Abstract
The chicken brain is more than twice as big as the bobwhite quail brain in adulthood. To determine how this species difference in brain size emerges during development, we examined whether differences in neurogenesis timing or cell cycle rates account for the disparity in brain size between chickens and quail. Specifically, we examined the timing of neural events (e.g. neurogenesis onset) from Nissl-stained sections of chicken and quail embryos. We estimated brain cell cycle rates using cumulative bromodeoxyuridine labelling in chickens and quail at embryonic day (ED) 2 and at ED5. We report that the timing of neural events is highly conserved between chickens and quail, once time is expressed as a percentage of overall incubation period. In absolute time, neurogenesis begins earlier in chickens than in quail. Therefore, neural event timing cannot account for the expansion of the chicken brain relative to the quail brain. Cell cycle rates are also similar between the two species at ED5. However, at ED2, before neurogenesis onset, brain cells cycle faster in chickens than in quail. These data indicate that chickens have a larger brain than bobwhite quail mainly because of species differences in cell cycle rates during early stages of embryonic development.
Keywords: neurogenesis, cell cycle, brain, size, development
1. Introduction
An influential model of brain evolution and development proposed by Finlay and Darlington has shown that, among mammals, the duration of brain development increases with absolute brain size (Sacher & Staffeldt 1974; Finlay & Darlington 1995). Moreover, the schedule of neurogenesis tends to be uniformly stretched as gestation period increases among mammals (Clancy et al. 2001). These findings suggest that evolutionary increases in brain size arise mainly because of a lengthening of brain development, which entails predictable changes in neurogenesis schedules.
Among birds, the length of embryonic development also correlates with brain size (Portmann 1947b). However, the brain of chickens (Gallus gallus domesticus) is significantly larger than that of bobwhite quail (Colinus virginianus; Boire & Baron 1994) even though the normal incubation time of chickens is shorter than that of bobwhite quail (21 versus 23 days at the same incubation temperature). Furthermore, both species are similar in degree of maturity at hatching (Starck & Ricklefs 1998). Therefore, the disparity in brain size between chickens and quail cannot be owing to the uniform stretching or compressing of neurogenesis schedules that forms the core of Finlay and Darlington's model.
In search of an alternative explanation for the observation that hatchling chickens have larger brains than hatchling quail, we examined the timing of neural events (i.e. neurogenesis onset, maturational milestones) and cell cycle rates in the brains of embryonic chickens and bobwhite quail. We report that most aspects of brain maturation occur at similar times in these two species, if time is expressed as percentage of incubation. Therefore, species differences in neurogenesis timing cannot account for the differences in brain size. Instead, the disparity in adult brain size between chickens and quail is owing to species differences in cell cycle rates at very early stages of development (i.e. ED2).
2. Material and methods
We first discuss how embryos were collected, how neural events were scored and how brain size was measured. We then discuss how we compared cell cycle rates across species.
(a). Analysis of neural events
Fertile eggs were obtained from several commercial suppliers. Bobwhite quail (n > 50) and chicken (n > 50) embryos ranging from 3 to 11 days of incubation were immersion-fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid). The next day, embryos were stored in 70 per cent ethanol and embedded in paraffin (Paraplast Plus, Fisher, Fair Lawn, NJ, USA). The embryos were sectioned horizontally or transversely at a thickness of 20 µm. We mounted 29–65 regularly spaced sections through each specimen and stained with Giemsa. These sections were examined to estimate the timing of a neural event, which we define as a relatively rapid, qualitative transformation in brain morphology. We restrict our analysis to transformations that can be readily compared across different species. To estimate the timing of a neural event, we noted the earliest age by which an event had occurred and the oldest embryo in which the event had not yet occurred (electronic supplementary material, table S1). The time of a neural event was estimated by averaging those days.
The neural events include maturational milestones and neurogenesis onset in selected brain regions (figure 1). To determine neurogenesis onset, we took advantage of the fact that, as progenitor cells exit the cell cycle, they tend to exit the ventricular zone (VZ) and form a less dense, post-proliferative zone (see Striedter & Charvet 2008). The appearance of a post-proliferative zone marked the onset of neurogenesis in several brain regions.
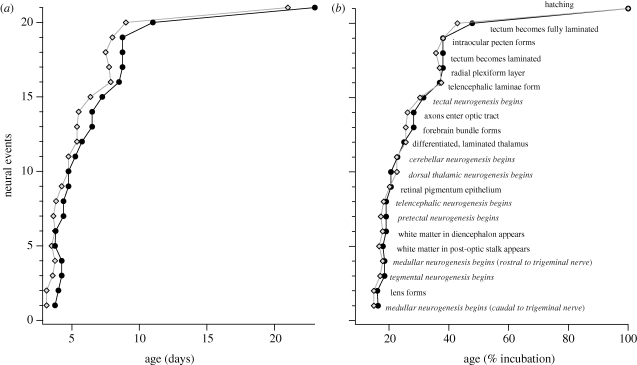
Figure 1.
(a) When compared in absolute days, neural events generally occur later in bobwhite quail than in chickens. (b) When compared in percentage of normal incubation period, the timing of neural events overlaps in chickens and quail. Neurogenesis onset events are represented in italics. Hatching was not considered a neural event in our statistical analysis. Diamonds, chicken; filled circles, quail.
(b). Analysis of brain growth
We measured brain size at various embryonic stages in the two species to determine when in development the chicken brain enlarges relative to that of quail. Chicken (n = 10) and bobwhite quail (n = 13) embryos were blotted dry and weighed on a precision balance (Ohaus Adventurer SL), immersion-fixed in methacarn, embedded in paraffin and sectioned horizontally at 20 µm. Brain and whole embryo areas were summed across sections and multiplied by the section spacing. Because fixation causes tissue to shrink, we divided volume estimates by an embryo-specific shrinkage factor, which was calculated by dividing the embryo's reconstructed body volume by the embryo's fresh weight, divided by the fresh tissue's estimated specific gravity of 1.04 g cm−3 (Stephan et al. 1981). Some of these embryos were collected for previous studies (Striedter & Charvet 2008; Charvet & Striedter 2009b; Charvet et al. in press).
(c). Cell cycle marker detection
To determine if cell cycle rates differ between chickens and quail, we used an antibody to label mitotic cells (phosphorylated histone-H3 (pH3); Hendzel et al. 1997) and an antibody to label all proliferating cells (proliferating cell nuclear antigen; PCNA). Although pH3+/PCNA+ cell counts cannot be used to estimate cell cycle rates, they suffice to determine whether cell cycle rates differ between species, as long as M-phase duration is assumed to be invariant (see Charvet & Striedter 2008).
Quail (n = 6; ED2.75) and chickens (n = 6; ED2.5) at 11 per cent of incubation were immersion-fixed in methacarn and embedded in paraffin. Brains were sectioned horizontally or sagittally at a thickness of 5 µm. Regularly spaced sections were mounted onto slides (Fisher Scientific). The sections were denatured with 0.5 M of hydrochloric acid for 30 min, incubated with an antibody against pH3 for 30 min (clone: Ser 10; species: rabbit; dilution: 1 : 100; source: Upstate, Temecula, CA, USA), followed by a biotinylated secondary antibody for 30 min (goat anti-rabbit IgG; dilution: 1 : 2; Chemicon, Temecula, CA, USA), processed with Vectastain ABC standard kit (Vector Laboratories, Burlingame, CA, USA) and reacted with diaminobenzidine (DAB; Vector Laboratories). The sections were then incubated with an antibody against PCNA for 30 min (clone: PC10; species: mouse; source: dilution: 1 : 100; Zymed, CA, USA), followed by a secondary antibody for 30 min (anti-mouse IgG; 1 : 200; Vector Laboratories), processed with Vectastain ABC standard kit (Vector Laboratories) and reacted with Vector SG (Vector Laboratories).
Estimates of pH3+/PCNA+ cell counts were obtained by placing a rectangular grid on photomicrographs of evenly spaced sections through the tectum and telencephalon. Randomly selected high-power photomicrographs of a region were taken with a digital colour camera (Spot Insight; Diagnostics Instruments, Sterling Heights, MI) attached to an Olympus BH-2 microscope. Several high-power photomicrographs were focused at different planes through the section of interest in order to identify cells at different depths within the VZ. A stack of these images was compiled and cell counts were made with the program ImageJ (Rasband 1997–2007).
Randomly selected rectangular counting frames were aligned along the ventricular surface. All frames were 50 µm in width but the height of the frames varied with the thickness of the ventricular (i.e. PCNA+) zone. Two sides of the frames served as exclusion lines to avoid overcounting. The pH3+ cells were counted as mitotic cells within the frame. The sum of the pH3+ and PCNA+ cells was scored as the number of PCNA+cells (i.e. total proliferating cells). We used an independent samples two-tailed t-test to detect species differences in pH3+/PCNA+ ratios.
(d). Cumulative bromodeoxyuridine labelling
Cumulative bromodeoxyuridine (BrdU) labelling was used to estimate brain cell cycle rates of chickens and quail. Quail (n = 13; ED2.75) and chickens (n = 7; ED2.5) at 11 per cent of incubation received 4 µg of BrdU and 5 µg of BrdU, respectively. Quail (n = 11; ED5) and chickens (n = 8; ED5) at 22 per cent of incubation received 10 µg of BrdU. The ED5 quail were collected for a previous study (Charvet & Striedter 2008).
The BrdU was dissolved in phosphate buffer and applied onto the embryo through a hole in the shell. After BrdU application, the hole was covered with tape and returned to a non-rotating incubator at 37°C. The embryos were sacrificed 0.5, 3, 6, 8–9 or 12 h after the initial application of BrdU. Previous work has shown that, unlike mammals, thymidine analogues are not rapidly metabolized in avian embryos (Striedter & Keefer 2000). Still, to ensure that high levels of unincorporated BrdU remained available within the egg, embryos sacrificed 6, 9 or 12 h after the initial BrdU application received additional doses of BrdU at 3 h intervals.
The embryos were immersion-fixed in methacarn, embedded in paraffin and sectioned at a thickness of 5 µm. Serial sections through the telencephalon and tectum were collected for immunohistochemistry. The sections were denatured with 0.5 M hydrochloride acid for 30 min, incubated with anti-BrdU for 30 min (IU4; 1 : 100; Caltag, Burmingame, CA), reacted with the biotinylated anti-mouse IgG for 30 min (anti-mouse IgG; 1 : 200; Vector Laboratories), processed with the Vectastain ABC standard kit (Vector Laboratories) and DAB (Vector Laboratories). The sections were counterstained with Giemsa. Sections from embryos that did not receive BrdU were processed to confirm antibody specificity. These procedures are identical to those used previously (Charvet & Striedter 2008).
BrdU+ and BrdU−cells within the VZ were counted in the tectum and the telencephalon of each embryo in a manner similar to that described for the pH3+/PCNA+ cell counts. In total, 5–10 counting frames per region were randomly selected for a given embryo. Within these frames, the number of BrdU+ cells and total VZ cells were estimated. The number of BrdU+ cells divided by the total number of VZ cells is referred to as the labelling index (LI; Fujita 1963). LIs were averaged for each survival time in each species to estimate cell cycle rates within the telencephalon or tectum. Regression lines were then fitted through the data points for survival times at which the LI had not saturated. We combined cell cycle rates for the telencephalon and tectum because we are interested in the overall brain growth. We averaged the telencephalon and tectum LIs for each survival time before the regression analysis. Statistical analyses were implemented in the software packages IGOR (Wavemetrics, Lake Oswego, OR, USA) and JMP (SAS, Cary, NC, USA).
3. Results
We first describe our finding that brain maturation is highly predictable in chickens and quail, as long as time is expressed as a percentage of normal incubation period. We then report our findings on species differences in brain growth and cell cycle rates.
(a). Conserved schedules of brain maturation
If the timing of neural events is expressed in days of incubation, 19 of 20 neural events occur later in bobwhite quail than in chickens (figure 1a). However, the timing of neural events overlaps considerably in the two species if time is expressed as a percentage of incubation (figure 1b). To quantify this difference, we divided each event time in quail (expressed in days of incubation or per cent of incubation) by the corresponding value for chickens. This analysis revealed that neural events occur on average 15 per cent later in quail than in chickens (s.e.m. = 1.3%; n = 20 events) when time is expressed in absolute days, but only 5 per cent later (s.e.m. = 1.2%; n = 20) when time is expressed relative to incubation period. A two-tailed t-test revealed this difference to be statistically significant (t =− 5.78; p < 0.0001). These findings indicate that age expressed as a percentage of normal incubation period is a better predictor of event timing than age expressed in days of incubation.
(b). Species differences in brain size
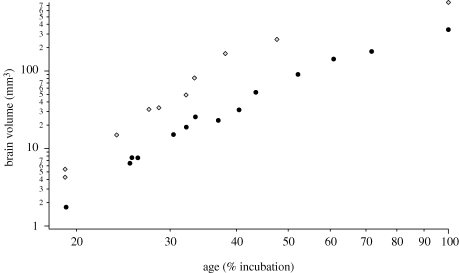
We calculated brain size at different stages of embryonic development in both species to assess when in development chicken brains become larger than quail brains. We found that at 19 per cent of incubation (when neurogenesis begins), the chicken's brain is already bigger than the quail's brain by a factor of 2–3. This size difference is similar to the one observed at hatching (figure 2). Therefore, the species difference in brain size must emerge before neurogenesis begins.
Figure 2.
Brain volumes of chickens and quail plotted against age expressed as a percentage of normal incubation period. By 19% of incubation, the chicken brain is already more than twice as big as the incubation-matched quail brain. The species difference in brain size persists throughout embryonic development. Diamonds, chicken; filled circles, quail.
(c). Early species differences in cell cycle rates
The finding that species differences in brain size are evident before neurogenesis onset suggests that events prior to neurogenesis account for the disparities in brain size of hatchling chickens and quail. We used pH3+/PCNA+ ratios and cumulative BrdU labelling to detect species differences in cell cycle rates in the telencephalon and tectum of quail and chickens at 11 per cent of incubation. We focus on the telencephalon and tectum because these structures account for more than 60 per cent of the brain in each of the two species (Boire & Baron 1994). Because we are interested in species difference in cell cycle rates in the overall brain, we combine results from the telencephalon and tectum. (We report cell cycle rates for telencephalon and tectum individually in the electronic supplementary material, table S2.)
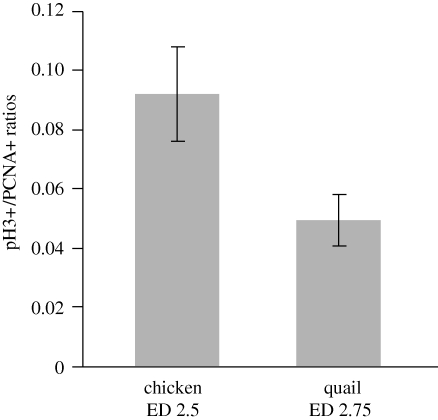
At 11 per cent of incubation, brain pH3+/PCNA+ ratios are nearly twice as high in chickens at ED2.75 (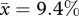 ; s.e.m. = 1.63; n = 6) than in quail at ED2.5 (
; s.e.m. = 1.63; n = 6) than in quail at ED2.5 (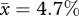 ; s.e.m. = 1.05; n = 6). This species difference is statistically significant (t = −2.28; p < 0.05). Assuming that the mitotic phase is invariant across these species, our findings suggest that, at 11 per cent of incubation, the cell cycle rate is nearly twice as fast in chickens than in quail (figure 3).
; s.e.m. = 1.05; n = 6). This species difference is statistically significant (t = −2.28; p < 0.05). Assuming that the mitotic phase is invariant across these species, our findings suggest that, at 11 per cent of incubation, the cell cycle rate is nearly twice as fast in chickens than in quail (figure 3).
Figure 3.
At 11% of incubation, the brain pH3+/PCNA+ ratios is higher in chickens than in quail. These findings suggest that brain cells cycle faster in chickens when compared with quail at 11% of incubation. Values are expressed as means and standard errors.
We used cumulative BrdU labelling to estimate the cell cycle rate at 11 per cent of incubation in both species. The principle of the cumulative BrdU labelling method is that the proportion of BrdU+ cells (i.e. the labelling index; LI) increases with time after BrdU application, up to a saturation level that is known as the growth fraction (Fujita 1962, 1963). The average cell cycle duration is estimated by fitting a line to the pre-saturation segment of the labelling index versus survival time, taking the inverse of the slope of this line, and then multiplying this value by the growth fraction. S-phase duration is estimated by dividing the y-intercept of the fitted line by the labelling index slope and, then, multiplying this value by the growth fraction (Takahashi et al. 1993).
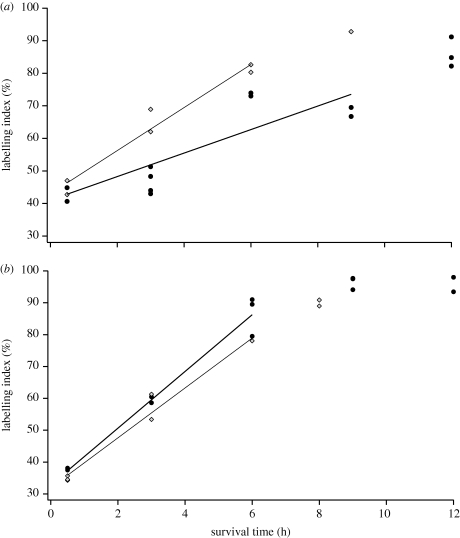
In the brain VZ of the ED2.75 quail, the LI increased approximately linearly from 0.5 to 9 h (figure 4a). At 12 h, the LI reached a saturation level of approximately 86 per cent (i.e. 86% of the cells within the VZ were BrdU+). The best-fit line for the pre-saturation segment for the quail brain has a slope of 3.61 ± 1.46 and a y-intercept of 41.05 ± 8.18. These data indicate that the brain's cell cycle duration is 23.8 h for the ED2.75 quail brain. The S phase lasts approximately 9.8 h. Assuming that pH3 exclusively labels mitotic cells, M-phase duration can be estimated as a product of the total cell cycle duration and the corresponding pH3+/PCNA+ ratio. Accordingly, M-phase lasts about 1.1 h in the quail brain.
Figure 4.
These graphs show how the fraction of BrdU+ cells in the brain (i.e. tectum, telencephalon) VZ increases with survival time after BrdU application in chickens and quail at (a) 11% of incubation (i.e. ED2–3) and (b) 22% of incubation (i.e. ED5). The slopes of these lines are inversely proportional to cell cycle duration. Furthermore, the LIs saturate at similar levels in the two species. These data indicate that (a) brain precursor cells cycle faster in chickens than in quail at 11% of incubation. (b) However, brain precursor cells cycle at a similar rate in chickens and quail at 22% of incubation. Diamonds, chicken brain; filled circles, quail brain.
In the brain VZ of ED2.5 chickens, the LI increased approximately linearly from 0.5 to 6 h after the BrdU application (figure 4a). At 9 h, the LI of the chicken brain reached a saturation level of approximately 92.8 per cent. The best-fit line for the pre-saturation segment for the chicken telencephalon has a slope of 6.61 ± 0.83 and y-intercept of 43.03 ± 3.2. These data indicate that the cell cycle duration is 14 h in the chicken brain and that S phase lasts approximately 6 h. Estimates of the pH3+/PCNA+ ratios in the chicken brain suggest that the M-phase lasts approximately 1.4 h. Thus, our pH3/PCNA ratios and cumulative BrdU labelling demonstrate that, at 11 per cent of incubation, the brain cell cycle rate is nearly twice as fast in chickens (Tc = 14 h) than in quail (Tc = 23.8 h).
(d). Cell cycle rates at ED5
Although we found differences in cell cycle rates between ED2.5 chickens and ED2.75 quail, these differences do not persist. This is evident from the observation that the volume difference between chicken and quail brains remains roughly constant (at a factor of 2–3) between 19 per cent of incubation and hatching. Furthermore, at ED5 (i.e. 22% of incubation), brain cell cycle rates are highly similar in chickens and quail (figure 4b). In the ED5 quail brain, the LI increased approximately linearly from 0.5 to 6 h and eventually reached a saturation level of approximately 96 per cent. The best-fit line for the pre-saturation LI has a slope of 8.89 ± 0.09 and a y-intercept of 33.17 ± 0.37. Thus, the cell cycle rate is approximately 10.8 h and the S phase lasts approximately 3.6 h. In the ED5 chicken brain, the best-fit line for the LI has a slope of 7.83 ± 0.60 and a y-intercept of 31.95 ± 2.34. Therefore, the cell cycle rate lasts 11.5 h and S phase lasts approximately 3.7 h.
4. Discussion
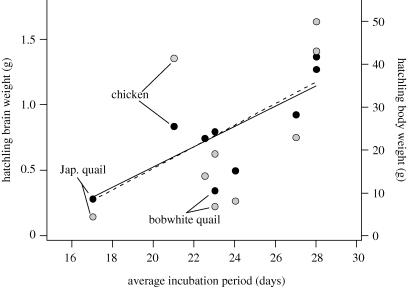
The chicken brain is more than twice the size of the bobwhite quail brain at hatching and in adulthood. This is not surprising, given that the chicken body is also much larger than that of quail (Portmann 1947a,b; Boire & Baron 1994). However, it is surprising that chickens grow these larger brains within a shorter time than quail. Chickens hatch after 21 days of incubation, whereas bobwhite quail hatch after 23 days of incubation. Thus, the chicken brain gets bigger than the quail brain within a shorter incubation period (figure 5). This observation runs counter to Passingham's (1985) finding that brains (at least mammalian brains) grow at constant rates. If the brain of chickens and quail grew at constant rates, then chicken brains should be smaller than quail brains. How, then, can we explain that hatchling chickens have larger brains than those of quail?
Figure 5.
Hatchling brain and body weights correlate positively with incubation period in some galliform birds, but this correlation is noisy. Bobwhite quail have a longer incubation period than chickens yet hatchling brains of chickens are larger than those of quail. Data are from Portmann (1947b). Black-filled circles, brain weights; grey-filled circles, body weights.
One possible explanation is that chickens delay neurogenesis relative to bobwhite quail. In this scenario, the chicken brain would undergo more rounds of precursor cell divisions and, therefore, expand relative to the quail brain. This hypothesis is plausible because the expansion of the telencephalon in parrots and songbirds is associated with delays in telencephalic neurogenesis (Striedter & Charvet 2008; Charvet & Striedter 2009a). However, our comparative analysis of neural event timing in chickens and quail indicates that neurogenesis occurs earlier in chickens than in quail when age is expressed in days post-incubation onset. Importantly, neurogenesis timing in several brain regions is highly predictable in chickens and quail once age is expressed as a percentage of normal incubation time. These findings are consistent with the notion that schedules of neurogenesis uniformly stretch or compress as developmental periods lengthen or shorten (Clancy et al. 2001). However, these findings cannot explain why chickens have larger brains than those of quail.
A second possibility is that brain cells cycle faster in chickens than in bobwhite quail throughout development. This is conceivable given that some mammals and some birds exhibit species differences in cell cycle rates during neurogenesis (Kornack & Rakic 1998; Charvet & Striedter 2008). However, this hypothesis is inconsistent with our brain growth curves for chickens and quail. By the time neurogenesis begins, the chicken brain is already larger than the quail brain by a factor similar to that observed at hatching. If brain progenitor cells uniformly cycle faster in chickens compared with quail, then chicken brains should gradually become larger than quail brains during embryonic development. Such a divergence in brain growth curves is not observed.
A third possibility is that chicken progenitor cells cycle faster than quail progenitor cells at specific stages of development. Our brain growth curves suggest that such a time-limited species difference in cell cycle kinetics might exist before 19 per cent of incubation is complete. Our estimates of pH3+/PCNA+ and cumulative BrdU labelling independently confirm that, at 11 per cent of incubation (i.e. ED2.5 chicken, ED2.75 quail), brain cells cycle faster in chickens than in quail. We also note that the quail brain's cell cycle rate increases between ED2.75 and ED5, whereas chickens maintain a more uniform cell cycle rate between ED2.5 and ED5. These findings are consistent with previous studies (Corliss & Robertson 1963; Wilson 1973).
The length of incubation generally correlates with hatchling brain size among galliform birds (figure 5). However, hatchling chicken brains are larger than expected from this general scaling relationship and bobwhite quail brains are smaller than expected. Thus, chickens may have accelerated their pre-neurogenetic cell cycle rates, whereas bobwhite quail may have lengthened them. However, further comparative studies of brain cell cycle rates is needed to determine which pattern of brain growth is primitive within galliform birds, and which is derived.
Our main finding is that an early growth spurt in chicken brains gives them a head start relative to bobwhite quail brains. Because the chicken's growth spurt emerges before neurogenesis begins, the chicken's predictably compressed schedule of neurogenesis is superimposed on an expanded brain precursor pool. Such evolutionary alterations are interesting because they would not lead to the species differences in brain region proportions that Finlay and Darlington have referred to as ‘late makes large’ (Finlay et al. 2010). Indeed, the brain's major regions (e.g. telencephalon) are similar in proportional size in adult chickens and bobwhite quail (Portmann 1947a; Boire & Baron 1994). By contrast, uniform acceleration of cell cycle rates or delays in neurogenesis would cause late born regions (e.g. telencephalon) to enlarge disproportionately relative to brain regions that are born early (e.g. medulla). Chickens bypass these constraints on their way to making a larger brain relative to those of quail.
A potential limitation of our study is that we only compare cell cycle rates at two stages of development. We do not know precisely when in development chickens and quail begin to exhibit different cell cycle rates or how long those differences persist. Our findings only show that species differences in cell cycle rates disappear by ED5. Furthermore, our labelling indices at ED2.75 are quite variable. This variation may be owing to ‘temporal jitter’ in which embryos that have incubated for the same amount of time may vary in size (Hamburger & Hamilton 1951; Striedter & Charvet 2008). This ‘temporal jitter’ appears to be most evident at early stages of development, before neurogenesis begins. Because of this variability, we cannot offer precise cell cycle rate estimates. However, both pH3+/PCNA+ estimates and cumulative BrdU labelling indicate that the cell cycle rate differs in the two species at 11 per cent of incubation.
Overall, our data show that there are several mechanisms for evolutionary alterations in brain size. Previous studies focused on the length of development and neurogenesis timing (Finlay & Darlington 1995; Clancy et al. 2001; Dyer et al. 2009). We show that species differences in cell cycle rates prior to neurogenesis account for at least some adult species differences in brain size. Whether such early alterations in cell cycle rates are common among vertebrates is not yet clear. Several studies have compared brain growth rates across species (Starck & Ricklefs 1998), but few studies have compared cell cycle kinetics in very young embryos (Blunn & Gregory 1935). We suspect that findings similar to ours will emerge from studies on species that develop for similar lengths of time, exhibit similar brain region proportions, but vary in absolute brain size.
Acknowledgements
This work was supported by an NSF grant no. IOS-0744332. We thank Shyam Srinivasan and Luke McGowan for helpful comments on the manuscript.
References
- Blunn C. T., Gregory P. W.1935The embryological basis of size inheritance in the chicken. J. Exp. Zool. 70, 397–414 (doi:10.1002/jez.1400700304) [Google Scholar]
- Boire D., Baron G.1994Allometric comparison of brain and main brain subdivisions in birds. J. Hirnforsch. 35, 49–66 [PubMed] [Google Scholar]
- Charvet C. J., Striedter G. F.2008Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain. Behav. Evol. 72, 295–306 (doi:10.1159/000184744) [DOI] [PubMed] [Google Scholar]
- Charvet C. J., Striedter G. F.2009aDevelopmental origins of mosaic brain evolution: morphometric analysis of the developing zebra finch brain. J. Comp. Neurol. 514, 203–213 (doi:10.1002/cne.22005) [DOI] [PubMed] [Google Scholar]
- Charvet C. J., Striedter G. F.2009bDevelopmental basis for telencephalon expansion in waterfowl: enlargement prior to neurogenesis. Proc. R. Soc. B 276, 3421–3427 (doi:10.1098/rspb.2009.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C. J., Sandoval A. L., Striedter G. F.In press Phylogenetic origins of early alterations in brain region proportions. Brain. Behav. Evol. (doi:10.1159/000300573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B., Darlington R. B., Finlay B. L.2001Translating developmental time across mammalian species. Neuroscience 105, 7–17 (doi:10.1016/S0306-4522(01)00171-3) [DOI] [PubMed] [Google Scholar]
- Corliss C. E., Robertson G. G.1963The pattern of mitotic density in the early chick neural epithelium. J. Exp. Zool. 153, 125–140 (doi:10.1002/jez.1401530205) [DOI] [PubMed] [Google Scholar]
- Dyer M. A., Martins R., da Silva Filho M., Muniz J. A., Silveira L. C., Cepko C. L., Finlay B. L.2009Developmental sources of conservation and variation in the evolution of the primate eye. Proc. Natl Acad. Sci. USA 106, 8963–8968 (doi:10.1073/pnas.0901484106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. L., Darlington R. B.1995Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584 (doi:10.1126/science.7777856) [DOI] [PubMed] [Google Scholar]
- Finlay B. L., Clancy B., Darlington R. B.2010Late stills equals large. Brain Behav. Evol. 75, 4–6 (doi:10.1159/000295350) [Google Scholar]
- Fujita S.1962Kinetics of cellular proliferation. Exp. Cell Res. 28, 52–60 (doi:10.1016/0014-4827(62)90311-7) [DOI] [PubMed] [Google Scholar]
- Fujita S.1963The matrix cell and cytogenesis in the developing central nervous system. J. Comp. Neurol. 120, 37–42 (doi:10.1002/cne.901200104) [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L.1951A series of normal stages in the development of the chick embryo. J. Morphol. 88, 231–272 [PubMed] [Google Scholar]
- Hendzel M. J., Wei Y., Mancini M. A., Van Hooser A., Ranalli T., Brinkley B. R., Bazett-Jones D. P., Allis C. D.1997Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (doi:10.1007/s004120050256) [DOI] [PubMed] [Google Scholar]
- Kornack D. R., Rakic P.1998Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc. Natl Acad. Sci. USA 95, 1242–1246 (doi:10.1073/pnas.95.3.1242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. E.1985Rates of brain development in mammals including man. Brain Behav. Evol. 26, 167–175 (doi:10.1159/000118773) [DOI] [PubMed] [Google Scholar]
- Portmann A.1947aÉtude sur la cérébralisation chez les oiseaux. II. Les indices intracérébraux. Alauda 15, 1–15 [Google Scholar]
- Portmann A.1947bÉtude sur la cérébralisation chez les oiseaux. III. Cérébralisation et mode ontogénétique. Alauda 15, 161–171 [Google Scholar]
- Rasband W. S.1997–2007ImageJ. Bethesda, MD: US National Institutes of Health [Google Scholar]
- Sacher G. A., Staffeldt E. F.1974Relation of gestation time to brain weight for placental mammals: implications for the theory of vertebrate growth. Am. Nat. 108, 593–615 (doi:10.1086/282938) [Google Scholar]
- Starck J. M., Ricklefs R. E.1998Avian growth and development: evolution within the altricial-precocial spectrum. New York, NY: Oxford University Press [Google Scholar]
- Stephan H., Frahm H., Baron G.1981New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 35, 1–29 (doi:10.1159/000155963) [DOI] [PubMed] [Google Scholar]
- Striedter G. F., Charvet C. J.2008Developmental origins of species differences in telencephalon and tectum size: morphometric comparisons between a parakeet (Melopsittacus undulatus) and a quail (Colinus virgianus). J. Comp. Neurol. 507, 1663–1675 (doi:10.1002/cne.21640) [DOI] [PubMed] [Google Scholar]
- Striedter G. F., Keefer B. B.2000Cell migration and aggregation in the developing telencephalon: pulse-labeling chick embryos with bromodeoxyuridine. J. Neurosci. 20, 8021–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr1993Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J. Neurosci. 13, 820–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B.1973Chronological changes in the cell cycle of chick neuroepithelial cells. J. Embryol. Exp. Morphol. 29, 745–751 [PubMed] [Google Scholar]