correlate with average TKOLA 1–2 and 3–6 years previously, respectively (r = 0.52 and 0.37, n = 84 and 80, both p < 0.001), and then used lagged TKOLA as proxy for delayed climate effects through subadult herring and cod. The results showed that TKOLA in the spawning year affected recruitment success of all species positively, that TKOLA 1–2 years preceding spawning (representing effects through HER1–2) had significant negative effect on capelin, while TKOLA 3–6 years preceding spawning (representing COD3–6) showed no significant effects.
4. Discussion
(a). Unexpected intra-cohort effects
Positive effects of cohort size and negative effect of length-at-age on growth (or, more precisely, on subsequent length-at-age) were unexpected. The causal bases for these effects are unclear, but we hypothesize that they may be related to effects of climate not captured by the environmental variables. Alternatively, positive density-dependent growth is conceivable, for example, if fish larvae go deeper to avoid visual predators when larval densities are low.
(b). Climate effects on growth and feeding conditions
Positive associations between temperature and recruitment success of cod, haddock and herring in the area studied are well documented, but the causal basis has remained elusive (Ottersen et al. 2004). Disentangling the roles of temperature and zooplankton has been particularly difficult because the temperature index used in most studies (TKOLA) has been considered as an indicator of both temperature and zooplankton availability in the study system (Ottersen & Sundby 1995; Ottersen & Loeng 2000), and because multi-annual zooplankton data have largely been lacking (but see Ellertsen et al. 1989). Several non-exclusive mechanisms have been suggested to explain the temperature–recruitment associations, including physiological effects of temperature on growth (Ottersen & Loeng 2000), increased on-shelf advection of copepods in warm years (Sundby 2000) and a better spatio-temporal match between the fish larvae and their zooplanktonic food during warm years (Ellertsen et al. 1989; Fossum 1996). Our analysis of long-term monitoring series (figure 1) suggests quantitatively important effects of both ambient zooplankton biomass and local temperature per se (figure 3). The general pattern emerging is that temperature mainly predicts growth in length, whereas zooplankton mainly predicts survival.
Zooplankton predicted survival during one or more life stages for all fish species (figure 3) and provided significantly better predictions than ambient sea surface temperature indices and TKOLA for capelin, cod and haddock (see electronic supplementary material, table S1.6 in appendix S1). Possible mechanisms behind these positive zooplankton-survival links include both direct starvation and indirect effects of food limitation (on, for example, predation risk, as the fish must spend more time in upper water layers when food is scarce; Walters & Martell 2004; Fiksen et al. 2007).
Ambient sea surface temperature indices predicted growth of cod and haddock (figure 3) and were also significant as alternatives to TKOLA as predictor of growth of herring and egg-to-larvae survival of haddock (see electronic supplementary material, table S1.6). The combination of positive sea surface temperature effects on growth (cod, haddock and herring) and survival (haddock) and positive growth effects on survival (haddock) supports the temperature–growth–predation hypothesis: higher temperature leads to higher metabolic rates and, provided that there is sufficient food, to faster growth. Fast growth may causally lead to higher survival, for instance, by reducing the time span of vulnerability to predation (Ottersen & Loeng 2000). The simultaneous detection of temperature effects on growth and zooplankton effects on survival (found for cod) may seem paradoxical, as it suggests that the number of survivors was food limited, while the growth of those that survived was not. However, this could be explained through behavioural responses (Fiksen et al. 2007), spatial heterogeneity in zooplankton density or asymmetric competition among the fish larvae.
TKOLA generally provided better predictions than ambient sea surface temperature and zooplankton indices for spring-to-summer growth and survival of cod, haddock and herring (figure 3). This illustrates the difficulty of measuring the relevant environmental factors that affect individuals locally and that indices of large-scale climate phenomena often account better for ecological processes (Stenseth & Mysterud 2005). Both the sea surface temperature and the zooplankton variables were probably only rough indices of the growth and feeding conditions experienced by the fish. Larval diets in spring (§2a) and effects of the timing of zooplankton production in spring (Ellertsen et al. 1989; Fossum 1996) may, for example, have been poorly captured by the indices.
(c). Delayed climate effects through interspecific interactions
The inclusion of interspecific and inter-cohort competition and predation effects allows indirect climate effects through predators and competitors to be separated from effects of temperature and zooplankton.
Our results suggest that the quantitatively most important predator group studied is subadult cod, which showed significant negative effects on survival of three of the species: on capelin spawners (capelin biomass is estimated before the main cod predation occurs, Hjermann et al. 2004a; Dingsør et al. 2007), on juveniles (0.5–3 year olds) of haddock and on juveniles (0.5–3 year olds) of their own species (i.e. cannibalistic effects; Hjermann et al. 2004a, 2007; Dingsør et al. 2007). Subadult herring showed a negative effect on capelin survival to the larval stage, consistent with predation on the larvae (Hjermann et al. 2004a). We further found a positive effect of predatory juvenile cod (alternatively, subadult herring; see electronic supplementary material, table S1.6) on capelin growth. This finding could be explained through size-selective feeding, but other explanations, for example, confounding with climatic effects, are also possible. Results suggest two strong competition effects: a competition effect of cod on haddock in the juvenile-recruitment period is consistent with the similar biology of the two species (Dalpadado et al. 2009), the strong intraspecific competition during this period of the life cycles of both species (figure 3) and the numerical dominance of cod compared with haddock. A competition effect of subadult herring on capelin is consistent with similarities in diets (i.e. zooplankton; Huse & Toresen 1996; Orlova et al. 2008; Dalpadado et al. 2009) and with the herring being located ‘up-stream’ in the inflowing zooplankton-rich Atlantic waters compared with capelin. We are, however, not aware of studies providing direct support for the implication that subadult herring feeding has a large-scale effect on zooplankton biomass.
Our analysis implies that all four species experience improved growth and feeding conditions when temperature is high in the Lofoten–Barents Sea system (as shown by the positive immediate effects of TKOLA in figure 4a,b). We would therefore expect that strong cohorts of cod and herring born in warm years might, when reaching subadult ages, have predation and competition effects on subsequent cohorts and thereby lead to delayed negative effects of high temperature. This was only partly confirmed (figure 4). Although the stage-wise analysis demonstrated negative effects of subadult cod on capelin, cod and haddock (figure 3), only the effect on cod is supported by the multiple imputation analysis (figure 4a), and delayed negative temperature effects through subadult cod are not demonstrated (figure 4b). The significant correlation between lagged TKOLA and COD3–6 suggests that the lack of delayed temperature effects is not solely caused by fishing dampening the temperature signal in the cod cohort after 3 years age. In contrast, a delayed negative temperature effect on capelin through subadult herring is clearly shown (figure 4a,b). For capelin, the net effect of warmer conditions in the Barents Sea therefore depends strongly on the time scale; positive effects from the rapidly responding zooplankton will later be absorbed by negative predation and competition effects from the increased population of subadult herring, which responds to climate with a time lag.
(d). Climate effects on population growth: modulated by density dependence
The strength and timing of density dependence determine how climate effects operating at early life stages influence cohort strength at later ages. Specifically, competition within a cohort may lead to increased mortality at high density and density-dependent regulation of cohort size (b > 0, equation (2.1)). Compensatory mortality then reduces the proportional effects of climate factors operating before the life stage in which the competition occurs. We found that the strength of density dependence differed between life stages and species (figure 3). From the stage-wise analysis we thus predict that climate factors affecting survival early in the pre-recruitment period are relatively more important for herring, intermediate for capelin and haddock and least important for cod.
The results of the spawning stock–recruitment models (figure 4a) partly conformed to these expectations. Largely in line with expectations, the statistical support for effects of predictor variables associated with spring and summer (including TKOLA effects and COD3–6 effect on capelin, see figure 3) was strongest for herring, intermediate for haddock and weakest for capelin and cod. However, the significant effects of TKOLA on cod and haddock recruitment and the non-significant effects of predictor variables associated with the juvenile-recruitment period for haddock and herring (figure 4a) were unexpected. Note that the spawning stock–recruitment models represent longer time spans than the stage-wise models. The unexpected findings may partly reflect effects of climate changing with time, which has been shown for the cod stock studied (Ottersen et al. 2006), failure to identify the relevant causal factors and low statistical power in some of the analyses. Note that standard errors in imputation models might have been reduced by removal of correlated predictor variables, but that figure 4a summarizes what we know and do not know about the general effects of the different factors.
(e). Insights from the integrative approach
In the present study, complex ecosystem dynamics have been simplified to inter-relationships between relatively few factors—ignoring other factors such as, for example, turbulence (Sundby & Fossum 1990) and advection rate (Vikebø et al. 2007), which thus act as noise in the analysis. Despite the noise, clear patterns have emerged, which show the key processes through which climate influences the populations. We show that climate affects the early survival of the four species through several pathways, including growth-mediated temperature effects and effects through prey, competitors and predators (figure 3). However, only a few of these pathways are shown to be important when it comes to explaining interannual variability in recruitment, and thus population growth, at longer time scales (figure 4a). This is partly because density dependence reduces the importance of climate effects operating early in life. While figures 3 and 4a point to mechanisms, figure 4b shows net effects of climate fluctuations; warmer temperature in the Barents Sea boosts the recruitment of all species studied, but for the cold-water species capelin, delayed negative effects through more warm-water affiliated competitors/predators (herring) later absorb this effect.
Our findings contribute to improved understanding of the dynamics of four ecologically and commercially important fish stocks in the Barents Sea, but may also serve as a case study for exploration of how climate affects marine ecosystems in general. Specifically, our results contribute to disentangle direct and indirect effects of climate and illustrate how climate effects on growth and feeding conditions in combination with intra- and interspecific interactions determine how climate affects population growth rates at different time scales.
Acknowledgements
We are thankful to the late S. Timofeev and to I. Berchenko for making Russian plankton data accessible, to G. E. Dingsør for making zero-group data accessible, to C. T. Marshall for providing capelin biomass data, to Arvid Hylen and Kjell Nedreaas (IMR, Bergen) for providing the VPA estimates for northeast Arctic cod for the years 1913–1945 and to two anonymous reviewers for helpful comments. The Norwegian Research Council financed the study (project no. 178434/S40).
References
- Carrol R. J., Ruppert D., Stefanski L. A.1995Measurement error in nonlinear models. London, UK: Chapman and Hall [Google Scholar]
- Dalpadado P., Ingvaldsen R., Hassel A.2003Zooplankton biomass variation in relation to climatic conditions in the Barents Sea. Polar Biol. 26, 233–241 [Google Scholar]
- Dalpadado P., Bogstad B., Eriksen E., Rey L.2009Distribution and diet of 0-group cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) in the Barents Sea in relation to food availability and temperature. Polar Biol. 32, 1583–1596 (doi:10.1007/s00300-009-0657-7) [Google Scholar]
- Dingsør G. E., Ciannelli L., Chan K.-S., Ottersen G., Stenseth N. C.2007Density dependence and density independence during the early life stages of four marine fish stocks. Ecology 88, 625–634 [DOI] [PubMed] [Google Scholar]
- Ellertsen B., Fossum P., Solemdal P., Sundby S.1989Relation between temperature and survival of eggs and first-feeding larvae of northeast Arctic cod (Gadus morhua L.). Rapp. P.-v. Réun. Cons. perm. int. Explor. Mer. 191, 209–219 [Google Scholar]
- Fiksen Ø., Jørgensen C., Kristiansen T., Vikebø F., Huse G.2007Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dispersal. Mar. Ecol. Prog. Ser. 347, 195–205 (doi:10.3354/meps06978) [Google Scholar]
- Fossum P.1996A study of first-feeding herring (Clupea harengus L.) larvae during the period 1985–1993. ICES J. Mar. Sci. 53, 51–59 (doi:10.1006/jmsc.1996.0005) [Google Scholar]
- Hjermann D. Ø., Ottersen G., Stenseth N. C.2004aCompetition among fishermen and fish causes the collapse of Barents Sea capelin. Proc. Natl Acad. Sci. USA 101, 11 679–11 684 (doi:10.1073/pnas.0402904101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjermann D. Ø., Stenseth N. C., Ottersen G.2004bIndirect climatic forcing of the Barents Sea capelin: a cohort effect. Mar. Ecol. Prog. Ser. 273, 229–238 (doi:10.3354/meps273229) [Google Scholar]
- Hjermann D. Ø., Bogstad B., Eikeset A. M., Ottersen G., Gjøsæter H., Stenseth N. C.2007Food web dynamics affect Northeast Arctic cod recruitment. Proc. R. Soc. B 274, 661–669 (doi:10.1098/rspb.2006.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich C. M., Tsai L.1991Regression and time series model selection in small samples. Biometrika 76, 297–307 (doi:10.1093/biomet/76.2.297) [Google Scholar]
- Huse G., Toresen R.1996A comparative study of the feeding habits of herring (Clupea harengus, Clupeidae L.) and capelin (Mallotus villosus, Osmeridae, Müller) in the Barents Sea. Sarsia 81, 143–153 [Google Scholar]
- Hylen A.2002Fluctuations in abundance of Northeast Arctic cod during the 20th century. ICES Mar. Sci. Symp. 215, 543–550 [Google Scholar]
- ICES 2007aReport of the Arctic Fisheries Working Group (AFWG). ICES CM 2007/ACFM:16, 18–27 April, Vigo, Spain [Google Scholar]
- ICES 2007bReport of the Working Group on Northern Pelagic and Blue Whiting Fisheries (WGNPBW). ICES CM 2007/ACFM:29, 27 August–1 September, Vigo, Spain [Google Scholar]
- Lima M., Berryman A.2006Predicting nonlinear and non-additive effects of climate: the Alpine ibex revisited. Clim. Res. 32, 129–135 (doi:10.3354/cr032129) [Google Scholar]
- Marshall C. T., Yaragina N. A., Ådlandsvik B., Dolgov A. V.2000Reconstructing the stock–recruit relationship for Northeast Arctic cod using a bioenergetic index of reproductive potential. Can. J. Fish. Aquat. Sci. 57, 2433–2442 (doi:10.1139/cjfas-57-12-2433) [Google Scholar]
- Matyshov G., Zuev A., Golubev V., Adrov N., Slobodin V., Levitus S., Smoliar I. Murmansk—Silver Spring. MMBI—NOAA; 1998. Climatic Atlas of the Barents Sea 1998. Temperature, salinity, oxygen. See http://www.nodc.noaa.gov/OC5/barsea/bardoc.html . [Google Scholar]
- Mukhina N. V.1992Results of the ichthyoplankton investigations in the Norwegian and Barents Seas in 1959–1990. In Ekologicheskie problemy Barentseva moria (ed. Belikov S. V.), pp. 62–102 Murmansk, Russia: PINRO.[In Russian.] [Google Scholar]
- Mukhina N. V., Marshall C. T., Yaragina N. A.2003Tracking the signal in year-class strength of Northeast Arctic cod through multiple survey estimates of egg, larval and juvenile abundance. J. Sea Res. 50, 57–75 (doi:10.1016/S1385-1101(03)00046-7) [Google Scholar]
- Nesterova V. N.1990Biomassa planktona na putiakh dreifa lichinok treski (spravochnyi material). Murmansk, Russia: PINRO.[In Russian.] [Google Scholar]
- Orlova E. L., Dolgov A. V., Rudneva G. B., Oganin I. A., Konstantinova L. L.2008Trophic relations of capelin Mallotus villosus and polar cod Boreogadus saida in the Barents Sea as a factor of impact on the ecosystem. Deep-Sea Res. II 56, 2054–2067 (doi:10.1016/j.dsr2.2008.11.016) [Google Scholar]
- Ottersen G., Loeng H.2000Covariability in early growth and year-class strength of Barents Sea cod, haddock and herring: the environmental link. ICES J. Mar. Sci. 57, 339–348 (doi:10.1006/jmsc.1999.0529) [Google Scholar]
- Ottersen G., Sundby S.1995Effects of temperature, wind and spawning stock biomass on recruitment of Arcto-Norwegian cod. Fish. Oceanogr. 4, 278–292 (doi:10.1111/j.1365-2419.1995.tb00073.x) [Google Scholar]
- Ottersen G., Alheit J., Drinkwater K., Friedland K., Hagen E., Stenseth N. C.2004The responses of fish populations to ocean climate fluctuations. In Marine ecosystems and climate variation: the North Atlantic (eds Stenseth N. C., Ottersen G., Hurrell J., Belgrano A.), pp. 73–94 Oxford, UK: Oxford University Press [Google Scholar]
- Ottersen G., Hjermann D., Stenseth N. C.2006Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod stock. Fish. Oceanogr. 15, 230–243 (doi:10.1111/j.1365-2419.2006.00404.x) [Google Scholar]
- R Development Core Team 2006R: a language and environment for statistical computing. Austria: Vienna; See http://www.R-project.org. R Foundation for Statistical Computing [Google Scholar]
- Rijnsdorp A. D., Peck M. A., Engelhard G. H., Mollmann C., Pinnegar J. K.2009Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 66, 1570–1583 (doi:10.1093/icesjms/fsp056) [Google Scholar]
- Sætre R., Toresen R., Anker-Nilssen T.2002Factors affecting the recruitment variability of the Norwegian spring-spawning herring (Clupea harengus L.). ICES J. Mar. Sci. 59, 725–736 [Google Scholar]
- Stenseth N. C., Mysterud A.2005Weather packages: finding the right scale and composition of climate in ecology. J. Anim. Ecol. 74, 1195–1198 (doi:10.1111/j.1365-2656.2005.01005.x) [Google Scholar]
- Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K.-S., Lima M.2002Ecological effects of climate fluctuations. Science 297, 1292–1296 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- Stige L. C., Lajus D. L., Chan K.-S., Dalpadado P., Basedow S. L., Berchenko I., Stenseth N. C.2009Climatic forcing of zooplankton dynamics is stronger during low densities of planktivorous fish. Limnol. Oceanogr. 54, 1025–1036 [Google Scholar]
- Sundby S.2000Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia 85, 277–298 [Google Scholar]
- Sundby S., Fossum P.1990Feeding conditions of Arcto-Norwegian cod larvae compared with the Rotschild–Osborn theory on small-scale turbulence and plankton contact rates. J. Plankton Res. 12, 1153–1162 (doi:10.1093/plankt/12.6.1153) [Google Scholar]
- Tereschenko V. V. Seasonal and year-to-year variations of temperature and salinity along the Kola meridian transect. 1996 ICES CM 1996/C:11. Reykjavik, Iceland: ICES. [Google Scholar]
- Toresen R., Østvedt O.2000Variation in abundance of Norwegian spring-spawning herring (Clupea harengus, Clupeidae) throughout the 20th century and the influence of climatic fluctuations. Fish Fish. 1, 231–256 (doi:10.1046/j.1467-2979.2000.00022.x) [Google Scholar]
- Van Buuren S.2007Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Meth. Med. Res. 16, 219–242 (doi:10.1177/0962280206074463) [DOI] [PubMed] [Google Scholar]
- Vikebø F., Jørgensen C., Kristiansen T., Fiksen Ø.2007Drift, growth and survival of larval Northeast Arctic cod with simple rules of behaviour. Mar. Ecol. Prog. Ser. 347, 207–219 (doi:10.3354/meps06979) [Google Scholar]
- Walters C. J., Martell S. J. D.2004Fisheries ecology and management. Princeton, NJ: Princeton University Press [Google Scholar]
- Wood S. N.2006Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC [Google Scholar]
- Worley S. J., Woodruff S. D., Reynolds R. W., Lubker S. J., Lott N.2005ICOADS Release 2.1 data and products. Int. J. Climatol. 25, 823–842 (doi:10.1002/joc.1166) [Google Scholar]