Abstract
Mountains, especially in the tropics, harbour a unique and large portion of the world's biodiversity. Their geographical isolation, limited range size and unique environmental adaptations make montane species potentially the most threatened under impeding climate change. Here, we provide a global baseline assessment of geographical range contractions and extinction risk of high-elevation specialists in a future warmer world. We consider three dispersal scenarios for simulated species and for the world's 1009 montane bird species. Under constrained vertical dispersal (VD), species with narrow vertical distributions are strongly impacted; at least a third of montane bird diversity is severely threatened. In a scenario of unconstrained VD, the location and structure of mountain systems emerge as a strong driver of extinction risk. Even unconstrained lateral movements offer little improvement to the fate of montane species in the Afrotropics, Australasia and Nearctic. Our results demonstrate the particular roles that the geography of species richness, the spatial structure of lateral and particularly vertical range extents and the specific geography of mountain systems have in determining the vulnerability of montane biodiversity to climate change. Our findings confirm the outstanding levels of biotic perturbation and extinction risk that mountain systems are likely to experience under global warming and highlight the need for additional knowledge on species' vertical distributions, dispersal and adaptive capacities.
Keywords: climate change, dispersal, extinction risk, geographical range, global warming, mountain biodiversity
1. Introduction
Mountain regions above 1000 m in elevation represent roughly 20 per cent of the Earth's surface (figure 1a) and are an important feature of the biosphere. They support a large portion of the world's biological diversity and a host of critical ecosystem services (Körner & Spehn 2002). Mountains in many cases harbour evolutionarily unique and species-rich assemblages (Lomolino 2001; Körner & Spehn 2002; Jetz et al. 2004), a characteristic that is especially pronounced in tropical mountains, which are renowned for their highly diverse assemblages of vertebrates and plants (Ceballos & Ehrlich 2006; Grenyer et al. 2006; Barthlott et al. 2007; Buckley & Jetz 2008). Mountain biodiversity also delivers primary ecosystem services such as water provision and erosion prevention (Körner & Spehn 2002) and important natural resources to humans (Messerli & Ives 1997).
Figure 1.
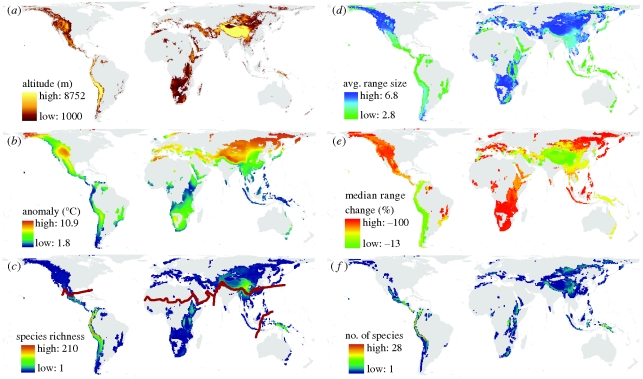
Maps summarizing current and projected geographical and ecological patterns within montane regions globally. (a) Terrestrial areas occurring above 1000 m in altitude. Within these areas, (b) the projected IPCC A2 temperature anomaly between 1980–1999 and 2080–2099, (c) species richness of montane birds (n = 1009; realm boundaries delineated by red lines), and (d) average log geographical range size (lateral range extent) of montane birds. (e) Median per cent change in range size for montane birds and (f) the number of montane birds projected to lose 50% or more of their range resulting in range sizes less than 20 000 km2 under the no-dispersal (ND) scenario. All maps, except for (a), which is at 1 km resolution, were summarized within an equal-area grid with a cell area of 3091 km2. Colour ramps use Jenk's natural breaks classification.
Recent climate change (Karl & Trenberth 2003) has already impacted biological systems worldwide (Parmesan & Yohe 2003; Root et al. 2003; Parmesan 2006; Rosenzweig et al. 2008) and mountain ecosystems are considered especially susceptible (Diaz et al. 2003; Nogués-Bravo et al. 2007). Recent work indicates the greatest relative impacts of global warming are likely to occur within tropical mountains and mountains at high northern latitudes (Still et al. 1999; Williams et al. 2007; Pepin & Lundquist 2008). There is evidence that many montane taxa have already responded to global warming by dispersing to higher elevations (Parmesan 2006). This trend, with some exceptions (Wilson et al. 2007; Moritz et al. 2008), has been documented primarily for woody plants at their leading or upper altitudinal range boundaries (Jump et al. 2009). As species move up in elevation, dispersal may be hampered by declines in the quantity and quality of habitable land area (electronic supplementary material, figure S1) and the development of vertical gaps between current and future suitable elevation bands (range-shift gaps), which may be particular acute for species with narrow vertical distributions (Colwell et al. 2008). With such constraints, population sizes are certain to decline (Schaffer 1981), ultimately leading to mountaintop extinctions (Colwell et al. 2008; Sekercioglu et al. 2008). The spatial variation in the structure and extent of orographic features (figure 1a), projected temperature changes (figure 1b) and species' vertical range extents and lateral range extents (or geographical range size) are all expected to jointly delineate geographical range contractions and extinction risk, but as yet it is unclear how.
With accelerating carbon dioxide emission rates (Raupach et al. 2007) and the likely irreversibility of climate change (Solomon et al. 2009), there is a critical need to generate broad-scale estimates of species' vulnerability to climate change to support conservation planning (Williams et al. 2008). Here, we provide a global baseline assessment of the magnitude and geography of expected global warming-induced range contractions and extinction risk of high-elevation specialists and of the relative roles of its determinants. We base the evaluation of the susceptibility of montane species to climate change on the strong association between temperature and elevation, which averages 6.2°C km−1 across continents (Mokhov & Akperov 2006), the availability of these gradients for montane species (Colwell et al. 2008) and the growing evidence that montane species are currently tracking their climatic niches over time (Tingley et al. 2009). Our assessment therefore focuses on the effect of global warming on species' susceptibility while keeping constant all other environmental or ecological factors that could come into play under climate change. We consider three dispersal scenarios that illustrate a gradient of vertical dispersal (VD) and lateral dispersal (LD) opportunities. We use both simulated and empirical distributions of montane species to assess potential temperature-driven changes in geographical range size between 1980–1999 and 2080–2099 and to isolate biological from physiographic drivers of susceptibility.
2. Material and methods
We used a combination of data sources to estimate current and future geographical range sizes for empirical and simulated montane species under three dispersal scenarios. Specifically, we used current elevation associations to refine species' geographical distributions based on 100 m elevational bands and projected future elevation associations and distributions given regional lapse rates and temperature projections (see electronic supplementary material, figure S2, for an outline of the analysis). All analyses were conducted between 60° S and 80° N latitude within a grid having a cylindrical equal-area projection and a cell area of 3091 km2. We excluded the continents of Antarctica and Greenland and only retained grid cells with more than 50 per cent terrestrial surface.
(a). Climate data
Projected temperature anomalies were calculated for the Intergovernmental Panel on Climate Change (IPCC) A2 Special Report on Emissions Scenario (Nakićenović & Swart 2000) between two 20 year time periods, 1980–1999 and 2080–2099. Values for 1980–1999 were based on the IPCC climate of the twentieth century experiment (20C3M). Temperature anomalies were defined as the difference in average monthly mean near-surface air temperature between 1980–1999 and 2080–2099. The A2 emission scenario was selected because there is strong evidence that alternative scenarios may no longer be relevant under current greenhouse gas emission rates (Raupach et al. 2007; Beaumont et al. 2008). We compiled and analysed temperature anomalies from 18 atmosphere–ocean general circulation models (AOGCMs) using data from the first run for each model (Randall et al. 2007); we used the National Center for Atmospheric Research CCSM 3.0 model as the showcase AOGCM (electronic supplementary material, figure S3). We acquired annual mean tropospheric lapse-rate values at a resolution of 2.5° (approx. 278 km at the equator) for the period 1948–2001 (Mokhov & Akperov 2006). Gridded climate data were bilinearly interpolated to match the resolution of the 3091 km2 equal-area grid. Projected shifts in altitudinal temperature profiles were estimated for each grid cell by dividing the projected temperature anomaly by the cell's tropospheric lapse rate. Each grid cell therefore had a projected vertical distance in which changes in the surface temperature profile (or, by extension dominant montane vegetation; Kelly & Goulden 2008) would be manifested.
(b). Elevation data and treeline
We acquired elevation data from the Shuttle Radar Topographic Mission (SRTM v. 3) digital elevation model (DEM) for terrestrial regions located between 60° S and 60° N latitude at a resolution of 3 arc-seconds (approx. 90 m at the equator; Jarvis et al. 2006). For terrestrial regions located between 60° N and 80° N latitude, we used the USGS GTOPO30 DEM at a resolution of 30 arc-seconds (approx. 1 km at the equator). The GTOPO30 DEM was bilinearly interpolated to a resolution of 3 arc-seconds to match the SRTM resolution. Slopes were estimated using the average maximum technique and a 3 × 3 cell neighbourhood (Burrough 1986). Median slope and non-planimetric surface area (i.e. a measure of surface area that considers spatial variation in slope) were summarized within 100 m elevation bands (centred on 1000, 1100, 1200, …, 8800 m) within each equal-area cell (3091 km2). Non-planimetric surface area was estimated by treating each elevation band as a square, horizontal surface and tilting this surface based on the median slope and recalculating the area.
We defined treeline using the global latitudinal treeline relationship (Körner 2007), which we modelled linearly. We first defined a maximum treeline of 3500 m between 30° S and 40° N latitude. In the Northern Hemisphere, we defined a linearly decreasing treeline occurring at 3500 m at 40° N and dropping down to 0 m at 70° N latitude. In the Southern Hemisphere, we defined a linearly decreasing treeline occurring at 3500 m at 30° S and dropping down to 0 m at 55° S latitude.
(c). Empirical and simulated and montane species data
We acquired breeding ranges and elevation associations for the global breeding avifauna from a variety of sources (electronic supplementary material, table S1). We defined montane bird species as montane specialists with current minimum elevation associations that are greater than 1000 m and current maximum elevation associations that occur anywhere above 1000 m below the median treeline estimated within the range. The first criterion was selected based on evidence that 1000 m demarcates lowland from montane bird communities in the tropics (Herzog et al. 2005; Romdal & Rahbek 2009), the region where the majority of the montane bird species in our assessment occurred (figure 1c). The second criterion allowed us to identify species that were associated with montane environments independent of latitudinal gradients in treeline, in particular, the treeline gradient as represented in the Northern Hemisphere. In species with large lateral extents, altitudinal associations sometimes vary geographically, and we aimed for the species-level elevation range to be approximately representative for the range midpoint. While more detailed knowledge of species exact elevational occurrences would of course be desirable, we do not expect there to be strong trends in these inaccuracies that would affect our conclusions. A total of 1009 montane species were identified (approx. 10% of the global avifauna), which we placed into six biogeographic realms (Udvardy 1975) based on the realm that contained the greatest proportion of each species' current range (electronic supplementary material, table S2). To simplify the analysis, three species occurring on the Hawaiian Islands were categorized as Nearctic.
Simulated ranges were generated by first identifying a range centre placed randomly within montane regions globally (figure 1a). The radius of the range was expanded from the centre and new montane cells added iteratively until the selected non-planimetric range size was achieved based on the selected vertical range extent. We considered four unique combinations of two lateral and two vertical range extents, in each case using 1000 simulated species: small and large vertical range extents (800 and 1600 m) and small and large lateral range extents (104 and 105 km2). These values were derived from the associations observed for montane birds (electronic supplementary material, figure S4a,b). The values for large vertical and large lateral range extents were roughly the median values for montane birds. The value for small vertical extent was half the value for large vertical extent and the value for small lateral extent was an order of magnitude smaller than the value for large lateral extent.
(d). Changes in range size for empirical and simulated species
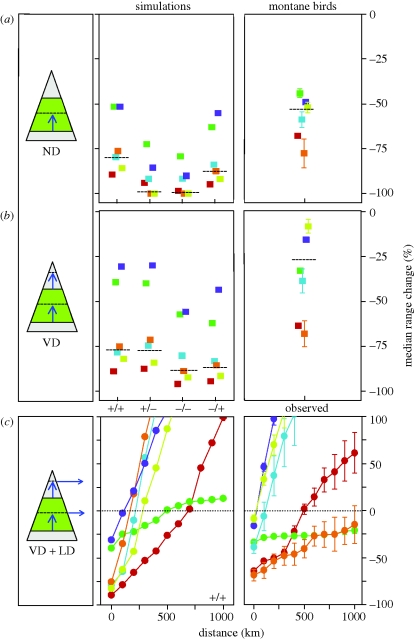
We estimated changes in geographical range size under global warming for empirical and simulated species using three dispersal scenarios that captured a gradient of increasing VD and LD opportunities (figure 2). The first no-dispersal (ND) scenario allowed only the lower elevational range boundary to shift in response to warming temperatures. The second VD scenario allowed both the lower and upper elevational range boundaries to shift. The third vertical dispersal plus lateral dispersal (VD + LD) scenario started with the geographical outcome of the second scenario and allowed LD at concentric 100 km intervals to a maximum distance of 1000 km from the edge of the range.
Figure 2.
Median per cent change in range size for simulated ranges (n = 1000) and empirical montane bird ranges (n = 1009; ±bootstrapped s.e.) summarized within six biogeographic realms under three dispersal scenarios. Red symbols, Afrotropics; green symbols, Australasia; light blue symbols, Indo Malaya; orange symbols, Nearctic; dark blue symbols, Neotropics; yellow, Palaearctic. The dashed line segments represent the overall median values across realms. The scenarios are (a) no-dispersal (ND), (b) vertical dispersal (VD), and (c) vertical plus lateral dispersal (VD + LD). Cartoons illustrate current elevation associations (green) and dispersal patterns (blue arrows) for species under each dispersal scenario. The four simulations are defined by four unique combinations of two parameters (lateral/vertical range extent where (−) and (+) identify small and large extents, respectively). Only the large-lateral/large-vertical simulation (+/+) is shown for the VD + LD scenario (see electronic supplementary material, figure S7, for remaining simulations).
We estimated the area within each species' range based on the non-planimetric area measured within 100 m elevation bands containing slopes of less than 45° using the global 90 m resolution DEM. We used projected changes in altitudinal temperature profiles to estimate the elevational associations for each species in each cell within (ND, VD) and outside (VD + LD) its current geographical range. The projected elevation associations were then used to estimate the non-planimetric area within each cell in the projected range. We used differences in area between the current and projected ranges to estimate the proportional change in range size for each species under each scenario. Using the IUCN Red List criteria for categorizing global extinction risk (IUCN 2001), we identify montane birds of special conservation concern whose projected range losses are 50 per cent or more, resulting in non-planimetric range sizes less than 20 000 km2.
(e). Correlates of changes in range size for montane birds
Ordinary least-squares regression was used to examine the relationship between the proportional projected change in range size per species and the following predictors: lateral range extent (or geographical range size), vertical range extent, the maximum elevation of the terrestrial surface within the region encompassed by the range (only included with the VD scenario), the average IPCC A2 temperature anomaly within the region encompassed by the range and biogeographic realm. After an assessment of distributional assumptions, lateral and vertical range extents were both log transformed. An assessment of variance inflation factors (VIFs) and values from the partial correlation (PC) matrix indicated all the predictors were statistically independent and could be included within the linear models (VIF < 3.1 and |PC| < 0.55). The most parsimonious collection of explanatory variables was selected for each model using a bootstrap stepwise (forward and backward) model selection procedure based on Akaike information criteria (AIC) scores (Austin & Tu 2004). Variables in each model were retained if they were selected more than 95 per cent of time within 10 000 bootstrap samples. Partial residuals plots (Larsen & McCleary 1972) were used to display the relationship between the selected predictors and the response after accounting for the effects of the remaining predictors in each model.
3. Results and discussion
Dispersal is a fundamental biological process that shows strong variation among species and regions (Clobert et al. 2001), but its connection with persistence under climate change requires further work and for most species even basic information is lacking (Watkinson & Gill 2001). In the absence of better knowledge and in light of various ecological and geographical barriers to dispersal, assuming a lack of dispersal opportunities at the leading edge of the range appears both pragmatic and precautionary for conservation prioritization (Jetz et al. 2007). In an initial ND scenario, we only allow range retractions to occur at species' lower altitudinal range boundaries (figure 2a). We assume in this scenario that the lower boundary will fully track the upward shift of abiotic and biotic conditions under warming temperatures.
We find that, in the ND scenario, simulated species with small vertical range extents fare very poorly with median losses in range sizes of 100 per cent, irrespective of lateral range size; species with large vertical range extents retain more of their range but median losses still exceed 75 per cent (figure 2a). These findings highlight the importance of elevational associations (Sekercioglu et al. 2008) and are in contrast to the typical conservation perspective where lateral range extent is considered the primary predictor of extinction risk (Mace et al. 2008). The Afrotropics and Palaearctic are the most, and the Neotropics and Australasia the least, affected realms, reflecting how spatial variation in orographic features (figure 1a) and warming projections (figure 1b) strongly affects range loss even in the absence of biological gradients.
Next, we compare these neutral patterns with those of montane birds, a real world taxon with a distinct biogeography. Species richness, vertical and lateral range extents for montane birds are far from uniform across species or space (electronic supplementary material, figure S1). Montane bird diversity shows striking peaks in the Andean, Himalayan and New Guinean mountains (figure 1c). Lateral range extent varies from 6 × 101 to 4 × 106 km2 (median = 1 × 105 km2) and small lateral ranges occur predominantly on mountains that are geographically isolated or high in species richness (figure 1d and table 1). Vertical range extents vary from 150 to 4500 m (median = 1500 m), broadly reflecting geographical variation in orographic features (table 1 and electronic supplementary material, figure S1). Species with large vertical range extents also tend to have large lateral extents (Spearman's ρ = 0.54, p < 0.001; electronic supplementary material, figure S4c). Vertical range extents of montane birds show only a slight decrease towards the equator (McCain 2009), much weaker than that observed for lateral ranges (Spearman's ρ = 0.14, p < 0.001 and ρ = 0.36, p < 0.001, respectively, for the Northern and Southern Hemispheres combined; electronic supplementary material, figure S4d,e).
Table 1.
The number of montane bird species in six biogeographic realms, the median IPCC A2 temperature anomaly within the realm, the median lateral range extent (range size) and vertical range extent, the number of species occurring in the IUCN 2008 Red List that are classified as threatened (i.e. critically endangered, endangered or vulnerable), the median per cent change in range size under the no-dispersal (ND) and vertical dispersal (VD) scenarios, and the number of species losing 50% or more of their range resulting in range sizes less than 20 000 km2 under the two dispersal scenarios (for species-level results, see electronic supplementary material, table S2).
| realm | species | median anomaly (°C) | median lateral range extent (1000 km2) | median vertical range extent (m) | species (%) currently threatened | median % change in range size |
species (%) losing ≥50% of range and resulting range size <20 000 km2 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ND | VD | ND |
VD |
||||||||
| Afrotropics | 130 | 3.5 | 104 | 1500 | 15 (11) | −68 | −64 | 52 | (40) | 50 | (38) |
| Australasia | 89 | 4.1 | 53 | 1520 | 2 (2) | −44 | −33 | 27 | (30) | 20 | (22) |
| IndoMalaya | 70 | 3.6 | 121 | 1800 | 6 (9) | −59 | −39 | 22 | (31) | 17 | (24) |
| Nearctic | 28 | 5.6 | 302 | 1300 | 8 (29) | −78 | −68 | 12 | (43) | 9 | (32) |
| Neotropics | 525 | 3.7 | 60 | 1400 | 67 (13) | −49 | −16 | 182 | (35) | 70 | (13) |
| Palaearctic | 167 | 5.5 | 389 | 1700 | 13 (8) | −52 | −9 | 32 | (19) | 18 | (11) |
| World | 1009 | 4.8 | 100 | 1500 | 111 (11) | −54 | −27 | 327 | (32) | 184 | (18) |
Under the ND scenario, global warming has a severe impact on montane birds with projected median range sizes declining by 54 per cent worldwide and losses 44 per cent or more in every realm (figure 2a, table 1 and electronic supplementary material, figure S3). The strongest range contractions are projected for mountains at high northern latitudes, within Africa, and within the IndoMalaya archipelago (table 1 and figure 1e). The Afrotropics and Nearctic contain the greatest proportion and the Neotropics the greatest number of species losing 50 per cent or more of their range, resulting in range sizes less than 20 000 km2 (table 1 and figure 1f). A total of 327 species fall into this category, of which 54 are projected to lose 100 per cent of their range, of which 73 are currently listed as threatened (table 1 and electronic supplementary material, table S2). These impacts are less severe than those found for simulated species owing to the high concentration of montane birds within the largest mountain systems, where climate change projections are weaker and vertical ranges are more broadly filled (figure 1a–c). The ranking of median range loss per realm is similar to that found for the neutral groups (figure 2a) with the exception of the Palaearctic where bird diversity in regions with strong warming projections is relatively low (figure 1b,c).
Despite many instances of physical, environmental, biotic and especially anthropogenic limitations to dispersal under climate change (Watkinson & Gill 2001), species may respond to warming by the upward movement of both the trailing and leading edges of the range. While dispersal lags with species and their biotic niches may hamper such shifts, VD has already been demonstrated in some instances, most broadly with woody plants (Jump et al. 2009). Therefore, a second scenario allows unconstrained VD within species' current lateral range extents (figure 2b). We expect the availability of higher elevation area within the species range to be a main driver of net range loss under this scenario (electronic supplementary material, figure S1). Across the four groups of simulated species, median range losses are now somewhat less severe than in the ND scenario and vary between 77 and 90 per cent; more in line with our expectations, the importance of vertical range extent is diminished and lateral extent is a stronger determinant of range loss (figure 2b). Among montane birds, median losses in range size decrease to 27 per cent and no species loses 100 per cent of its range (figure 2b, table 1 and electronic supplementary material, figure S3). Geographical patterns of range loss are similar to those under the ND scenario (table 1 and electronic supplementary material, figure S5). The number of species losing 50 per cent or more of their range resulting in range sizes of less than 20 000 km2 is approximately halved to 184, of which 41 are currently listed as threatened (table 1 and electronic supplementary material, table S2). As expected, the extensive high-elevation areas in the Neotropics and Palaearctic buffer montane species (figure 2b and table 1). Vertical range-shift gaps were evident for 42 species with a median gap of 149 m; the majority (69%) and smallest vertical range-shift gaps on average occurred within the tropics (electronic supplementary material, figure S6). The limited number and magnitude of vertical range-shift gaps suggest this phenomenon might not be a broad deterrent to VD. However, for species with narrow vertical range extents, especially in the tropics where VD is likely to be the primary geographical response to global warming, the development of vertical range-shift gaps could hinder dispersal and increase extinction risk (Colwell et al. 2008).
Mountains are often characterized by their relative geographical isolation, a main driver for the evolution of their unique biotas (Körner & Spehn 2002). Surrounding lowlands are known to severely constrain LD for montane taxa. Nevertheless, LD is a broadly documented response to climate change (Parmesan 2006), with birds providing some of the broadest geographical evidence (La Sorte & Thompson 2007), suggesting movements within and among mountain systems are conceivable. However, LD under climate change has been documented primarily in temperate regions. The potential for LD in the tropics is likely to be much weaker owing to the lack of latitudinal temperature gradients and weak seasonality in temperature (Janzen 1967; Ghalambor et al. 2006), an outcome that has been observed even with very mobile groups such as birds (Moore et al. 2008). Accordingly, the third scenario allows for both VD and LD to a maximum distance of 1000 km from the edge of the range (VD + LD; figure 2c).
The potential for LD buffers against range loss in simulated species, with the strong exception of Australasia and to a degree the Afrotropics where mountain systems are more isolated (figure 2c and electronic supplementary material, figure S7). For montane birds, these regional differences are exacerbated (figure 2c and electronic supplementary material, figure S3). Species in IndoMalaya, Neotropics and the Palaearctic experience the greatest benefits from LD. To compensate for elevational range losses, LD to mountains at least 300 and 600 km away is necessary for montane species in the Afrotropics and Nearctic, respectively, whereas LD out to 1000 km provides no benefits in Australasia. As stated above, an important caveat for species in the Afrotropics, IndoMalaya and Neotropics is that opportunities for LD could be hampered by the lack of latitudinal and seasonal temperature gradients. These results highlight the fundamental role of the geography of mountain systems in determining expected biodiversity loss.
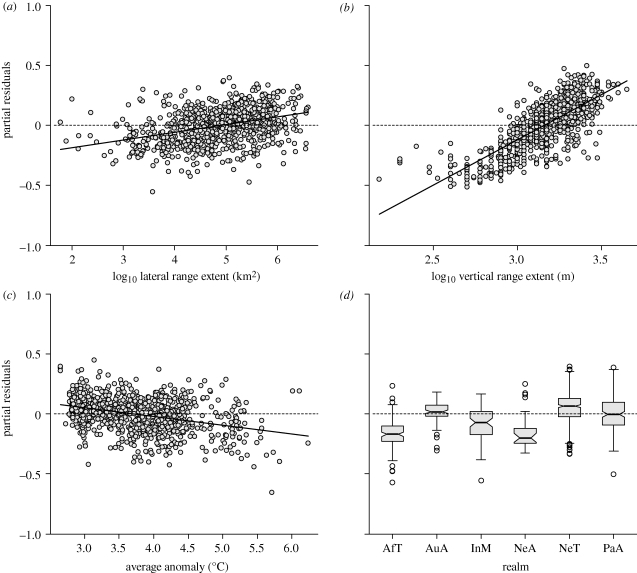
Understanding the drivers of montane species extinction risk is critical for the prioritization of research and conservation efforts (Brook et al. 2008). Our data allow a quantitative comparison of core factors (projected warming, lateral and vertical range extents, orographic characteristics) in a multi-predictor modelling framework with species' projected proportional change in range size as a response. In the ND scenario, vertical range extent has by far the greatest significance for projected species' extinction risk (ΔAIC = 641; figure 3 and electronic supplementary material, table S3). Regional characteristics and, notably, even projected temperature anomalies only play a secondary role (ΔAIC = 324 and 54, respectively). Surprisingly, lateral range extent is of only minor importance (ΔAIC = 79). More specifically, a doubling of vertical range extent is associated with an approximately 23 per cent decrease in average range loss, a doubling of lateral range extent with an approximately 2 per cent decrease in average range loss and one unit decrease in projected temperature anomaly with an approximately 8 per cent decrease in average range loss. Therefore, a 3°C decrease in projected average temperature anomaly within the range—a relatively large change (figure 3c)—is required to match the effect of a doubling of vertical range extent. This finding reaffirms the role of vertical range extent as a main criterion for threat categorization (Sekercioglu et al. 2008). It also highlights the critical need to advance our knowledge of species' current elevational ranges.
Figure 3.
Partial residuals from a four-predictor model of projected proportional change in range size per species (n = 1009) under the no-dispersal (ND) scenario. The predictors include (a) the species' lateral range extent (range size) and (b) vertical range extent, (c) the average IPCC A2 temperature anomaly between 1980–1999 and 2080–2099 within the species' range, and (d) the species' biogeographic realm (AfT, Afrotropics; AuA, Australasia; InM, IndoMalaya; NeA, Nearctic; NeT, Neotropics; PaA, Palaearctic).
For the case of unconstrained VD, we additionally assess the importance of the maximum elevation available within the geographical range, which emerges as the most important predictor of projected range loss (ΔAIC = 253), followed by realm (ΔAIC = 124) and lateral range extent (ΔAIC = 17; electronic supplementary material, figure S8 and table S3). Under this scenario, a 100 m increase in maximum elevation within the range is associated with an approximately 2 per cent decrease in average range loss and a doubling of lateral range extent with an approximately 2 per cent increase in average range loss. The latter association probably reflects the greater prevalence of small ranged species in the tallest mountain systems. The geographical differences in projected warming (figure 1b), only of minor importance in the ND scenario, lose significance altogether in the VD case.
Given the broad scale, these analyses only offer a first baseline evaluation of biodiversity susceptibility to global warming under what could be considered worst- (ND) to best-case (VD + LD) scenarios. We note that a broad array of environmental and ecological contingencies will probably shape individual species' responses to climate change (Jackson et al. 2009), with demographic traits and metapopulation processes affecting distributional shifts at leading and trailing edges of species' ranges (Anderson et al. 2009). Changes in other climatic factors such as temperature or precipitation regimes, which are currently more difficult to predict than changes in average temperature, are likely to be particularly relevant in defining species' responses to climate change (Knapp et al. 2008). Moreover, the link between elevation and temperature, as examined in this study, represents a component of a species' climatic niche (Soberón & Nakamura 2009) whose characteristics could be redefined under potentially novel future climatic conditions (Williams et al. 2007). For montane species, plasticity and adaptations (Gienapp et al. 2007; Skelly et al. 2007) and local-scale environmental heterogeneity (Luoto & Heikkinen 2008; Randin et al. 2009) could at least in principle lessen impacts. Existing and future anthropogenic land-use changes, which are likely to be particularly severe at elevations greater than 1000 m (Sala et al. 2000; Jetz et al. 2007; Nogués-Bravo et al. 2008), are bound to increase projected range losses in the ND and especially the VD scenarios. Further, changes in species interactions will probably add additional variability to species' geographical responses (Tylianakis et al. 2008). We posit that many uncertainties still underlie our current understanding on how biological systems will respond to climate change and how and whether communities perturbed by climate change may be able to reassemble (Williams & Jackson 2007; La Sorte et al. 2009).
4. Conclusions
Our findings highlight the use of a combined global- and species-level perspective and reaffirm the need to address both physiological and physiographic factors in order to comprehensively understand the potential impacts of global warming on biodiversity. We conjecture that in montane birds, range losses in the twenty-first century will certainly be worse than in the best-case scenario (VD + LD), and in many cases better than the worst-case scenario (ND). With our current knowledge, other terrestrial vertebrate taxa (and probably most others) have smaller lateral range sizes than birds (median: birds = 5.3 × 105 km2; amphibians = 3.1 × 104; mammals = 2.2 × 105), and correspondingly, probably narrower vertical ranges (electronic supplementary material, figure S4c). Even with different biogeographic patterns of diversity and range extents in other taxa, from our simulations and empirical analyses we can make the following generalizations. (i) Owing to geographical patterns of projected warming and the extent, orientation and isolation of mountains, the montane biodiversity of Australasia, the Afrotropics and the Nearctic are exceptionally susceptible to climate change and deserve particular attention. (ii) In a world of limited dispersal opportunities, the vertical range of elevations occupied by a species is the most critical driver of montane biodiversity extinction risk, more than any other factor (including a several degree Celsius difference in projected warming). (iii) Species threat assessment will therefore benefit strongly from extending and including the knowledge of species' vertical ranges. (iv) Fine-scale modelling of the potential for upward movement of vegetation is necessary to gauge the explicit feasibility of vertical animal dispersal. (v) VD opportunities are often not explicitly considered in correlative distribution models that predict species' distributions under climate change; the quality of model predictions would probably benefit by simultaneously considering both lateral and VD opportunities (La Sorte & Jetz 2010). (vi) Conservation efforts will benefit from the placement of reserves in highland locations and along key elevational corridors to promote connectivity (Pressey et al. 2007), especially in high-altitude regions with intensive human land use (Hodgson et al. 2009).
In total, our findings emphasize the strong, irreversible and undeniable threat faced by montane biota in a future warmer world. These threats extend to the services provided by montane biodiversity and to the human livelihoods that rely on them (Messerli & Ives 1997). Next to arctic ecosystems (MacDonald 2010), there is probably no other terrestrial global biological system that is more extensively and demonstrably threatened by impending climate change, and no other that offers fewer excuses for scientific or conservation inaction.
Acknowledgements
We thank I. I. Mokhov for providing global lapse-rate values and Ch. Körner for advice on treeline estimates; J. Belmaker, A. Boyer, N. Cooper, R. Colwell, D. Kissling, H. Kreft and C. Sekercioglu and two anonymous reviewers for helpful comments on the manuscript; T. M. Lee, C. McCain and H. Wilman for valuable discussions; and C. Edward for assistance with data compilation. We acknowledge support from National Science Foundation Grant BCS-0648733.
References
- Anderson B. J., Akçakaya H. R., Araújo M. B., Fordham D. A., Martinez-Meyer E., Thuiller W., Brook B. W.2009Dynamics of range margins for metapopulations under climate change. Proc. R. Soc. B 276, 1415–1420 (doi:10.1098/rspb.2008.1681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P., Tu J.2004Bootstrap methods for developing predictive models. Am. Stat. 58, 131–137 (doi:10.1198/0003130043277) [Google Scholar]
- Barthlott W., Hostert A., Kier G., Küper W., Kreft H., Mutke J., Rafiqpoor M. D., Sommer J. H.2007Geographic patterns of vascular plant diversity at continental to global scales. Erdkunde 61, 305–315 (doi:10.3112/erdkunde.2007.04.01) [Google Scholar]
- Beaumont L. J., Hughes L., Pitman A. J.2008Why is the choice of future climate scenarios for species distribution modelling important? Ecol. Lett. 11, 1135–1146 [DOI] [PubMed] [Google Scholar]
- Brook B. W., Sodhi N. S., Bradshaw C. J. A.2008Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- Buckley L. B., Jetz W.2008Linking global turnover of species and environments. Proc. Natl Acad. Sci. USA 105, 17 836–17 841 (doi:10.1073/pnas.0803524105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrough P. A.1986Principles of geographic information systems for land resources assessment. New York, NY: Oxford University Press [Google Scholar]
- Ceballos G., Ehrlich P. R.2006Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl Acad. Sci. USA 103, 19 374–19 379 (doi:10.1073/pnas.0609334103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clobert J., Danchin E., Dhondt A. A., Nichols J. D.(eds)2001Dispersal. Oxford, UK: Oxford University Press [Google Scholar]
- Colwell R. K., Brehm G., Cardelús C. L., Gilman A. C., Longino J. T.2008Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- Diaz H. F., Grosjean M., Graumlich L.2003Climate variability and change in high elevation regions: past, present and future. Clim. Change 59, 1–4 (doi:10.1023/A:1024416227887) [Google Scholar]
- Ghalambor C. K., Huey R. B., Martin P. R., Tewksbury J. J., Wang G.2006Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- Gienapp P., Leimu R., Merila J.2007Responses to climate change in avian migration time: microevolution versus phenotypic plasticity. Climate Res. 35, 25–35 (doi:10.3354/cr00712) [Google Scholar]
- Grenyer R., et al. 2006Global distribution and conservation of rare and threatened vertebrates. Nature 444, 93–96 (doi:10.1038/nature05237) [DOI] [PubMed] [Google Scholar]
- Herzog S. K., Kessler M., Bach K.2005Elevational gradient in Andean bird species richness at the local scale: a foothill peak and a high-elevation plateau. Ecography 28, 209–222 (doi:10.1111/j.0906-7590.2005.03935.x) [Google Scholar]
- Hodgson J. A., Thomas C. D., Wintle B. A., Moilanen A.2009Climate change, connectivity and conservation decision making: back to basics. J. Appl. Ecol. 46, 964–969 (doi:10.1111/j.1365-2664.2009.01695.x) [Google Scholar]
- IUCN 2001IUCN Red List categories and criteria, version 3.1, p. 30 Gland, Switzerland and Cambridge, UK: IUCN Species Survival Commission [Google Scholar]
- Jackson S. T., Betancourt J. L., Booth R. K., Gray S. T.2009Ecology and the ratchet of events: climate variability, niche dimensions, and species distributions. Proc. Natl Acad. Sci. USA 106, 19 685–19 692 (doi:10.1073/pnas.0901644106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen D. H.1967Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 (doi:10.1086/282487) [Google Scholar]
- Jarvis A., Reuter H. I., Nelson A., Guevara E.2006Hole-filled SRTM for the globe version 3, from the CGIAR-CSI SRTM 90m database. See http://srtm.csi.cgiar.org [Google Scholar]
- Jetz W., Rahbek C., Colwell R. K.2004The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol. Lett. 7, 1180–1191 (doi:10.1111/j.1461-0248.2004.00678.x) [Google Scholar]
- Jetz W., Wilcove D. S., Dobson A. P.2007Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, e157 (doi:10.1371/journal.pbio.0050157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump A. S., Mátyás C., Peñuelas J.2009The altitude-for-latitude disparity in range retractions of woody species. Trends Ecol. Evol. 24, 694–701 (doi:10.1016/j.tree.2009.06.007) [DOI] [PubMed] [Google Scholar]
- Karl T. R., Trenberth K. E.2003Modern global climate change. Science 302, 1719–1723 (doi:10.1126/science.1090228) [DOI] [PubMed] [Google Scholar]
- Kelly A. E., Goulden M. L.2008Rapid shifts in plant distributions with recent climate change. Proc. Natl Acad. Sci. USA 105, 11 823–11 826 (doi:10.1073/pnas.0802891105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. K., et al. 2008Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58, 811–821 (doi:10.1641/B580908) [Google Scholar]
- Körner C.2007Climatic treelines: conventions, global patterns, causes. Erdkunde 61, 316–324 (doi:10.3112/erdkunde.2007.04.02) [Google Scholar]
- Körner C., Spehn E. M. (eds) 2002Mountain biodiversity: a global assessment. Boca Raton, FL: Parthenon Publication Group [Google Scholar]
- Larsen W. A., McCleary S. J.1972The use of partial residual plots in regression analysis. Technometrics 14, 781–790 (doi:10.2307/1267305) [Google Scholar]
- La Sorte F. A., Jetz W.2010Avian distributions under climate change: towards improved projections. J. Exp. Biol. 213, 862–869 (doi:10.1242/jeb.038356) [DOI] [PubMed] [Google Scholar]
- La Sorte F. A., Thompson F. R.2007Poleward shifts in winter ranges of North American birds. Ecology 88, 1803–1812 [DOI] [PubMed] [Google Scholar]
- La Sorte F. A., Lee T. M., Wilman H., Jetz W.2009Disparities between observed and predicted impacts of climate change on winter bird assemblages. Proc. R. Soc. B 276, 3167–3174 (doi:10.1098/rspb.2009.0162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomolino M. V.2001Elevation gradients of species-density: historical and prospective views. Glob. Ecol. Biogeogr. 10, 3–13 (doi:10.1046/j.1466-822x.2001.00229.x) [Google Scholar]
- Luoto M., Heikkinen R. K.2008Disregarding topographic heterogeneity biases species turnover assessments based on bioclimatic models. Glob. Change Biol. 14, 1–12 [Google Scholar]
- MacDonald G. M.2010Global warming and the Arctic: a new world beyond the reach of the Grinnellian niche? J. Exp. Biol. 213, 855–861 (doi:10.1242/jeb.039511) [DOI] [PubMed] [Google Scholar]
- Mace G. M., Collar N. J., Gaston K. J., Hilton-Taylor C., Akçakaya H. R., Leader-Williams N., Milner-Gulland E. J., Stuart S. N.2008Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22, 1424–1442 (doi:10.1111/j.1523-1739.2008.01044.x) [DOI] [PubMed] [Google Scholar]
- McCain C. M.2009Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 12, 550–560 (doi:10.1111/j.1461-0248.2009.01308.x) [DOI] [PubMed] [Google Scholar]
- Messerli B., Ives J. D. (eds) 1997Mountains of the world: a global priority. New York, NY: Parthenon [Google Scholar]
- Mokhov I. I., Akperov M. G.2006Tropospheric lapse rate and its relation to surface temperature from reanalysis data. Izv. Atmos. Ocean. Phys. 42, 430–438 (doi:10.1134/S0001433806040037) [Google Scholar]
- Moore R. P., Robinson W. D., Lovette I. J., Robinson T. R.2008Experimental evidence for extreme dispersal limitation in tropical forest birds. Ecol. Lett. 11, 960–968 (doi:10.1111/j.1461-0248.2008.01196.x) [DOI] [PubMed] [Google Scholar]
- Moritz C., Patton J. L., Conroy C. J., Parra J. L., White G. C., Beissinger S. R.2008Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264 (doi:10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- Nakićenović N., Swart R.2000Emissions scenarios. In IPCC special report, p. 570 Cambridge, UK: IPCC [Google Scholar]
- Nogués-Bravo D., Araújo M. B., Errea M. P., Martínez-Rica J. P.2007Exposure of global mountain systems to climate warming during the 21st century. Glob. Environ. Change 17, 420–428 [Google Scholar]
- Nogués-Bravo D., Araujo M. B., Romdal T. S., Rahbek C.2008Scale effects and human impact on the elevational species richness gradients. Nature 453, 216–220 (doi:10.1038/nature06812) [DOI] [PubMed] [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Pepin N. C., Lundquist J. D.2008Temperature trends at high elevations: patterns across the globe. Geophys. Res. Lett. 35, L14701 (doi:10.1029/2008GL034026) [Google Scholar]
- Pressey R. L., Cabeza M., Watts M. E., Cowling R. M., Wilson K. A.2007Conservation planning in a changing world. Trends Ecol. Evol. 22, 583–592 (doi:10.1016/j.tree.2007.10.001) [DOI] [PubMed] [Google Scholar]
- Randall D. A., et al. 2007Climate models and their evaluation. In Climate Change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Randin C. F., Engler R., Normand S., Zappa M., Zimmermann N. E., Pearman P. B., Vittoz P., Thuiller W., Guisan A.2009Climate change and plant distribution: local models predict high-elevation persistence. Glob. Change Biol. 15, 1557–1569 (doi:10.1111/j.1365-2486.2008.01766.x) [Google Scholar]
- Raupach M. R., Marland G., Ciais P., Le Quéré C., Canadell J. G., Klepper G., Field C. B.2007Global and regional drivers of accelerating CO2 emissions. Proc. Natl Acad. Sci. USA 104, 10 288–10 293 (doi:10.1073/pnas.0700609104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romdal T. S., Rahbek C.2009Elevational zonation of Afrotropical forest bird communities along a homogenous forest gradient. J. Biogeogr. 36, 327–336 (doi:10.1111/j.1365-2699.2008.01996.x) [Google Scholar]
- Root T. L., Price J. T., Hall K. R., Schneider S. H., Rosenzweig C., Pounds J. A.2003Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- Rosenzweig C., et al. 2008Attributing physical and biological impacts to anthropogenic climate change. Nature 453, 353–357 (doi:10.1038/nature06937) [DOI] [PubMed] [Google Scholar]
- Sala O. E., et al. 2000Biodiversity: global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- Schaffer M. L.1981Minimum population sizes and species conservation. Bioscience 31, 131–134 [Google Scholar]
- Sekercioglu C. H., Schneider S. H., Fay J. P., Loarie S. R.2008Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150 (doi:10.1111/j.1523-1739.2007.00852.x) [DOI] [PubMed] [Google Scholar]
- Skelly D. K., Joseph L. N., Possingham H. P., Freidenburg L. K., Farrugia T. J., Kinninson M. T., Hendry A. P.2007Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355 (doi:10.1111/j.1523-1739.2007.00764.x) [DOI] [PubMed] [Google Scholar]
- Soberón J., Nakamura M.2009Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19 644–19 650 (doi:10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S., Plattner G.-K., Knutti R., Friedlingstein P.2009Irreversible climate change due to carbon dioxide emissions. Proc. Natl Acad. Sci. USA 106, 1704–1709 (doi:10.1073/pnas.0812721106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still C. J., Foster P. N., Schneider S. H.1999Simulating the effects of climate change on tropical montane cloud forests. Nature 398, 608–610 [Google Scholar]
- Tingley M. W., Monahan W. B., Beissinger S. R., Moritz C.2009Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643 (doi:10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A.2008Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- Udvardy M. D. F.1975A classification of the biogeographical provinces of the world. In IUCN Occasional Paper, vol. 18 Morgues, Switzerland: IUCN [Google Scholar]
- Watkinson A. R., Gill J. A.2001Climate change and dispersal. In Dispersal ecology (eds Bullock J. M., Kenward R. E., Hails R. S.), pp. 410–428 Oxford, UK: Blackwell Publishing [Google Scholar]
- Williams J. W., Jackson S. T.2007Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (doi:10.1890/070037) [Google Scholar]
- Williams J. W., Jackson S. T., Kutzbach J. E.2007Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (doi:10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. E., Shoo L. P., Isaac J. L., Hoffmann A. A., Langham G.2008Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, e325 (doi:10.1371/journal.pbio.0060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J., Gutiérrez D., Gutiérrez J., Monserrat V. J.2007An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob. Change Biol. 13, 1873–1887 (doi:10.1111/j.1365-2486.2007.01418.x) [Google Scholar]