Abstract
Phenotypic plasticity plays a key role in modulating how environmental variation influences population dynamics, but we have only rudimentary understanding of how plasticity interacts with the magnitude and predictability of environmental variation to affect population dynamics and persistence. We developed a stochastic individual-based model, in which phenotypes could respond to a temporally fluctuating environmental cue and fitness depended on the match between the phenotype and a randomly fluctuating trait optimum, to assess the absolute fitness and population dynamic consequences of plasticity under different levels of environmental stochasticity and cue reliability. When cue and optimum were tightly correlated, plasticity buffered absolute fitness from environmental variability, and population size remained high and relatively invariant. In contrast, when this correlation weakened and environmental variability was high, strong plasticity reduced population size, and populations with excessively strong plasticity had substantially greater extinction probability. Given that environments might become more variable and unpredictable in the future owing to anthropogenic influences, reaction norms that evolved under historic selective regimes could imperil populations in novel or changing environmental contexts. We suggest that demographic models (e.g. population viability analyses) would benefit from a more explicit consideration of how phenotypic plasticity influences population responses to environmental change.
Keywords: reaction norm, evolutionary trap, environmental stochasticity, cue reliability, persistence, population dynamics
1. Introduction
Stochastic factors exert a strong influence on ecological and evolutionary dynamics (Lande et al. 2003; Lenormand et al. 2009). Temporal variation in climate, for example, can affect individual performance, patterns and intensity of natural selection, and density-dependent interactions, by driving physical habitat changes and altering the distribution and abundance of interacting species (Coulson et al. 2001; Grant & Grant 2002; Stenseth et al. 2002). A central goal of modern population biology is therefore to understand how stochastic environmental variation affects individual fitness and, in turn, population and evolutionary dynamics (Coulson et al. 2006; Tuljapurkar et al. 2009).
Phenotypic plasticity and environmental canalization represent two contrasting biological phenomena linking individual-level and population-level responses to environmental variation (Schlichting & Pigliucci 1998). Phenotypic plasticity refers to the ability of a single genotype to produce different phenotypes under different environmental conditions. Environmental canalization, in contrast, occurs when genetic expression is insensitive to the environment—the same phenotype is produced regardless of environmental variation (Debat & David 2001).
Plastic responses such as changes in development, behaviour and allocation of resources to competing demands potentially allow individuals to match their phenotypes (or those of their offspring, in the case of plastic maternal effects) to spatial or temporal variations in their abiotic and biotic environments (Bradshaw 1965; Scheiner 1993; Gotthard & Nylin 1995; Schlichting & Pigliucci 1998; Sultan 2003). In order for phenotypic plasticity to be effective, however, organisms must often be able to accurately assay or forecast environmental challenges affecting their individual fitnesses (Levins 1963; Bradshaw 1986; Moran 1992; Scheiner 1993; De Jong 1999; Tufto 2000; Lande 2009). To do so, they often use indirect cues. Seasonal environments, for example, are characterized by predictable sequences of change in environmental variables such as photoperiod and temperature, but also by random variation in selective factors across years (e.g. timing of optimal temperatures for growth and survival, peak food availability, predation pressure). By sensing cues early in the season, organisms can anticipate the best time to initiate seasonal reproduction, migration, dormancy, etc., or to produce a particular seasonal morph, thereby matching their phenotypes to the expected conditions.
Although the relevance of intragenerational environmental predictability (or intergenerational, in the case of adaptive maternal effects) to the evolution of phenotypic plasticity is well established (Moran 1992; Scheiner 1993; Mousseau & Fox 1998; De Jong 1999; Tufto 2000), its importance for population dynamics has arguably been under-appreciated. One potential reason for this is that the distinction between effects of phenotypic changes on absolute fitness and relative fitness is rarely made explicit. Selective pressures on phenotypic plasticity are governed by variability in the relative fitnesses of phenotypes across environments, with plastic genotypes being favoured when reliable cues allow close phenotype–environment matching. Consequences of plasticity for population growth and persistence, on the other hand, depend on how it affects absolute fitness (mean per capita offspring production per time step), which in turn influences the sensitivity of demographic parameters to stochastic environmental changes occurring within the lifetimes of individuals.
Caswell (1983) suggested that adaptive plasticity in regulatory traits might act to reduce variance in highly elastic demographic parameters (those for which relatively small changes can have a large impact on fitness), providing a mechanism by which absolute fitness can be buffered against random temporal environmental fluctuations (see also Pfister 1998; Gaillard & Yoccoz 2003). The potential fitness-buffering effects of plasticity, however, should be a function of the reliability of environmental cues, which can change when cues and selective filters become decoupled. Climate change, habitat alterations or species introductions, for example, can reduce the reliability of cues as indicators of optimal behavioural or life-history decisions, rendering previously adaptive plastic responses less effective, or even maladaptive, in the new environmental context (Schlaepfer et al. 2002). Extreme or novel environmental stresses can also directly induce maladaptive plastic responses, independent of whether cue reliability changes (Ghalambor et al. 2007).
Here we present a stochastic, individual-based model to test the hypothesis that the effect of plasticity on population viability in a stochastic environment depends on the reliability of environmental cues. We focus on plasticity, independent of evolution, in order to complement numerous other studies that have considered the role of evolution in population persistence, independent of plasticity (reviewed in Kinnison & Hairston 2007). Environmental heterogeneity was characterized by two components: magnitude and predictability of stochastic fluctuations. The former was simulated as stochastic temporal variations in the optimal phenotype (cf. Lynch & Lande 1993; Lande & Shannon 1996), while the latter was modelled as the correlation between the value of an arbitrary environmental cue, with its own stochastic component, and that of the optimal phenotype (figure 1). In this case, ‘predictability’ reflects reliability of an indirect environmental cue as an indicator of the optimum phenotype in the present or at some future time period. Such predictability should not be confused with the ability of a plastic phenotype to produce the optimum phenotype when cues are reliable, which instead determines the absolute fitness and demographic contributions of various plastic phenotypes in our model.
Figure 1.
Illustration of three different scenarios of environmental predictability (simulated data). In each panel, phenotypic optima are plotted against cue values, simulated by drawing each from a standard bivariate normal distribution (means of 0 and standard deviations of 1), with a different correlation between the cue and the optimum in each panel. The optimal reaction norm in each case is shown as a solid black line, and the reaction norms of two individuals are also shown: a non-plastic genotype that produces the same phenotype in every environment (dashed line), and a plastic genotype (dotted line; slope = 0.5). (a) The correlation between the cue and the optimum is perfect (r = 1), so the optimal reaction norm has a slope of 1 and intercept of 0. The plastic genotype has higher average expected fitness than the fixed genotype (the fitness of a genotype is maximized by minimizing the sum of squared deviations between environment-specific phenotypes and corresponding optimal phenotypes). (b) The cue and the optimum are completely decoupled (r = 0), so the optimal reaction norm has a slope of 0 as well as an intercept of 0. The fixed genotype has higher expected fitness than the plastic genotype under these circumstances. (c) The cue and the optimum are 40 per cent correlated, so the optimal reaction norm has a slope of 0.4. The plastic genotype has a slope closer to the optimum slope, and consequently higher fitness, than a non-plastic genotype.
We used the model to address the following questions, which are difficult to answer quantitatively using existing models and theory. Under what conditions does adaptive plasticity have a stabilizing effect on population dynamics? How strong must the correlation between the cue and selective optimum be for plasticity to significantly reduce extinction risk? Can phenotypic responses towards the current environmental optimum have adverse demographic consequences for the population under conditions of altered cue reliability? Our results show that reaction norm slope, cue reliability, magnitude of stochastic variation and density dependence interact in nonlinear ways to affect population dynamic processes and persistence.
2. Material and Methods
(a). Model overview
We simulated a closed but freely mixing finite sexual population, with discrete generations (e.g. an annual plant or animal). The model kept track of information on individuals, such as their ontogenetic stage (juvenile, sub-adult or mature adult), phenotypic values and fecundity. Ordering of events in the model was (i) reproduction, (ii) density-dependent juvenile mortality, and (iii) phenotypic selection on offspring that survived density-dependent mortality.
Individual relative fitness was determined by a single continuously distributed character, which responded plastically to environmental variation. Timing traits are good examples of plastic quantitative characters—such as germination timing in annual plants (Freas & Kemp 1983), spawn timing in fish (Genner et al. 2010) or laying date in birds (Visser et al. 2009)—each of which might be triggered by photoperiod, temperature or some other cue. Mean (absolute) fitness in the population  per generation had three components:
per generation had three components:
| 2.1 |
where  is the mean survival of juveniles (density-dependent),
is the mean survival of juveniles (density-dependent),  is the mean survival of sub-adults (phenotype-dependent) and
is the mean survival of sub-adults (phenotype-dependent) and  is the mean per capita fecundity. λ is therefore the multiplicative population growth rate per generation. The model was parametrized such that expected geometric mean λ ≈ 1 (on average, the population replaced itself) when the mean phenotype was at the optimum and there was moderate stabilizing selection (see below). Any change in the optimum therefore resulted in a drop in mean fitness within a generation, in the absence of a plastic response.
is the mean per capita fecundity. λ is therefore the multiplicative population growth rate per generation. The model was parametrized such that expected geometric mean λ ≈ 1 (on average, the population replaced itself) when the mean phenotype was at the optimum and there was moderate stabilizing selection (see below). Any change in the optimum therefore resulted in a drop in mean fitness within a generation, in the absence of a plastic response.
(b). Characterizing plasticity
We adopted a linear reaction norm approach to model plasticity (Tufto 2000; Lande 2009; Chevin & Lande 2010), where individual phenotypic responses to the environment were characterized by an elevation (expected trait value in the average environment, which varied around zero) and slope (degree to which the phenotype changes as the environment changes). The reaction norm slope in this case can be conceived either as a separate quantitative character, potentially correlated at the genetic level with the trait elevation, or as the outcome of environment-dependent gene regulation (Via et al. 1995).
The model was seeded with 1000 juveniles at the beginning of each simulation run. The initial phenotype of each individual was formulated as
| 2.2 |
where zi is the realized trait value for the ith individual. Here, pi (an individual's intercept—analogous to a ‘permanent’ environment effect in the standard quantitative genetics model) represents an individual's deviation from the population mean phenotype that is independent of E. The initial mean phenotype in the average environment,  , was set to 0 (which corresponded to the expected optimal phenotype across environments; see below). βi is an individual's plastic response to the environmental cue E. Environmental fluctuations were expressed as random deviations from the initial environmental state Et=0 = 0. The mean plastic response
, was set to 0 (which corresponded to the expected optimal phenotype across environments; see below). βi is an individual's plastic response to the environmental cue E. Environmental fluctuations were expressed as random deviations from the initial environmental state Et=0 = 0. The mean plastic response  was designated at the beginning of each run, and Et varied across generations within runs but was kept constant within generations (coarse-grained temporal environmental heterogeneity).
was designated at the beginning of each run, and Et varied across generations within runs but was kept constant within generations (coarse-grained temporal environmental heterogeneity).
The random variables pi and βi were drawn from independent Gaussian distributions with means  = 0 and
= 0 and  , respectively, and standard deviations σp = 1 and σβ = 0.1 (the main effects reported in the results were qualitatively insensitive to these dispersion parameters). Note that although we model developmentally fixed plasticity (once expressed, the trait does not change over the lifetime of individuals), our model is general and can easily be extended to plastic traits that are developmentally labile or reversible (Nussey et al. 2007).
, respectively, and standard deviations σp = 1 and σβ = 0.1 (the main effects reported in the results were qualitatively insensitive to these dispersion parameters). Note that although we model developmentally fixed plasticity (once expressed, the trait does not change over the lifetime of individuals), our model is general and can easily be extended to plastic traits that are developmentally labile or reversible (Nussey et al. 2007).
(c). Survival
Individuals were passed through two survival filters, the first being density-dependent survival WJ from the juvenile stage to the sub-adult stage. Here, we used a stage-specific Beverton–Holt function (Moussalli & Hilborn 1986):
NJ is the number of juveniles, S the intrinsic survival from the juvenile to sub-adult stage (survival at very low density) and K the carrying capacity of sub-adults. Survival through this stage was independent of phenotype.
Subsequent survival WS from sub-adult to adulthood was a function of phenotype, and independent of population density and phenotype frequency (hard viability selection). We used a Gaussian fitness function to model stabilizing selection (Lynch & Lande 1993):
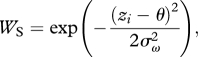 |
where θ is the optimal value of z and σω is the width (standard deviation) of the fitness function. When the mean phenotype was at the optimum (i.e.  = θ), the strength of stabilizing selection was inversely proportional to σ2ω, and individuals with phenotype zi = θ had a survival of 1 (however, with any phenotypic variance, the mean survival at this stage was less than 1). We used a selection strength of σω = 3 (i.e. width of fitness function = 3 phenotypic standard deviations), which is equivalent to moderate selection (Kingsolver et al. 2001; Estes & Arnold 2007).
= θ), the strength of stabilizing selection was inversely proportional to σ2ω, and individuals with phenotype zi = θ had a survival of 1 (however, with any phenotypic variance, the mean survival at this stage was less than 1). We used a selection strength of σω = 3 (i.e. width of fitness function = 3 phenotypic standard deviations), which is equivalent to moderate selection (Kingsolver et al. 2001; Estes & Arnold 2007).
Beverton–Holt parameters were chosen such that at carrying capacity the juvenile survival was approximately 0.5 (default values were S = 0.75 and K = 1500). A hypothetical perfectly adapted population with equal survival probability for all individuals would have a total survival to adulthood of 0.5, and with a mean per capita fecundity of 2 (the value used in all simulations) would stabilize at approximately 500 individuals. Sensitivity of the results to Beverton–Holt parameters is described in the electronic supplementary material.
(d). Environmental stochasticity and predictability
Environmental stochasticity was modelled as a stationary white noise process (Lande & Shannon 1996), by assuming that the optimal phenotype varies stochastically around some expectation. The optimum θ and the environmental cue E were drawn at each time step from a zero-mean bivariate normal distribution with equal variances and covariance σθ,E = ρσθσE, where ρ is the correlation between the optimum and the cue. In the case of a perfect correlation (ρ = 1), the theoretical optimal plastic response perfectly tracks fluctuations in the selective environment (figure 1a). Conversely, with no relationship between the cue and the optimum (i.e. ρ = 0), individuals cannot reliably predict the fitness consequences of producing different phenotypes, and plasticity is theorized to have no adaptive benefit, and potentially a net cost (figure 1b; Moran 1992).
For simplicity, we assumed that variance in the cue and the optimum was the same, reflecting a situation where interannual variation in plasticity cues (e.g. temperature early in the season) is approximately on scale with interannual variation in the factor(s) determining fitness (e.g. temperature or food supply later in the season). Stochastic changes in the optimum (hereafter termed environmental stochasticity) were in squared units of phenotypic standard deviations. To put this in context, consider a hypothetical example of temperature-sensitive flowering date in an annual plant. If the phenotypic standard deviation in flowering dates among individual plants within a year is 7 days, and the standard deviation in optimal flowering date across years is 21 days, then the variance in θ would be 9 units (i.e. the square of 3 phenotypic standard deviation units). If the between-year variance in temperatures is also 9 (°C2), then in any given year there is an approximately 34 per cent chance that the temperature might be 3°C higher than usual (i.e. 1 s.d. above the mean). A plastic genotype with a reaction norm slope of +0.5 would be expected to flower 3.5 days (half a phenotypic standard deviation) later in such a year, relative to its expected flowering date in an average year. Note that the units for reaction norm slopes are phenotypic standard deviations per unit change in the cue.
(e). Scenarios explored
We considered a reaction norm's direction to be nominally ‘adaptive’ when the phenotypic response to a reliable cue is in the direction of the optimum for the current environment (Ghalambor et al. 2007). Nominally adaptive norms of reaction occur in our model when both ρ and the reaction norm slope (β) are positive. Outcomes were the same when the symmetrical situation of a negative correlation and negative reaction norm slope was modelled and are thus not shown. Extant populations are not expected to have evolved plastic responses that result in phenotypes further from the fitness optimum than would be produced by weaker plastic or canalized responses. However, anthropogenic environmental changes (e.g. reductions in cue reliability) could render previously adaptive reaction norms sub-optimal in new environmental contexts. Hence, it is important to examine demographic outcomes for a range of combinations of reaction norm slope, environmental stochasticity and cue reliability.
We examined a number of population-level response metrics, including average degree of phenotypic mismatch (the average absolute deviation of the observed mean phenotype from the optimum phenotype, calculated across 150 generations), likelihood of extinction (proportion of 500 replicate populations where zero-reproducing adults remained after 150 generations), arithmetic mean population size across the last 100 generations and coefficient of variation (CV) in population size across the last 100 generations. The independent variables were reaction norm slope (which could vary from 0 to +1), environmental stochasticity (six values explored, from 1 to 11 in increments of 2) and cue reliability (which could vary from 0 to 1). The model was coded in C++.
3. Results
The demographic effects of plasticity depended strongly on cue reliability. Arithmetic mean population size, N, and the magnitude of intergenerational fluctuations in population size for a given magnitude of environmental stochasticity depended on the match between the mean phenotype and the optimum phenotype. Without plasticity, the mean phenotype was invariant regardless of changes in the optimum, and populations were therefore phenotypically mismatched much of the time (figure 2, black line). The initial drop in mean absolute fitness caused by this mismatch resulted in negative population growth. Non-plastic populations were often able to stabilize and persist at smaller N following an initial drop in population size (because juvenile survival was compensatory) so long as environmental stochasticity was not too great (see the electronic supplementary material). Because fecundity was held constant, changes in population growth (whether positive or negative) from one generation to the next solely reflected the product of density-dependent juvenile survival and sub-adult survival, the latter being inversely proportional to the degree of phenotypic mismatch.
Figure 2.
Average absolute deviation (mismatch) per generation (calculated across 150 generations) between the observed mean phenotype and the optimum phenotype, plotted as a function of cue reliability. The y-axis units are phenotypic standard deviations. Environmental stochasticity was fixed at 5 units. Different colours represent different reaction norm slopes (black = no plasticity, pink = slope of 0.11, blue = slope of 0.33, red = slope of 0.66, green = slope of 0.99; see main text for explanation of units).
Without plasticity (flat reaction norm), the mean and variance of population size depended solely on the magnitude of environmental stochasticity (electronic supplementary material) and cue reliability had no effect because the phenotype was unresponsive to cues (figure 3a,b). However, when the reaction norm slope was positive, and cue reliability was positive and high, the mean population size was close to carrying capacity and fluctuations in population size were greatly reduced (figure 3d). In contrast, when cue reliability was low, strong plasticity had a negative effect on the mean population size, and caused larger population fluctuations (higher SD and CV of population size; figure 3c). Figure 2 shows why: the mean phenotype of populations exhibiting strong plasticity deviated more, on average, from the environmentally determined optimum phenotype than did populations with less plasticity, and consequently suffered cumulative reductions in absolute fitness.
Figure 3.
Effects of plasticity on population dynamics as a function of cue reliability (correlation between cue and environmental optimum). The magnitude of environmental stochasticity was fixed at 3 units (see main text for explanation), and cue reliability was fixed at either (a,c) 0.2 or (b,d) 0.8. In each panel, temporal fluctuations in the abundance of four randomly chosen replicate populations are shown. (a,b) Trends for populations with no plasticity (flat reaction norms); (c,d) trends for populations with strongly positive reaction norm slopes (here, β = 0.99).
In general, higher environmental stochasticity led to greater reductions in population size, increased variance in population growth rates and higher extinction risk (figure 4; electronic supplementary material). These effects were strongly modulated, however, by the degree of plasticity, and the effects of plasticity in turn depended strongly on cue reliability. At high levels of environmental stochasticity and low cue reliability, stronger plasticity (large positive reaction norm slopes) greatly increased the likelihood of extinction (figure 4). Extinction risk for plastic populations dropped off rapidly with increasing cue reliability. At intermediate levels of environmental stochasticity and cue reliability greater than approximately 0.6, extinction risk was essentially nil for populations exhibiting any capacity for plasticity, compared with around 20 per cent for non-plastic populations (figure 4c). At cue reliabilities less than approximately 0.5 and intermediate levels of environmental stochasticity, however, extinction risk was considerably higher for more plastic populations compared with less plastic populations (figure 4c,d, compare green and red curves with blue and pink curves).
Figure 4.
The proportion of replicate populations (out of a total of 500) that went extinct within 150 generations, plotted against cue reliability. The panels show outcomes for different magnitudes of environmental stochasticity (variance in the cue and the optimum; see main text for explanation), and different colour curves represent different strengths of plasticity (as indexed by β, the reaction norm slope; black: β = 0, pink: β = 0.11, blue: β = 0.33, red: β = 0.66, green: β = 0.99). (a) Stochasticity = 1; (b) stochasticity = 3; (c) stochasticity = 5; (d) stochasticity = 7; (e) stochasticity = 9; (f) stochasticity = 11.
Decreased extinction risk with higher cue reliability was relatively gradual for low and medium plasticity populations at intermediate levels of environmental stochasticity (figure 4c,d, pink and blue curves), but very steep for highly plastic populations (red and green curves). At higher levels of environmental stochasticity (figure 4e,f), extinction probability was close to 1 for non-plastic and low-plasticity populations (black and pink curves) regardless of cue reliability, and also for medium- to high-plasticity populations at cue reliabilities less than approximately 0.4 (blue, red and green curves). At higher cue reliabilities, high-plasticity populations had much higher persistence probabilities (figure 4f, red and green curves). At low levels of environmental stochasticity, populations could persist without any plasticity (figure 5a,b). At higher levels of stochasticity, the minimum amount of plasticity required for persistence was much lower when cue reliability was higher (figure 5c,d).
Figure 5.
Minimum plasticity (slope of reaction norm) required for persistence, as a function of cue reliability and environmental stochasticity. The panels show outcomes for six different magnitudes of environmental stochasticity. Three levels of quasi-persistence are depicted in each panel: 50% (darkest shade of grey; ≥50% of replicate populations persisted), 75% (intermediate shade of grey; >75% of replicate populations persisted) and 95% (lightest shade of grey; >95% of replicate populations persisted). Black areas are regions of parameter space where >50% of replicate populations went extinct. (a) Stochasticity = 1; (b) stochasticity = 3; (c) stochasticity = 5; (d) stochasticity = 7; (e) stochasticity = 9; (f) stochasticity = 11.
Varying the strength of density dependence affected absolute extinction probabilities, but did not substantially alter the relative effects of plasticity on population viability (electronic supplementary material). Varying the stage at which density dependence was implemented (from juvenile to sub-adult or adult) also did not alter results or conclusions.
4. Discussion
Ecologists and conservation biologists have long debated the relative importance of factors affecting population persistence in variable environments. Empirical studies of wild populations clearly show that stochastic changes in density-independent factors (e.g. weather) can be a major driver of temporal fluctuations in population size (Coulson et al. 2001; Stenseth et al. 2002) and, in some cases, population extirpations (Ehrlich et al. 1980; McLaughlin et al. 2002). Yet organisms occupying highly variable environments display a remarkable range of adaptations that allow them to maintain positive fitness across a broad range of conditions, achieved through a combination of homeostatic, plastic and bet-hedging mechanisms (Caswell 1983). The idea that individuals can adaptively adjust their behaviour, development and allocation of resources to competing demands is a cornerstone of behavioural ecology (Krebs & Davies 1997). Given the strong emphasis currently placed on questions of species persistence in the face of global environmental change, it is somewhat surprising that the demographic consequences of plasticity have received so little attention in the population ecology literature.
We present a flexible model for conceptualizing and quantifying the effects of plasticity on population dynamics and persistence, where the key parameter linking proximate cues and ultimate costs of selection is the correlation between an indirect environmental cue and the optimal phenotype each generation (cue reliability). Two major results emerged from our analysis. First, we found that when cue reliability is high and environmental stochasticity is moderate, a little plasticity can go a long way: even a relatively weak plastic response (low reaction norm slope) has a strong positive effect on population persistence under these circumstances (figure 5). An adaptive plastic response (plasticity towards the optimum under a reliable cue) allows individuals to tightly match their phenotype to the variable conditions encountered (figure 2), and therefore maintain high survival. As a result, population dynamics are more stable: mean population size remains close to carrying capacity, fluctuations in population size are greatly reduced and extinction risk is low.
Second, we found that demographic consequences of plasticity depend in a nonlinear, non-additive way on cue reliability and the magnitude of environmental stochasticity, both of which can change as a result of natural causes or anthropogenic impacts. When environmental stochasticity is high but cue reliability is low, plastic phenotypes do a poor job tracking environmental fluctuations. The average absolute deviation of the mean phenotype from the optimum phenotype is larger for populations exhibiting strong plasticity under these circumstances, compared with populations with reduced plasticity or canalized (non-plastic) populations (figure 2). These populations over-respond to an unreliable cue and hence more frequently ‘overshoot’ the optimum. The fitness function was symmetrical and stabilizing about an optimum that fluctuated across generations. Consequently, deviations in the mean phenotype either side of the optimum reduced mean absolute fitness, and the greater the absolute deviation, the greater the per-generation reduction in fitness. This negative demographic effect of strong plasticity outweighs its positive effects under these circumstances, and consequently population dynamics become less stable (figure 3, lower mean and higher variance in population size) and extinction risk increases (figure 4).
(a). The importance of reliable cues
Although our model focuses on absolute fitness and its demographic consequences, it nonetheless produces some findings consistent with past explorations of the evolution of plasticity based on relative fitness. Environmental predictability has long been emphasized in the evolutionary literature on phenotypic plasticity (Levins 1963; Bradshaw 1986; Gabriel & Lynch 1992; Moran 1992; Gavrilets & Scheiner 1993; Scheiner 1993; De Jong 1999; Tufto 2000). Using a simple population genetic model involving two discrete environmental states, Moran (1992) showed that in the absence of predictive cues, random assignment of phenotypes to each environment would achieve a better level of phenotype matching than plasticity. Our findings are in broad agreement with these early theoretical results, and our modelling framework may be considered analogous in many parts to that of Tufto (2000), who showed using an analytical quantitative genetic model that incomplete phenotype–environment matching evolves when developmental cues and selective optima are only partially correlated (see also Gavrilets & Scheiner 1993; de Jong 1999; Lande 2009).
As noted by Caswell (1983), phenotypic plasticity provides a potential mechanism by which organisms can buffer key vital rates against temporal or spatial environmental heterogeneity. If this is true, one would expect plastic populations to have lower variance in fitness in a stochastic environment (and hence higher geometric mean fitness, even if the arithmetic mean is the same) compared with non-plastic populations. Our model shows that this basic prediction is upheld only when cues are reliable. When cues are less informative (e.g. cue reliabilities less than approx. 0.5 for intermediate magnitudes of environmental stochasticity; figure 2), the demographic costs of strong plasticity can outweigh the benefits, and extinction risk increases as a nonlinear function of cue reliability. Notably, these demographic costs occur in our model even without explicit consideration of intrinsic costs of plasticity (e.g. production, maintenance and information-acquisition costs; DeWitt et al. 1998). The results suggest that strong plasticity should be rare in situations where temporal covariation between indirect cues and environmental factors affecting individual fitnesses is low (e.g. plastic responses involving long time lags, relative to the time scale of environmental variability; Padilla & Adolph 1996). To the extent that greater variability in the vital rates of plastic phenotypes reduces their geometric mean fitness when cues are completely unreliable, plasticity is expected to be disfavoured in lieu of relative trait canalization, or bet-hedging, in unpredictable environments (Gillespie 1974; Moran 1992).
A major challenge for many taxa is that climate change and other forms of anthropogenic disturbance might disrupt correlations between proximate cues and ultimate fitness-determining factors, such as that between seasonally changing day length and thermally regulated invertebrate abundance (Visser et al. 2004; Nussey et al. 2005; Visser 2008), reducing the accuracy of phenotype–environment matching, and in some cases threatening population viability (e.g. Both et al. 2006). Although such empirical examples of cue disruption often involve fitness costs of limited or maladaptive plasticity under directional environmental change, our model clearly suggests that net environmental change is not a prerequisite for concern. Many environmental time series are inherently ‘noisy’ and show considerable year-to-year variation relative to long-term trends. In such cases, environmental stochasticity coupled to reduced reliability of cues could also imperil populations. In the field of conservation biology, such cue failures have been termed ‘evolutionary traps’ (Schlaepfer et al. 2002), recognizing that evolved responses to environmental cues can ensnare populations when anthropogenic disturbances make cues unreliable indicators of optimal responses. Over time frames relevant to management (generally less than 50 years), many populations could face greater demographic costs from imperfect plasticity (as a result of reduced reliability of cues) coupled with strong environmental stochasticity than they might from limits on evolutionary or plastic responses to comparatively subtle environmental trends. In such cases, natural selection would be expected to favour compensatory changes in reaction norms or the use of alternative, more reliable cues.
(b). General applicability and potential refinements of the model
We made a number of simplifying assumptions in our model. First, we assumed that reaction norms were linear—a typical assumption when modelling characters that are not themselves major fitness components (Gavrilets & Scheiner 1993; Tufto 2000; Lande 2009; Chevin & Lande 2010). For characters more directly linked to fitness, nonlinear reaction norms might be more realistic (e.g. thermal performance curves; Gabriel & Lynch 1992; Kingsolver et al. 2004), and further modelling would be required to assess the consequences for population dynamics. Second, we assumed that plastic responses and selective pressures were density- and frequency-independent. In some situations, both plastic responses and their fitness consequences might be dependent on population density or the frequency of other genotypes adopting similar strategies, with potentially complex consequences for population dynamics (Ernande & Dieckmann 2004; Plaistow & Benton 2009).
Third, we did not model genetic variation in elevation or slope of reaction norms (genotype by environment interactions). We sought to assess the effects of plasticity independent of evolution, so as to complement models that have considered evolution independent of plasticity (reviewed in Kinnison & Hairston 2007). Nonetheless, this distinction is somewhat artificial, and the capacity for evolution of plasticity might be a crucial factor affecting population persistence, as suggested under directional environmental change (Visser 2008; Lande 2009; Chevin & Lande 2010). Lande (2009) and Chevin & Lande (2010) recently showed, for example, that following an abrupt directional change in the environment, plasticity (even if only partially adaptive) can significantly reduce the demographic cost of maladaptation, while subsequent evolution of the reaction norm slope can restore adaptation much quicker than evolution not affecting the slope of the norm (i.e. elevation evolution). Somewhat counterintuitively, however, our simulations under stochastic environmental variability (with no directional change component) show that if cues and environmental filters become increasingly uncoupled, then genotypes with reduced plasticity might in fact have higher absolute fitness and odds of persistence than those with greater plasticity. Although stronger plasticity might be expected to evolve when environments change or become more variable, selective pressures on reaction norms will depend heavily on cue reliability and relative magnitudes of directional environmental change and stochastic environmental variation (i.e. the signal-to-noise ratio).
In conclusion, we show that the effect of environmental stochasticity on population dynamics is modulated by phenotypic plasticity. Potential buffering effects of plasticity on demography hinge on the existence of reliable cues that allow organisms to match their phenotypes to the conditions encountered. Given that environments might become both more variable and unpredictable in the future owing to anthropogenic influences, incorporating these phenotypic mechanisms into techniques such as population viability analysis could improve our ability to predict how populations might respond.
Acknowledgements
This work was made possible by generous funding from the Gordon and Betty Moore Foundation, and facilitation from the Natural Center for Ecological Synthesis and Analysis, Santa Barbara, CA, USA. The model in this paper was designed and implemented in collaboration with SimBiotic Software (www.simbio.com) using their SimUText modelling framework. We thank Dan Nussey and two anonymous reviewers for constructive criticisms on previous versions of the manuscript.
References
- Both C., Bouwhuis S., Lessells C. M., Visser M. E.2006Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- Bradshaw A. D.1965Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155 [Google Scholar]
- Bradshaw W. E.1986Pervasive themes in insect life cycle strategies. In The evolution of insect life cycles (eds Taylor F., Karban R.). London, UK: Springer-Verlag [Google Scholar]
- Caswell H.1983Phenotypic plasticity in life-history traits—demographic effects and evolutionary consequences. Am. Zool. 23, 35–46 [Google Scholar]
- Chevin L. M., Lande R.2010When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150 (doi:10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- Coulson T., Catchpole E. A., Albon S. D., Morgan B. J. T., Pemberton J. M., Clutton-Brock T. H., Crawley M. J., Grenfell B. T.2001Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292, 1528–1531 (doi:10.1126/science.292.5521.1528) [DOI] [PubMed] [Google Scholar]
- Coulson T., Benton T. G., Lundberg P., Dall S. R. X., Kendall B. E., Gaillard J. M.2006Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B 273, 547–555 (doi:10.1098/rspb.2005.3357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debat V., David P.2001Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561 (doi:10.1016/S0169-5347(01)02266-2) [Google Scholar]
- De Jong G.1999Unpredictable selection in a structured population leads to local genetic differentiation in evolved reaction norms. J. Evol. Biol. 12, 839–851 (doi:10.1046/j.1420-9101.1999.00118.x) [Google Scholar]
- DeWitt T. J., Sih A., Wilson D. S.1998Costs and limits of phenotypic plasticity. Trends in Ecology & Evolution 13, 77–81 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- Ehrlich P. R., Murphy D. D., Singer M. C., Sherwood C. B., White R. R., Brown I. L.1980Extinction, reduction, stability and increase—the responses of checkerspot butterfly (Euphydryas) populations to the California drought. Oecologia 46, 101–105 (doi:10.1007/BF00346973) [DOI] [PubMed] [Google Scholar]
- Ernande B., Dieckmann U.2004The evolution of phenotypic plasticity in spatially structured environments: implications of intraspecific competition, plasticity costs and environmental characteristics. J. Evol. Biol. 17, 613–628 (doi:10.1111/j.1420-9101.2004.00691.x) [DOI] [PubMed] [Google Scholar]
- Estes S., Arnold S. J.2007Resolving the paradox of stasis: models with stabilizing selection explain evolutionary divergence on all timescales. Am. Nat. 169, 227–244 [DOI] [PubMed] [Google Scholar]
- Freas K. E., Kemp P. R.1983Some relationships between environmental reliability and seed dormancy in desert annual plants. J. Ecol. 71, 211–217 [Google Scholar]
- Gabriel W., Lynch M.1992The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 5, 41–59 (doi:10.1046/j.1420-9101.1992.5010041.x) [Google Scholar]
- Gaillard J. M., Yoccoz N. G.2003Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84, 3294–3306 (doi:10.1890/02-0409) [Google Scholar]
- Gavrilets S., Scheiner S. M.1993The genetics of phenotypic plasticity. V. Evolution of reaction norm shape. J. Evol. Biol. 6, 31–48 (doi:10.1046/j.1420-9101.1993.6010031.x) [Google Scholar]
- Genner M. J., Halliday N. C., Simpson S. D., Southward A. J., Hawkins S. J., Sims D. W.2010Temperature-driven phenological changes within a marine larval fish assemblage. J. Plank. Res. 32, 699–708 [Google Scholar]
- Ghalambor C. K., McKay J. K., Carroll S. P., Reznick D. N.2007Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (doi:10.1111/j.1365-2435.2007.01283.x) [Google Scholar]
- Gillespie J.1974Natural selection for within-generation variance in offspring number. Genetics 76, 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard K., Nylin S.1995Adaptive plasticity and plasticity as an adaptation—a selective review of plasticity in animal morphology and life-history. Oikos 74, 3–17 (doi:10.2307/3545669) [Google Scholar]
- Grant P. R., Grant B. R.2002Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- Kingsolver J. G., Hoekstra H. E., Hoekstra J. M., Berrigan D., Vignieri S. N., Hill C. E., Hoang A., Gibert P., Beerli P.2001The strength of phenotypic selection in natural populations. American Naturalist 157, 245–261 [DOI] [PubMed] [Google Scholar]
- Kingsolver J. G., Izem R., Ragland G. J.2004Plasticity of size and growth in fluctuating thermal environments: comparing reaction norms and performance curves. Integr. Comp. Biol. 44, 450–460 (doi:10.1093/icb/44.6.450) [DOI] [PubMed] [Google Scholar]
- Kinnison M. T., Hairston N. G.2007Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 21, 444–454 (doi:10.1111/j.1365-2435.2007.01278.x) [Google Scholar]
- Krebs J. R., Davies N. B.1997Behavioral ecology: an evolutionary approach. Oxford, UK: Blackwell Science Limited [Google Scholar]
- Lande R.2009Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 (doi:10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- Lande R., Shannon S.1996The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50, 434–437 (doi:10.2307/2410812) [DOI] [PubMed] [Google Scholar]
- Lande R., Engen S., Saether E.2003Stochastic population dynamics in ecology and conservation. Stoch. Popul. Dyn. Ecol. Conserv. i-x, 1–212 [Google Scholar]
- Lenormand T., Roze D., Rousset F.2009Stochasticity in evolution. Trends Ecol. Evol. 24, 157–165 (doi:10.1016/j.tree.2008.09.014) [DOI] [PubMed] [Google Scholar]
- Levins R.1963Theory of fitness in a heterogeneous environment. 2. Developmental flexibility and niche selection. Am. Nat. 97, 75–90 [Google Scholar]
- Lynch M., Lande R.1993Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva P. M., Kingsolver J. G., Huey R. B.), pp. 234–250 Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- McLaughlin J. F., Hellmann J. J., Boggs C. L., Ehrlich P. R.2002Climate change hastens population extinctions. Proc. Natl Acad. Sci. USA 99, 6070–6074 (doi:10.1073/pnas.052131199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A.1992The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 [Google Scholar]
- Moussalli E., Hilborn R.1986Optimal stock size and harvest rate in multistage life-history models. Can. J. Fish. Aquat. Sci. 43, 135–141 (doi:10.1139/f86-014) [Google Scholar]
- Mousseau T. A., Fox C. W.1998Maternal effects as adaptations. Oxford, UK: Oxford University Press [Google Scholar]
- Nussey D. H., Postma E., Gienapp P., Visser M. E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- Nussey D. H., Wilson A. J., Brommer J. E.2007The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- Padilla D. K., Adolph S. C.1996Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol. Ecol. 10, 105–117 (doi:10.1007/BF01239351) [Google Scholar]
- Pfister C. A.1998Patterns of variance in stage-structured populations: evolutionary predictions and ecological implications. Proc. Natl Acad. Sci. USA 95, 213–218 (doi:10.1073/pnas.95.1.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaistow S. J., Benton T. G.2009The influence of context-dependent maternal effects on population dynamics: an experimental test. Phil. Trans. R. Soc. B 364, 1049–1058 (doi:10.1098/rstb.2008.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S. M.1993Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68 (doi:10.1146/annurev.es.24.110193.000343) [Google Scholar]
- Schlaepfer M. A., Runge M. C., Sherman P. W.2002Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480 (doi:10.1016/S0169-5347(02)02580-6) [Google Scholar]
- Schlichting C. D., Pigliucci M.1998Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates [Google Scholar]
- Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K. S., Lima M.2002Ecological effects of climate fluctuations. Science 297, 1292–1296 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- Sultan S. E.2003Phenotypic plasticity in plants: a case study in ecological development. Evol. Dev. 5, 25–33 [DOI] [PubMed] [Google Scholar]
- Tufto J.2000The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am. Nat. 156, 121–130 [DOI] [PubMed] [Google Scholar]
- Tuljapurkar S., Gaillard J. M., Coulson T.2009From stochastic environments to life histories and back. Phil. Trans. R. Soc. B 364, 1499–1509 (doi:10.1098/rstb.2009.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via S., Gomulkiewicz R., De Jong G., Scheiner S. M., Schlichting C. D., van Tienderen P. H.1995Adaptive phenotypic plasticity—consensus and controversy. Trends Ecol. Evol. 10, 212–217 (doi:10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- Visser M. E.2008Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Both C., Lambrechts M. M.2004Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 35, 89–110 (doi:10.1016/S0065-2504(04)35005-1) [Google Scholar]
- Visser M. E., Holleman L. J. M., Caro S. P.2009Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331 (doi:10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]







