Abstract
Natural selection leads to behavioural choices that increase the animal's fitness. The neuronal mechanisms underlying behavioural choice are still elusive and empirical evidence connecting neural circuit activation to adaptive behavioural output is sparse.
We exposed foraging juvenile crayfish to approaching shadows of different velocities and found that slow-moving shadows predominantly activated a pair of giant interneurons, which mediate tail-flips that thrust the animals backwards and away from the approaching threat. Tail-flips also moved the animals farther away from an expected food source, and crayfish defaulted to freezing behaviour when faced with fast-approaching shadows. Under these conditions, tail-flipping, an ineffective and costly escape strategy was suppressed in favour of freezing, a more beneficial choice. The decision to freeze also dominated in the presence of a more desirable resource; however, the increased incentive was less effective in suppressing tail-flipping when paired with slow-moving visual stimuli that reliably evoked tail-flips in most animals. Together this suggests that crayfish make value-based decisions by weighing the costs and benefits of different behavioural options, and they select adaptive behavioural output based on the activation patterns of identifiable neural circuits.
Keywords: escape, decision making, behavioural choice, neurons, crayfish
1. Introduction
There has been growing interest in understanding the neural computations underlying decision making in humans and non-human animals. It has been recognized that neurobiology and economic theory are mutually informative, and this has led to the emergence of a new discipline termed ‘neuroeconomics’ (Camerer et al. 2005; McCabe 2008). Neuroscientists aim to identify value-based decision making processes in the nervous system, which weigh the costs and benefits of behavioural alternatives and produce desirable behavioural output (Sugrue et al. 2005; Rangel et al. 2008). Functional imaging studies in humans and electrophysiological experiments in monkeys are providing important new insights into the neural underpinnings of decision making and behavioural choice (Schall 2001; Sanfey et al. 2003; Gold & Shadlen 2007). However, these approaches have certain limitations: motion artefacts seldom allow subjects to be tested while freely behaving, most studies are correlative and rarely permit causal inference and the signals obtained from imaging studies are often reflections of neuronal mass activity. While single-unit recordings in monkeys provide much better resolution, detailed descriptions of the neuronal interactions between sensory and motor systems are nevertheless, difficult to obtain owing to the complex multi-part structures of the primate brain (Kristan 2008; Logothetis 2008; Kable & Glimcher 2009).
Not all decisions are based on rational thought or conscious intent, however, and invertebrate species have emerged as productive model systems for studying different aspects of decision making and behavioural choice (Gillette et al. 2000; Kavaliers & Choleris 2001; Calabrese 2003; Briggman et al. 2005). In these models, easily quantifiable behaviours are controlled by ‘simple’ neural networks, and the neural activation of these identified circuits can be recorded non-invasively in freely behaving crayfish (Herberholz et al. 2004; Liden & Herberholz 2008; Herberholz 2009).
For crayfish and most other animals, producing effective escape behaviour is one of the most important decisions these animals face, and failure to do so immediately eliminates any future reproductive success (Lima & Dill 1990). However, when searching for food or mates, trade-offs have to be made between avoiding a predator and fulfilling important needs (Kavaliers & Choleris 2001).
Foraging juvenile crayfish respond to shadows that move towards them with one of two discrete and incompatible anti-predatory behaviours: they either freeze or produce powerful tail-flips thrusting them backwards and away from the approaching shadow. Tail-flipping is mediated by excitation of a pair of medial giant (MG) interneurons (Liden & Herberholz 2008). The MG neurons are the key elements of one of three escape circuits in crayfish, each controlling different types of tail-flips of different response latencies and movements (Wine & Krasne 1972, 1982; Edwards et al. 1999). The MG neurons can be activated by strong tactile stimuli directed to the head and the thorax as well as fast-moving visual stimuli, and a single action potential in the MG neurons is sufficient to activate flexor muscles in the abdomen and to produce the escape response that propels the animal backwards (Wine & Krasne 1972; Herberholz et al. 2004; Liden & Herberholz 2008). The unambiguous behavioural dichotomy observed in foraging juvenile crayfish in response to approaching shadows (tail-flipping or freezing), and the fact that activation of the MG neurons can be recorded in freely behaving crayfish, allowed us to directly relate changes in neural activity patterns to the costs and benefits of behavioural action.
2. Material and methods
(a). Animals and experimental design
Crayfish (Procambarus clarkii Girard) obtained from a commercial supplier (Atchafalaya Biological Supply Co.) were individually isolated in small water-filled plastic containers (H: 10 cm, L: 15 cm, W: 8 cm) for one week prior to experimental use. All animals were fed the same amount of food (Ocean Nutrition Formula One Shrimp pellets; Aqua Pets Americas) and were last fed one week before being tested. Animals were kept under a constant 12 L∶12 D cycle and all experiments were performed at approximately the same time each day. Animals were thoroughly checked for intactness and no animal was used that had moulted less than 2 days prior to the experiment. Each animal was used only once. A total of 259 juvenile crayfish of similar size (mean ± s.d.: 3.48 ± 0.16 cm; measured from rostrum to tail) were included in the analysis.
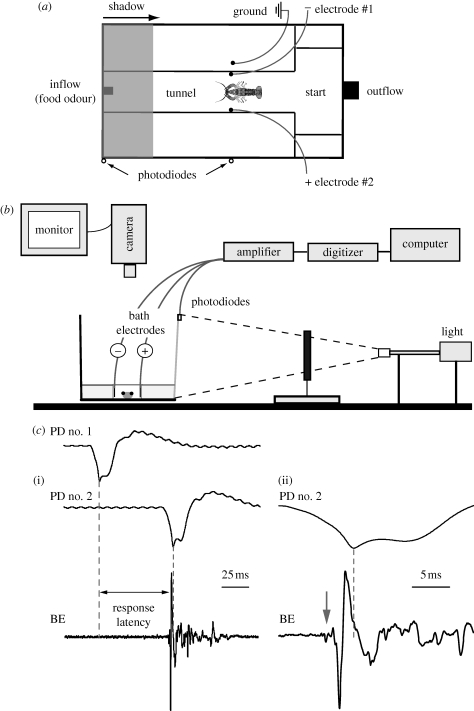
The experimental set-up (figure 1a) was modified from a version previously used (Liden & Herberholz 2008). It consisted of an experimental tank (H: 21 cm, L: 31 cm, W: 17 cm) separated into different compartments and filled with deionized water to a height of 5 cm. The tank design allowed water to flow from one end of a narrow tunnel (H: 4 cm, L: 24 cm, W: 5.5 cm) to a ‘start compartment’ (H: 21 cm, L: 6 cm, W: 12 cm) located at the other end. The start compartment was separated from the tunnel by a removable barrier. Water was directed into the tunnel using a 0.5 cm diameter polyethylene tube connected to a reservoir. Flow was regulated at a rate of 190 ml min−1 by means of a flow meter (Cole-Parmer Instrument Company). Water left the tank through a 1 cm round opening placed in the start compartment 5 cm above the bottom of the tank. A food odour stock solution was produced by dissolving 1 g of crushed medium-sized shrimp pellets (Ocean Nutrition Formula One, Aqua Pets Americas) in 1 l of deionized water, then filtering the solution to remove any particulates. Stock solution was made fresh every few days. For 5 l of standard experimental solution (‘low’), 200 ml of stock solution was mixed with 4.8 l of distilled water; for 5 l of higher (10×) concentrated solution (‘high’), 2 l of stock solution was mixed with 3 l of distilled water.
Figure 1.
Experimental set-up and signal recordings. (a) Top view of the experimental tank. Water containing the food odour flows into a tunnel on the left side and exits on the right. Animals enter the tunnel from the start compartment and approach the food odour release point. A pair of bath electrodes is attached to the tunnel walls, 8 cm from the tunnel entrance and 17.5 cm from the end. Shadows (indicated as the grey shaded area) always move from left to right over the tank. Photodiodes are placed on the front of the tank to measure shadow velocity and position. (b) Side view of the set-up. Animals inside the tank are filmed with a camera positioned above the tank. The camera is connected to a TV monitor. Bath electrodes and photodiodes are connected to an amplifier and digitizer and recorded signals are stored on a computer. The shadow is produced by moving a plastic rectangle through a light beam directed onto the front of the tank. The tank wall facing the light is covered with a white translucent paper. (c) Example of recorded signals from bath electrodes and photodiodes for a shadow moving at 2.5 m s−1. (i) Recording traces of two photodiodes spaced 175 mm apart. The first photodiode (PD no. 1) was placed at the front edge of the tank and recorded the shadow when it first became visible. The second photodiode (PD no. 2) was placed at the position of the bath electrodes in the tank, i.e. the position of the animal when the shadow was released. Response latency was measured between the peak response of PD no. 1 and the beginning of the field potential that was generated by the tail-flip response and recorded by the bath electrodes (BE). (ii) Traces from PD no. 2 and BE at higher temporal resolution. The animal initiated a tail-flip response (arrow) 4 ms before the shadow produced the peak response in PD no. 2. The first small deflection in the BE trace is owing to the activation of the MG neurons (arrow), while the large phasic potential and the smaller more erratic potentials that follow are owing to muscular activity during tail-flips.
(b). Physiological recordings
A pair of bath electrodes was attached to the tunnel walls, located 8 cm from the tunnel entrance and 17.5 cm from the end of the tunnel (figure 1a). Bath electrodes were used as previously described (Liden & Herberholz 2008). In short, the two bath electrodes of a pair were placed on opposite walls inside the tunnel to record field potentials generated during tail-flips. Electrodes were made from 2 × 2 mm gold pins soldered to 24 American wire gauge insulated copper wire (Belden CDT Inc.) and connected to an amplifier (A-M Systems). The bath was grounded using a copper ground wire. Amplified signals (×1000) were filtered, digitized and recorded with Axoscope software (Axon Instruments) on a personal computer. Identification of MG-mediated tail-flips is warranted by the initial large potentials (owing to the simultaneous activation of muscles by the giant motorneurons) and the immediately preceding MG giant neuron action potentials (figure 1c) paired with discrete behavioural appearance (Herberholz et al. 2001, 2004; Finley & Macmillan 2002; Liden & Herberholz 2008; Herberholz 2009).
(c). Shadows
The inside of the tunnel and the start compartment was painted white; the back side and the end of the tank opposite to the start compartment was painted black. The side of the tank facing the light source and a shadow-generating apparatus was covered with a translucent white paper (4.15 calliper, 87.5 opacity, 92 US brightness; Boise Inc.). Shadows were generated by moving a rectangular piece of black opaque plastic (18 × 9 cm) through a light beam generated by a goose-neck illuminator (Fibre Lite MI-150; Dolan Jenner Industries). The light beam was projected onto the white paper that covered the front of the experimental tank and it was focused to evenly illuminate the entire inside of the tank (figure 1b). The piece of plastic was moved through the light beam on a single-axis linear stepper forcer (Model STPM-SL-05-36-R; Optimal Engineering Systems) and the velocity controlled by a single-axis programmable stepper motor control system (Allegra-1-10; Optimal Engineering Systems). Crayfish inside the experimental tank were unable to see the light source or the apparatus that generated the shadow. A video camera (Canon ZR850) was positioned above the tank and connected to a TV monitor (Audiotronics 12VM968) to observe and record the animals' behaviour during trials (figure 1b). The light level inside the tank was measured each day with a light meter (SM 700; Milwaukee Instruments Inc.) before experiments were started. With the room lights turned on, the total light level of 190 lux before shadows was reduced by 50 per cent when the plastic rectangle completely covered the light beam. This arrangement was used for all experiments except when testing the effects of different food odour concentrations. For these experiments, the room lights were turned off, and the initial light level in the tank (100 lux) was reduced by 95 per cent when the light beam was fully covered; the modification was made to generate shadows of higher contrast which increased the number of tail-flip responses (Liden & Herberholz 2008).
Shadows were modelled to move at different non-accelerating velocities to resemble shadows produced by predators. Velocities were based on the fast-start (attack) swim speed of predacious fishes (Webb 1978; Harper & Blake 1991; Domenici & Blake 1997). Velocities of the shadows were calibrated with an array of six silicon photodiodes (Allied Electronics), each spaced 35 mm apart and positioned above the tank facing the illuminator. The photodiodes were arranged to cover a distance of 175 mm, ranging from one side of the tank (where the shadows first became visible) to the position of the bath electrodes. The diodes were coupled to an amplifier (A-M Systems) and signals were recorded on a personal computer with Axoscope software (Axon Instruments). Five repetitions were recorded for each shadow and average velocities (between each pair of diodes) were computed from these measurements. Shadow velocities were extremely consistent for each measurement and were repeatedly confirmed during the course of the experiments when two photodiodes (the first and last) were used for measurements (figure 1a). Average velocities were determined as 1.00 ± 0.01 m s−1 for the slow shadow, 2.01 ± 0.01 m s−1 and 2.51 ± 0.01 m s−1 for medium shadows and 4.01 ± 0.01 m s−1 for the fast shadow.
(d). Procedure and analysis
Each experiment was started by transferring a single animal from its home tank into the start compartment and allowing it to acclimate for 10 min. Following this period, the video camera positioned above the tank was turned on, the barrier separating the start compartment from the tunnel was carefully opened and the flow of food odour was started. At this time, the software programme that recorded the electronic signals from the bath electrodes and photodiodes was also started. Animals entered the tunnel shortly after the food odour was turned on and walked towards the end where the highest concentration of the food odour was present. As soon as the rostrum and eyes of the animal reached the location of the bath electrodes in the tank, the programme controlling the movement of the plastic rectangle was started, producing a shadow moving towards and then passing over the animal. Slow shadows (1 m s−1) were visible to the animals for 175 ms before they reached the bath electrodes (i.e. the location of the animals in the tank when the shadow was triggered), shadows moving at 2.0 m s−1 were visible for 87.5 ms, shadows moving at 2.5 m s−1 were visible for 70 ms, and the fastest tested shadows (4 m s−1) were only visible for 43.75 ms before they reached the bath electrodes.
Animals that were not in motion, had passed the bath electrodes by more than 10 mm, or had turned inside the tunnel by more than 40° when exposed to shadows were later excluded from the results. Each animal was exposed to only one shadow, and different groups of animals were exposed to different shadow velocities and food odour concentrations. All individual compartments of the experimental tank were thoroughly washed between each single experiment.
Behavioural frequencies (in percentage) for tail-flipping and freezing were determined using the video-recordings obtained during the experiments. Tail-flips rapidly propelled the animal backwards, sometimes back into the start compartment. Freezing was defined as complete cessation of forward locomotion. We use the term freezing rather than stopping for this behaviour because video-analysis suggested that most animals suppressed all body movements (including movements of the appendages).
Response latencies (in milliseconds) were calculated from photodiode and bath electrode measurements as the duration between the signal of the photodiode that recorded the first appearance of the shadow in the tank and the MG neurons' response to the shadow (figure 1c). Approach times were measured using single-frame video analysis and defined as the duration (in seconds) between the time when the shadow reached the bath electrodes and the time when the animals eventually arrived at the food odour release point. Recovery times were defined as the duration (in seconds) between the time when the shadow reached the bath electrodes and the moment when the animals resumed their foraging activity, i.e. started again to move towards the food odour release point.
Unless otherwise stated, data are presented as means with standard deviation (mean ± s.d.). Statistical software (SPSS versus 14.0; SPSS Inc.) was used for analysis and each applied statistical test is specified in the text.
3. Results
(a). Behavioural frequencies in response to different shadows
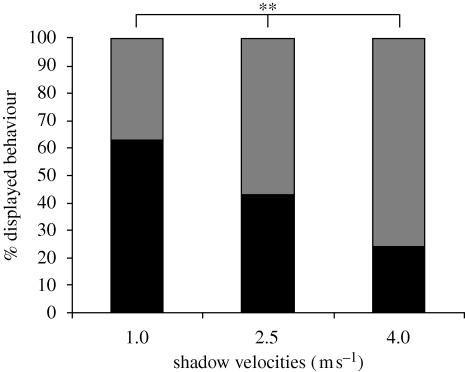
The frequency of MG-mediated tail-flips was highest in response to shadows that moved at a velocity of 1.0 m s−1 (figure 2). Sixty-three per cent of the animals tail-flipped and 37 per cent produced freezing behaviour (n = 46). Shadows that moved at 2.5 m s−1 elicited tail-flipping in 43 per cent of the tested animals whereas 57 per cent of the animals displayed freezing behaviour (n = 46). The fastest shadow moved at 4.0 m s−1 towards the animals and evoked tail-flipping in only 24 per cent of the animals whereas 76 per cent of the animals froze (n = 46). The measured frequencies for tail-flipping and freezing were significantly different for the three shadow velocities (χ2-test (2-sided): p ≤ 0.01). Animals produced significantly fewer tail-flips and significantly more freezing when exposed to shadows that moved at 4.0 m s−1 when compared with shadows that moved at 2.5 m s−1 and shadows that moved at 1 m s−1 (χ2-tests (2-sided): p ≤ 0.05 and p ≤ 0.01, respectively). Shadows that moved at 2.5 m s−1 elicited less tail-flipping and more freezing than shadows that moved at 1 m s−1 but the differences were not statistically significant although only marginally (χ2-test (2-sided): p = 0.060).
Figure 2.
Percentage of behaviours displayed in response to shadows of different velocities. Crayfish produce only one of two discrete defensive responses when exposed to approaching shadows: tail-flipping, which is always mediated by activity in the medial giant (MG) interneurons or freezing behaviour. Tail-flipping behaviour decreases and freezing behaviour increases as shadow velocities increase. The measured differences in displayed behavioural patterns are statistically significant. ** = p ≤ 0.01. Grey bars, freezing; black bars, tail-flipping.
(b). Response latencies to different shadows
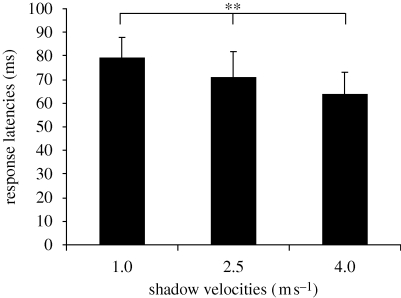
Response latencies were also significantly different (Kruskal-Wallis test: p ≤ 0.01) for all the three types of presented shadows (figure 3). Animals responded most quickly to the fastest shadow (4 m s−1; visibility before contact = 43.75 ms) with an average response latency of 64 ± 9 ms (range: 54–75 ms; n = 11). None of the animals that attempted to tail-flip away from the fastest shadow succeeded before the shadow reached the animal. Shadows of medium velocity (2.5 m s−1; visibility before contact = 70 ms) produced slower responses (71 ± 11 ms; range: 54–90 ms; n = 20) and 45 per cent of the animals that attempted tail-flips were able to initiate them before the shadows reached the animals. Tail-flip responses to slow-moving shadows (1.0 m s−1; visibility before contact = 175 ms) were initiated after longer latencies (79 ± 9 ms; range: 61–93 ms; n = 29). All animals that attempted to tail-flip away from slow shadows were able to execute tail-flips before the first contact with the shadows occurred. Responses to fast-moving shadows (4 m s−1) were significantly faster than responses to shadows that moved at 2.5 m s−1 (Mann–Whitney test (2-sided): p ≤ 0.05) and shadows that moved at 1 m s−1 (Mann–Whitney test (2-sided): p ≤ 0.01). Responses to shadows of medium velocity were significantly faster than responses to slow-moving shadows (Mann–Whitney test (2-sided): p ≤ 0.05).
Figure 3.
Latencies to initiate tail-flips in response to shadows of different velocities. Latencies are measured between the time when shadows first became visible and the time when the animals activated the MG neurons to produce a tail-flip. Response latencies are longer for slower shadows than faster shadows. The measured differences in response latencies are statistically significant. ** = p ≤ 0.01.
(c). Consequences of tail-flipping and freezing
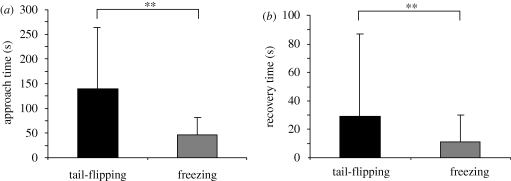
Approach times were significantly longer (Mann–Whitney test (2-sided): p ≤ 0.01) for animals that tail-flipped when compared with animals that froze (figure 4a). Animals that used tail-flips (n = 60) needed 140 ± 124 s to reach the food odour release point at the end of the tank whereas animals that displayed freezing behaviour (n = 78) needed only 47 ± 34 s (figure 4a). Recovery time was also longer for animals that tail-flipped in response to the shadows (29 ± 58 s) when compared with animals that froze (11 ± 19 s), a significant difference (Mann–Whitney test (2-sided): p ≤ 0.01; figure 4b). Most animals tail-flipped only once in response to the shadows but a small number of animals (12%) produced multiple tail-flips, or they briefly walked backwards after they had tail-flipped (17%) before they resumed foraging activity. When freezing behaviour was elicited by the shadows, very few animals (3%) walked backwards before they resumed their forward motion towards the food source. As a result, animals that tail-flipped were further removed from the food source (7.3 cm ± 2.7 cm; n = 60), took longer to resume foraging activity, and consequently their arrival at the food odour release point was significantly delayed compared with animals that displayed freezing behaviour.
Figure 4.
Consequences of tail-flipping and freezing. (a) Approach times after tail-flipping and freezing measured between the time of contact with the presented shadow and the eventual time of arrival at the food odour release point. The measured differences in approach times are statistically significant. ** = p ≤ 0.01. (b) Recovery time measured between the time of contact with the presented shadow and the time when foraging activity resumed. The measured differences in recovery times are statistically significant. ** = p ≤ 0.01.
(d). Effect of resource value
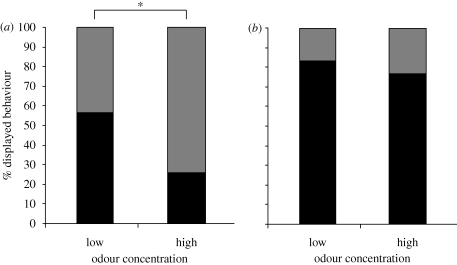
When shadows of high contrast and 2 m s−1 velocity were combined with different food odour concentrations (figure 5a), crayfish (n = 30) produced more MG-mediated tail-flips (57%) than freezing behaviour (43%) under standard (‘low’) odour conditions. Crayfish (n = 31) exposed to the same shadow paired with higher food odour concentration (10×) produced less tail-flipping (26%) and more freezing behaviour (74%). The measured frequencies for tail-flipping and freezing are significantly different for the two odour concentrations (χ2-test (2-sided): p ≤ 0.05; figure 5a). When exposed to slow shadows (1 m s−1) of high contrast and standard (‘low’) food odour concentration, crayfish (n = 30) predominately produced MG-mediated tail-flips (83%) and few animals froze (17%) in response to the shadow (figure 5b). When slow shadows were combined with high food odour concentration (n = 30), tail-flipping was reduced (77%) and freezing behaviour increased (23%); however, the response patterns were not statistically different for the two odour concentrations (χ2-test (2-sided): p = 0.52; figure 5b).
Figure 5.
Effect of food odour concentration on behavioural output. (a) Percentage of tail-flipping and freezing responses to shadows of high contrast that approached with a velocity of 2 m s−1 and low or high (10×) food odour concentration in the tank. The measured differences in behaviour are statistically significant. * = p ≤ 0.05. (b) Percentage of tail-flipping and freezing responses to shadows of high contrast that approached with a velocity of 1 m s−1 and low or high (10×) food odour concentration in the tank. The measured differences in behaviour are not statistically significant. (a,b) Grey bars, freezing; black bars, tail-flipping.
4. Discussion
Only limited data exists on escape behaviour of crayfish that are attacked by natural predators, and freezing has only been documented as an effective anti-predatory strategy in other species such as rodents (Eilam 2005). When attacked by dragonfly nymphs in the laboratory, juvenile crayfish use their fast and powerful tail-flips as an exclusive response to the predatory strikes of the nymphs, and they do so very successfully (Herberholz et al. 2004). Other predators of crayfish that are more likely to cast shadows during attacks are fishes, mammals and wading birds (Englund & Krupa 2000; Davis & Huber 2007). Although the exact properties of these predator-induced shadows are unknown, it is possible that they accelerate as predators approach the crayfish. Shadows in our current study were controlled to move at a constant velocity, and behavioural responses mirrored those from a previous study where accelerating shadows were used (Liden & Herberholz 2008). Thus, tail-flipping or freezing were the exclusive responses of juvenile crayfish to all shadows used in our studies, whether the shadows accelerated or not. Moreover, high-speed video recordings reveal that tail-flipping and freezing are true binary choices as tail-flips are not preceded by freezing but generated while the animals are still in forward locomotion (K. Florek & J. Herberholz 2010, unpublished data).
The main processes in value-based decision making are recognition of the present situation, determination of the values of competing actions (costs/benefits), action selection (based on valuations) and re-evaluation of the action based on the outcome (Doya 2008). Studies in humans and monkeys have identified brain areas devoted to these processes. However, the challenge is to better define specific roles for subcomponents of these large multi-part brain structures, to determine how different values are compared to make adaptive choices, and to identify the neural mechanisms for action selection (Rangel et al. 2008). We have now shown that crayfish, similar to organisms of higher complexity, integrate different sensory stimuli that are present in their environment, and they select a behavioural output (tail-flipping or freezing) according to the current values for each choice. All tail-flips were mediated by activation of a pair of MG interneurons, and if predator signals were sufficient to excite the MG neurons, fixed motor output invariably followed. Thus, the MG neurons are at the core of the decision making network representing the neuronal interface between sensory integration and motor action.
Although MG-mediated tail-flipping is a highly effective escape strategy in crayfish (Herberholz et al. 2004), it can be costly for foraging animals as it increases the distance between the animal and the food source; arriving significantly later at the food source after tail-flipping, as seen in our study, will reduce the likelihood of gaining access to the food. This is a major disadvantage because food is a limited resource for crayfish, and crayfish fight for access to food under both natural and laboratory conditions (Ranta & Lindström 1992; Bergman & Moore 2003; Herberholz et al. 2007).
Measurements of response latencies indicate that tail-flipping was suppressed when shadows approached at velocities that made timely tail-flip execution impossible. Thus, the low responsiveness of MG-mediated tail-flips to fast shadows reduces the costs for the animal because any benefits that tail-flipping may have over freezing are eliminated under these conditions. In rodents, freezing behaviour also occurs more often than fleeing when threats become inescapable (Blanchard et al. 2001). Alternatively, tail-flipping could be suppressed in response to fast shadows because they are simply less threatening than slow-moving shadows. However, fast shadows were never ignored and evoked tail-flipping in some animals. Moreover, suppressing tail-flipping in response to stimuli of low threat would be equally beneficial to the animals.
The observed differences in response latencies for different shadow velocities further suggest that activation of the MG neurons is based on threshold angular size of the stimulus (Fotowat & Gabbiani 2007), which is reached shortly after the shadows became visible in the tank but slightly earlier for faster moving shadows than slower moving shadows. Another possibility is that shadows were only detectable after they had travelled a certain distance in the tank. Since faster shadows would take less time to enter the visible range, they would have resulted in quicker response latencies. This seems unlikely though since the animals were always facing the approaching shadow from a maximum distance of 17.5 cm, and prior studies have shown that crayfish can detect small moving objects that are almost twice as far away in both air and underwater (Hernández-Falcón et al. 1999).
The costs of tail-flipping also increased (i.e. its value decreased) when higher concentrated food odour was present. Crayfish suppressed MG-mediated tail-flipping in this condition and produced more freezing instead. Activation of the lateral giant escape circuit in crayfish, on the other hand, is only suppressed during the consumption of food but seem to be unaffected during food search (Krasne & Lee 1988).
Our results indicate that when the respective values for tail-flipping and freezing change, crayfish adjust their choices accordingly, thus preserving adaptive action selection. Interestingly, high food odour concentration was less effective in reducing MG tail-flipping when combined with a slow visual signal that evoked tail-flips in most animals at regular odour concentration. As a result, a predator signal that strongly excites the MG neurons maintains its efficacy in the presence of more desirable resources suggesting that the risk of predation is carefully weighed against the expected reward. Ultimately, the selection of an appropriate defence strategy in the wild will also depend on variables that were impossible to measure in our study. Tail-flips may increase conspicuousness, while freezing may camouflage the animals. Tail-flips are energetically costly (Webb 1979) and combined with the high energetic costs of neural processing (Niven & Laughlin 2008), this could affect the trade-off between the two choices.
Crayfish make value-based decisions that are controlled by relatively simple neural circuitry. While this may preclude identification of complex cognitive processes, it allows for a better understanding of the basic neural mechanisms of decision making and behavioural choice that probably underlie more complex decision making systems. The MG circuit is accessible for intracellular electrophysiology and thus provides an excellent opportunity to further probe the cellular mechanisms controlling behavioural choice. In order to fully understand the decision making network, however, it will also be necessary to identify the circuit underlying freezing. Towards this end, activation of a single neuron that suppresses all ongoing movements has been identified in the brain connectives of crayfish (Bowerman & Larimer 1974), and future investigation of interactions between freezing circuitry and MG circuitry seems feasible. Whether action selection in crayfish is controlled by mutual inhibitory connections between command systems (Kovac & Davis 1977; Edwards 1991; Lo & Wang 2006), or by subtle differences in activity levels of neuronal populations, or single neurons that are shared among the circuits (Briggman et al. 2005) needs to be determined. Such experiments are planned for the future, and they will facilitate a more complete understanding of the neuronal mechanisms underlying value-based decision making.
Acknowledgements
This work is supported by National Science Foundation grant IOS-0919845 (J.H.). We would like to thank Ms Katrina Florek for her help.
References
- Bergman D. A., Moore P. A.2003Field observations of intraspecific agonistic behaviour of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 205, 26–35 [DOI] [PubMed] [Google Scholar]
- Blanchard D. C., Griebel G., Blanchard R. J.2001Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 25, 205–218 (doi:10.1016/S0149-7634(01)00009-4) [DOI] [PubMed] [Google Scholar]
- Bowerman R. F., Larimer J. L.1974Command fibres in the circumoesophageal connectives of crayfish. I. Tonic fibres. J. Exp. Biol. 60, 95–117 [Google Scholar]
- Briggman K. L., Abarbanel H. D. I., Kristan W. B., Jr2005Optical imaging of neuronal populations during decision-making. Science 307, 896–901 (doi:10.1126/science.1103736) [DOI] [PubMed] [Google Scholar]
- Calabrese R.2003Behavioral choices: how neuronal networks make decisions. Curr. Biol. 13, 140–142 [DOI] [PubMed] [Google Scholar]
- Camerer C., Loewenstein G., Prelec D.2005Neuroeconomics: how neuroscience can inform economics. J. Econ. Lit. 43, 9–64 (doi:10.1257/0022051053737843) [Google Scholar]
- Davis K. M., Huber R.2007Activity patterns, behavioural repertoires, and agonistic interactions of crayfish: a non-manipulative field study. Behaviour 144, 229–247 (doi:10.1163/156853907779947300) [Google Scholar]
- Domenici P., Blake R. W.1997The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178 [DOI] [PubMed] [Google Scholar]
- Doya K.2008Modulators of decision-making. Nat. Neurosci. 11, 410–416 (doi:10.1038/nn2077) [DOI] [PubMed] [Google Scholar]
- Edwards D. H.1991Mutual inhibition among neural command systems as a possible mechanism for behavioral choice in crayfish. J. Neurosci. 11, 1210–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. H., Heitler W. J., Krasne F. B.1999Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 22, 153–161 (doi:10.1016/S0166-2236(98)01340-X) [DOI] [PubMed] [Google Scholar]
- Eilam D.2005Die hard: a blend of freezing and fleeing as a dynamic defense—implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 29, 1181–1191 (doi:10.1016/j.neubiorev.2005.03.027) [DOI] [PubMed] [Google Scholar]
- Englund G., Krupa J. J.2000Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshw. Biol. 43, 75–83 (doi:10.1046/j.1365-2427.2000.00524.x) [Google Scholar]
- Finley L. A., Macmillan D. L.2002An analysis of field potentials during different tailflip behaviours in crayfish. Mar. Freshw. Behav. Physiol. 35, 221–234 (doi:10.1080/1023624021000019306) [Google Scholar]
- Fotowat H., Gabbiani F.2007Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behaviour. J. Neurosci. 27, 10 047–10 059 (doi:10.1523/JNEUROSCI.1515-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R., Huang R. C., Hatcher N., Moroz L. L.2000Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc. Natl Acad. Sci. USA 97, 3585–3590 (doi:10.1073/pnas.97.7.3585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J. I., Shadlen M. N.2007The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (doi:10.1146/annurev.neuro.29.051605.113038) [DOI] [PubMed] [Google Scholar]
- Harper D. G., Blake R. W.1991Prey capture and the fast start performance of northern pike Esox Lucius. J. Exp. Biol. 15, 175–192 [Google Scholar]
- Herberholz J.2009Recordings of neural circuit activation in freely behaving animals. J. Vis. Exp. 29 (doi:10.3791/1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberholz J., Issa F. A., Edwards D. H.2001Patterns of neural circuit activation and behavior during dominance hierarchy formation in freely behaving crayfish. J. Neurosci. 21, 2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberholz J., Sen M. M., Edwards D. H.2004Escape behavior and escape circuit activation in juvenile crayfish during prey–predator interactions. J. Exp. Biol. 207, 1855–1863 (doi:10.1242/jeb.00992) [DOI] [PubMed] [Google Scholar]
- Herberholz J., McCurdy C., Edwards D. H.2007Direct benefits of social dominance in juvenile crayfish. Biol. Bull. 213, 21–27 (doi:10.2307/25066615) [DOI] [PubMed] [Google Scholar]
- Hernández-Falcón J., Serrato J., Ramón F.1999Evoked potentials elicited by natural stimuli in the brain of unanesthetized crayfish. Physiol. Behav. 66, 397–407 [DOI] [PubMed] [Google Scholar]
- Kable J. W., Glimcher P. W.2009The neurobiology of decision: consensus and controversy. Neuron 63, 733–745 (doi:10.1016/j.neuron.2009.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M., Choleris E.2001Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci. Biobehav. Rev. 25, 577–586 (doi:10.1016/S0149-7634(01)00042-2) [DOI] [PubMed] [Google Scholar]
- Kovac M. P., Davis W. J.1977Behavioral choice: neural mechanisms in Pleurobranchaea. Science 198, 632–634 (doi:10.1126/science.918659) [DOI] [PubMed] [Google Scholar]
- Krasne F. B., Lee S. C.1988Response-dedicated trigger neurons as control points for behavioral actions: selective inhibition of lateral giant command neurons during feeding in crayfish. J. Neurosci. 8, 3703–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan W. B.2008Neuronal decision-making circuits. Curr. Biol. 18, 928–932 [DOI] [PubMed] [Google Scholar]
- Liden W. H., Herberholz J.2008Behavioral and neural responses of juvenile crayfish to moving shadows. J. Exp. Biol. 211, 1355–1361 (doi:10.1242/jeb.010165) [DOI] [PubMed] [Google Scholar]
- Lima S. L., Dill L. M.1990Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 (doi:10.1139/z90-092) [Google Scholar]
- Lo C. C., Wang X. J.2006Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci. 9, 956–963 (doi:10.1038/nn1722) [DOI] [PubMed] [Google Scholar]
- Logothetis N.2008What we can do and what we cannot do with fMRI. Nature 453, 869–878 (doi:10.1038/nature06976) [DOI] [PubMed] [Google Scholar]
- McCabe K. A.2008Neuroeconomics and the economic sciences. Econ. Philos. 24, 345–368 (doi:10.1017/S0266267108002010) [Google Scholar]
- Niven J. E., Laughlin S. B.2008Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 (doi:10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- Rangel A., Camerer C., Montague P. R.2008A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 9, 545–556 (doi:10.1038/nrn2357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta E., Lindström K.1992Power to hold sheltering burrows by juveniles of the signal crayfish, Pasifastacus leniusculus. Ethology 92, 217–226 (doi:10.1111/j.1439-0310.1992.tb00961.x) [Google Scholar]
- Sanfey A. G., Rilling J. K., Aronson J. A., Nystrom L. E., Cohen J. D.2003The neural basis of economic decision-making in the Ultimatum Game. Science 300, 1755–1758 (doi:10.1126/science.1082976) [DOI] [PubMed] [Google Scholar]
- Schall J. D.2001Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2, 33–42 (doi:10.1038/35049054) [DOI] [PubMed] [Google Scholar]
- Sugrue L. P., Corrado G. S., Newsome W. T.2005Choosing the greater of two goods: neural currencies for valuation and decision making. Nat. Rev. Neurosci. 6, 363–375 (doi:10.1038/nrn1666) [DOI] [PubMed] [Google Scholar]
- Webb P. W.1978Fast-start performance and body form in seven species of teleost fish. J. Exp. Biol. 74, 211–226 [Google Scholar]
- Webb P. W.1979Mechanics of escape responses in crayfish (Orconectes virilis). J. Exp. Biol. 79, 245–263 [Google Scholar]
- Wine J. J., Krasne F. B.1972The organization of escape behavior in the crayfish. J. Exp. Biol. 56, 1–18 [DOI] [PubMed] [Google Scholar]
- Wine J. J., Krasne F. B.1982The cellular organization of crayfish escape behavior. In The biology of Crustacea (eds Sandeman D. C., Atwood H. L.), pp. 241–292 New York, NY: Academic Press [Google Scholar]