Abstract
Reports of socially transmitted traditions based on behavioural differences between geographically separated groups of conspecifics are contentious because they cannot exclude genetic or environmental causes. Here, we report persistent differences between neighbouring groups of meerkats (Suricata suricatta) where extensive gene flow precludes genetic differentiation. Over 11 years, some groups consistently emerged later from their sleeping burrows in the morning than others, despite complete turnovers in group membership and the influx of immigrants. Group territories overlapped and, in many cases, the same sleeping burrows were used by different groups. Differences persisted even after accounting for effects of group size, weather and burrow characteristics, and were unrelated to food availability within territories. These results provide compelling evidence that the emergence times of meerkat groups represent conservative traditions.
Keywords: culture, meerkats, social learning, Suricata suricatta, traditions
1. Introduction
Individuals in social groups may acquire information from one another, giving rise to patterns of behaviour or traditions that are shared within groups and differ between groups. The occurrence of such traditions in non-human animals is of critical importance for our understanding of the origins of human culture, and has major ecological and evolutionary implications because traditions can dissociate behavioural traits from environmental conditions and modify selection pressures acting on groups (Whiten & van Schaik 2007; Laland et al. 2009). However, the existence of traditions in natural animal populations remains highly contentious (Laland & Janik 2006; Laland & Galef 2009). The majority of putative examples are based on behavioural differences between geographically separated populations (Whiten et al. 1999; Rendell & Whitehead 2001; Hunt & Gray 2003; Perry et al. 2003; van Schaik et al. 2003) and so cannot exclude the possibility that these differences are a result of contrasts in genetic or environmental factors rather than the social spread of information (Galef 1992; Laland & Janik 2006; Laland et al. 2009). For instance, reports of repertoires of traditions in chimpanzees in study sites across Africa (Whiten et al. 1999) have been criticized on the basis that allopatric populations may show substantial genetic differentiation and variation in ecological conditions (Laland & Janik 2006).
A small number of field experiments have confirmed that social information can spread in wild fish (Helfman & Schultz 1984; Warner 1988; Reader et al. 2003), birds (Lefebvre 1986; Langen 1996; Midford et al. 2000) and mammals (Thornton & Malapert 2009a,b; van de Waal et al. 2010). However, these have generally examined the spread of artificially introduced skills or information and tell us little about whether naturally observed differences between groups constitute traditions. Indeed, the only experimental studies to test the role of social learning in generating naturally occurring group differences in the wild are translocation experiments on reef fish, where local mating sites and migration routes appear to be maintained as local traditions (Helfman & Schultz 1984; Warner 1988). As such studies are unlikely to be feasible or ethical with other vertebrates, alternative approaches are needed to determine the occurrence of traditions.
One such approach is to investigate behavioural differences between neighbouring groups of conspecifics that occupy similar habitats, where dispersal between groups precludes genetic differentiation and provides a natural equivalent of experimental translocations. Here, we analyse differences in the time at which 15 meerkat groups within our long-term study population emerged from their underground sleeping burrows in the morning over an 11 year period. Meerkats are cooperatively breeding mongooses that live in groups of 2–50 individuals in the arid regions of southern Africa. Groups typically consist of a natal dominant female and an immigrant dominant male (who are responsible for the majority of breeding attempts in the group), a variable number of natal subordinates of both sexes and often one or more subordinate immigrant males (Griffin et al. 2003; Spong et al. 2008). Gene flow between groups is extensive, as males always breed outside their natal groups, either through attaining the dominant position in a different group or by mating with females during short prospecting forays (Griffin et al. 2003; Spong et al. 2008).
We used global positioning system (GPS) records of group movements to map meerkat groups' territories and burrow use. We then used multi-factorial statistics to examine differences in the emergence times of the 15 groups, controlling for repeated burrow use as well as the effects of variation in group size, habitat characteristics and meteorological conditions. Finally, we investigated the influence of individual group members and changes in group structure on group emergence times and used detailed records of dispersal patterns to explore whether emergence times changed following the arrival of immigrants.
2. Material and Methods
(a). Study site and data collection
Data were collected between November 1998 and March 2009 on 15 groups of 2–47 meerkats living in semi-desert in the South African Kalahari. Habitat at the study site consists of sparsely vegetated sand dunes and flat terraces intersected by the dry Kuruman River (see Clutton-Brock et al. 2001a for details of habitat and climate). Groups were located by radio-tracking collared individuals (Golabek et al. 2008) and all animals were identifiable through unique dye marks on their fur. All individuals were habituated to close observation (less than 1 m) and the majority (greater than 90%) could be weighed regularly by enticing them onto an electronic balance using crumbs of hard-boiled egg. Animals were weighed before they began foraging in the morning, and again after the cessation of foraging in the middle of the day (mean time between ‘morning’ and ‘afternoon’ weighing 3.40 ± 0.03 h).
Groups were visited in the early morning at least once every two weeks. Observers arrived at the sleeping burrow before sunrise and recorded the time that the first individual emerged from the burrow and its identity. The rest of the group typically emerged in quick succession, so the time of emergence of the first individual provides a good indicator of the emergence time for the group. Occasional instances where one individual (generally a prospecting male or a subordinate female being targeted for harassment and eviction by the dominant female) emerged substantially earlier (greater than 5 min) than the rest of the group were excluded from analyses.
As time of emergence may be affected by the characteristics of the sleeping burrow, we surveyed all the burrows used during the study period. For each burrow, we recorded the dominant vegetation type (open, grass, shrubs or under the canopy of a tree) and habitat (riverbed, flats or dunes) in the surrounding area, whether the burrow entrance was shaded in the morning, and the colour of the sand (white, pink, red) at the entrance. Sand colour reflects haematite and clay content, and provides a measure of specific heat capacity (Walden & White 1997; White et al. 2007). Emergence times may also be affected by weather conditions, so we recorded minimum overnight temperatures, wind and cloud cover. Meerkats generally avoid emerging from the burrow if it is raining, so rainy mornings were excluded from analyses.
(b). Home range analysis
From 2002, records of group movements were collected using a handheld eTrex (Garmin International Inc., Olathe, KS, USA) GPS every time the group was visited. Coordinates of group location were taken every 15 min throughout the day after groups left sleeping burrows in the morning. To maximize independence between GPS points, we extracted a single randomly selected coordinate from each observation session. These locations, along with sleeping burrow coordinates, were used to estimate group territories with the Hawth's Analysis Tools extension (Beyer 2004) in ArcMap 9.3 (Environmental Systems Research Institute, Redlands, CA, USA), using the 95 per cent fixed kernel method. This generates a region within which there is 95 per cent likelihood that the group will be found. Neighbouring groups are defined as those that shared overlapping regions of the territory at the 95 per cent kernel. As group territories shifted over time, separate maps of group territories were generated for each year (see electronic supplementary material, figure S1).
(c). Statistical analyses
Data were analysed in Genstat v. 10.1 (Rothamsted Experimental Station, Harpenden, UK). Multi-factorial analyses were conducted using linear mixed models (LMM), with random terms fitted to control for repeated measures (Schall 1991). Where necessary, response terms were normalized for analysis using Box–Cox power transformations. Initially, all probable explanatory variables were entered into models. Possible two-way interactions between them were investigated and terms were sequentially dropped until the minimal model contained only terms whose elimination would significantly reduce the explanatory power of the model (only significant interactions are presented in results tables). Wald statistics and probability values for significant terms were derived from having all significant terms in the model, and values for non-significant terms were obtained by adding each term individually to the minimal model. Full statistical tables for all mixed models with significant terms are given in electronic supplementary material.
(i). Factors affecting group emergence times
The time (in minutes) between sunrise and the emergence of the first group member (n = 12 214 emergence times) was used as the response term in an LMM. Precise sunrise times were obtained from the United States Naval Observatory (www.usno.navy.mil/USNO/astronomical-applications/data-services/rs-one-year-world). Group identity was fitted as an explanatory variable, along with season (January–March, April–June, July–September, October–December), measures of weather conditions (minimum temperature in the previous night in degrees celsius; cloud cover, recorded as fine or overcast; and whether it was windy) and burrow characteristics (vegetation, terrain, sand colour and shade). As emergence time might be affected by the number of meerkats in the group, group size was fitted as an additional explanatory variable (see electronic supplementary material, table S1 for changes in group size at all groups over the period of study). Group size refers to the number of individuals greater than 90 days old present that day, including those babysitting pups underground (i.e. those that were seen on previous and subsequent days, so were known to be alive when there were pups below ground). Burrow identity and month nested in year were fitted as random terms (electronic supplementary material, table S2).
(ii). Magnitude and consistency of group differences
We used the residuals of the LMM above, excluding group identity, to provide a measure of emergence times for each group relative to that expected given the season, group size, weather conditions and burrow characteristics on each day (hereafter ‘relative emergence time’). We then calculated the mean relative emergence times of each group in each season (‘seasonal relative emergence time’). The magnitude of differences in the seasonal relative emergence times of neighbouring groups are presented as mean differences with 95 per cent confidence intervals. Neighbouring groups are defined as those that had overlapping territories for at least 5 years. To examine the consistency of differences between neighbouring groups, we used paired sign tests. These provide a conservative assessment of whether a group consistently emerged later than a neighbouring group over several years.
(iii). Effects of food availability on relative emergence times
In addition to the factors discussed above, emergence time may be affected by food availability in the territory. We therefore used an LMM to investigate whether the mean seasonal rate of foraging intake for adult individuals affected seasonal relative emergence times (n = 445 seasonal relative emergence times; range = 6–42 seasons per group). The rate of foraging intake was measured as the mean change in mass (in grams per hour) of adult individuals during the period between morning and afternoon weighing sessions when meerkats forage intensively. Group identity was fitted as a random term (estimated variance component ± s.e.: 15.98 ± 7.33). As required foraging intake may vary with the mass of individuals, we also repeated the analysis using percentage weight gain per hour as the explanatory term (estimated variance components for random term ± s.e.: 15.83 ± 7.27).
(iv). Association between relative emergence times and distance between groups
We used Mantel tests to investigate the association between pairwise group differences in emergence times and the spatial proximity of group territories. For each year from 2002 to 2009, we compared matrices of pairwise group differences in mean yearly relative emergence times and pairwise distances between groups (measured in metres between the centroids of group territory polygons in ArcMap 9.3). Analyses were conducted on a yearly rather than seasonal basis to avoid interpretational issues arising from conducting large numbers of separate Mantel tests, and because GPS data over the course of a year generates more accurate measures of distance between groups.
(v). Individual influences on relative emergence times
As group emergence times could be driven by a subset of individuals who were consistently the first to emerge, we used LMMs to examine whether the mean and variance of seasonal relative emergence times was affected by the number of individuals recorded as being the first to emerge in that season. The analysis was restricted to instances where the identity of the first individual to emerge was known for at least 10 days within the season (range = 10–53 days; mean = 25.93 ± 0.55 days in a season; n = 352 seasonal emergence times). Group identity was included as a random term (estimated variance components ± s.e. for LMMs on mean and variance, respectively: 18.58 ± 8.33; 2255 ± 1272).
(vi). Time spent at the burrow in the mornings and evenings
Meerkats typically spend up to an hour sunning, grooming and playing at the sleeping burrow before setting off to forage in the morning and after returning in the evening. We used LMM analyses to investigate whether groups' daily relative emergence times influenced the amount of time they spent at the burrow in the mornings (time between emerging and leaving the burrow, in minutes; n = 11 374 morning periods) and evenings (time between arriving at the burrow in the evening and retreating below, in minutes; n = 5141 evening periods). Burrow identity, group identity and month nested in year were fitted as random terms, with season, group size, weather and burrow characteristics fitted as additional explanatory terms. As group movements may be affected by temperature in the preceding period, we considered minimum overnight temperature in the analysis of time spent at the burrow in the morning and maximum daytime temperature in the analysis of evening times (electronic supplementary material, table S3).
(vii). Time groups retreated below ground in the evening
Meerkats typically retreated into their sleeping burrows shortly after sunset. We used an LMM to examine the factors affecting the time groups went below ground. The response term was the time (in minutes) between sunset and the retreat of the last group member. Sample sizes and explanatory terms were as in the model of time spent at the burrow in the evening above (see electronic supplementary material, table S4).
(viii). Effects of immigrants on relative emergence times
The influx of immigrants might affect emergence times in their new groups. We investigated this using LMMs to examine whether the mean and variance of relative emergence times were affected by time period (before or after immigration events). As the arrival of immigrants can cause extensive social disruption for up to two months (Clutton-Brock et al. 2001b; Spong et al. 2008), we defined ‘before’ as the two months prior to the arrival of immigrants and ‘after’ as the two months following the two-month settling-down period. Group identity and a unique identifier for each immigration event were fitted as random terms (estimated variance components ± s.e.: 9.76 ± 16.06 and 4.16 ± 20.60, respectively).
Any change in emergence times following immigration events could be affected by the relative emergence times in immigrants' original groups. We therefore used an LMM where the response term was the change in mean emergence times between the ‘before’ and ‘after’ period, with the mean emergence time in immigrants' original groups (earlier, same or later) fitted as an explanatory term. Emergence times of original groups were defined as earlier or later if they differed significantly from those of new groups in paired analyses during the relevant season. The number of immigrants was fitted as an additional explanatory term, with group identity as a random term (estimated variance component ± s.e.: 44.7 ± 58.0). These analyses are based on 25 immigration events at 12 groups (1–3 events per group; mean = 2.08 ± 0.26) for which the relative emergence time of both groups was known. There were 1–15 immigrants per event (mean = 3.80 ± 0.71).
3. Results
(a). Factors affecting group emergence times
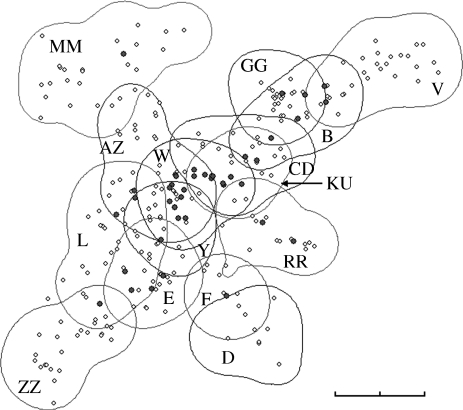
The meerkat groups in this study used 625 different sleeping burrows (31–125 burrows per group; mean = 67.5 ± 7.2). There was substantial overlap between the territories of neighbouring groups; 27 per cent of burrows were used by more than one group (figure 1 and electronic supplementary material, figure S1; different groups never used the same burrow simultaneously). An LMM analysis controlling for repeated burrow use (estimated variance component ± s.e. for random term: 0.01 ± 0.02) showed that emergence times differed significantly between groups over the period of study (χ2 = 212.52, p < 0.001; see electronic supplementary material, table S2). This effect persisted even after accounting for fluctuations in group size (χ2 = 59.86, p < 0.001), effects of seasonal variation (χ2 = 188.03, p < 0.001), meteorological conditions (minimum overnight temperature: χ2 = 281.93, p < 0.001; wind: χ2 = 41.79, p < 0.001; cloud cover: χ2 = 88.79, p < 0.001) and whether the burrow was shaded in the morning (χ2 = 6.82; p = 0.009). The habitat, vegetation type and sand type around the burrow did not have significant effects (p > 0.3; electronic supplementary material, table S2).
Figure 1.
Group territories in 2007 based on 95 per cent kernels from GPS coordinates of group movements. Letters indicate group names. Circles represent sleeping burrows; black circles are sleeping burrows that were used by more than one study group during the year. As territory boundaries shifted over time, maps for 2002–2009 are given in electronic supplementary material, figure S1. Scale bar, 2 km.
(b). Magnitude and consistency of group differences
Differences in emergence times between neighbouring groups were consistent over long periods. Paired comparisons of the mean seasonal relative emergence times of neighbouring groups with overlapping territories showed that in 10 out of 15 pairs, one group consistently emerged later than the other (figure 2a). For example, over 42 seasons spanning an 11 year period, group F emerged later than neighbouring groups D and E in 35 and 37 seasons, respectively (sign tests, p < 0.001; figure 2b). In contrast, group Y consistently emerged earlier than all its neighbouring groups (figure 2c).
Figure 2.
Differences in the emergence times of neighbouring groups that had overlapping territories for at least 5 years. (a) The graph on the left shows the magnitude (means with 95% CI) of differences in the seasonal relative emergence times of neighbouring groups. Numbers on the right provide an indication of the consistency of seasonal differences. ‘Total seasons’ is the number of seasons that both groups were present in the population and ‘group 1 later’ shows the number of seasons for which the mean relative emergence time of group 1 was later than that of group 2. p-values are derived from sign tests, providing a conservative assessment of whether a group consistently emerged later than a neighbouring group over several years. (b,c) Mean seasonal relative emergence times for neighbouring groups. Relative emergence times of zero indicate that groups emerged at precisely the expected time given the season, group size, weather conditions and burrow characteristics. (b) Group F (solid line) consistently emerged later in the morning than its neighbouring groups, D (dashed line) and E (dotted line). Circles indicate intervals between which no individuals present at the start remained in the group by the end (white: D; grey: E; black: F). (c) Group Y (solid line) consistently emerged earlier in the morning than its neighbouring groups, E (dotted line), GG (long dashed line), V (dashed line) and W (dashed–dotted line). Circles indicate intervals between which no individuals present at the start remained in the group by the end (grey: E; horizontal hatch: GG; diagonal hatch: V; white: W; black: Y).
Figure 2a presents results from multiple sign tests. If p-values were drawn from a normal distribution, significant values (at the 0.05 level) may occasionally arise by chance. However, the distribution of p-values differed significantly from normal (Kolmogorov–Smirov test: p < 0.01), indicating that it was unlikely to have arisen by chance. A subsequent jack-knifing procedure showed that distributions always remained significantly different from normal (p < 0.03) even when results from each individual group were sequentially excluded, confirming that the distribution was not being skewed by a single group.
(c). Effects of food availability on relative emergence times
The mean seasonal rate of weight gain had no significant effect on seasonal relative emergence times (LMMs: g h−1: χ2 = 1.69, p = 0.194; percentage weight gain per hour: χ2 = 1.84, p = 0.224), indicating that emergence times were unrelated to group members' foraging intake.
(d). Association between relative emergence times and distance between groups
There was no significant association between pairwise group differences in yearly relative emergence times and distances between groups in any year (Mantel tests with 10 000 permutations: 2002: r = 0.02, p = 0.450; 2003: r = −0.07, p = 0.499; 2004: r = 0.004, p = 0.481; 2005: r = 0.24, p = 0.119; 2006: r = −0.24, p = 0.911; 2007: r = 0.16, p = 0.147; 2008: r = 0.42, p = 0.067; 2009: r = 0.07, p = 0.374).
(e). Individual influences on relative emergence times
It is possible that group emergence times could be driven by a small subset of individuals who are consistently the first to emerge. However, all but one of the groups saw complete turnover in group membership during the study period (the exception is group MM, where the same dominant female was present since the group was founded in 2002). Of the individuals present in 2009, 162 out of 166 (97.6%) were born since 2004. Of the remaining four, only one was still in its natal group (the dominant female in group E, born in 2002, dominant since 2005). Moreover, there was substantial variation in the identity of the first individual to emerge, with between 2 and 20 individuals (mean = 7.45 ± 0.14), or 17 to 100 per cent of group members (mean = 46.5% ± 0.93%), being the first to emerge in any given season. Neither the mean nor the variance of seasonal relative emergence times was significantly influenced by the number of group members that emerged first during that season (LMMs: mean: χ2 = 0.22, p = 0.639; variance: χ2 = 0.53, p = 0.467).
(f). Time spent at the burrow in the mornings and evenings
Group size had a significant positive effect on the amount of time groups spent at the burrow before leaving to forage in the morning (LMM: χ2 = 9.31, p = 0.002; electronic supplementary material, table S3) and after returning in the evening (χ2 = 41.67, p < 0.001). Time at the burrow was also significantly influenced by the season, temperature, cloud cover, wind and sand type at the burrow (all factors: p < 0.05; electronic supplementary material, table S3). Controlling for these effects, relative emergence time had a significant negative effect on time spent at the burrow (LMMs: mornings: χ2 = 120.22, p < 0.001; evenings: χ2 = 4.17, p = 0.041; electronic supplementary material, table S3).
(g). Time groups retreated below ground in the evening
Large groups retreated into sleeping burrows in the evening later than small groups (LMM: χ2 = 87.64, p < 0.001). The time that groups went below ground was significantly influenced by temperature, cloud cover, wind (all factors: p < 0.001) and season (p = 0.032), but was unrelated to relative emergence time (χ2 = 1.06, p = 0.303; electronic supplementary material, table S4).
(h). Effects of immigrants on relative emergence times
Group emergence times were unaffected by the arrival of immigrants. LMM analyses revealed no difference in the mean or variance of group relative emergence times before and after the arrival of immigrants (mean: χ2 = 2.62, p = 0.119; variance: χ2 = 1.48, p = 0.234). The difference in mean emergence times before and after immigration events was unaffected by the number of immigrants or by the group emergence time in immigrants' original groups (LMM: number of immigrants: χ2 = 2.01, p = 0.173; emergence time in original group: χ2 = 4.23, p = 0.158; number of immigrants × emergence time in original group: χ2 = 0.36, p = 0.837).
4. Discussion
The majority of research on social learning has focused on behaviours such as foraging (Galef & Giraldeau 2001), mate choice (Freeberg 2000) and communication (Janik & Slater 2000). However, laboratory experiments have suggested that daily activity patterns in honeybee (Apis mellifera) colonies may also be affected by social learning, and could potentially give rise to differing patterns in different colonies (Kirchner 1987; cited in Leadbeater & Chittka 2007). The possibility that daily activity patterns might be maintained as traditions in free-living animal groups has not previously been investigated.
A number of lines of evidence suggest that emergence times represent conservative cultural traditions in neighbouring meerkat groups. First, although ecological explanations cannot be unequivocally ruled out in the absence of experimental manipulation, our analyses strongly suggest that group differences in emergence times did not result from ecological differences. Group emergence times were consistently different from one another over many years, even though group territories overlapped and, in many cases, different groups used the same sleeping burrows. Differences in emergence times were unrelated to the spatial proximity of group territories and persisted even when accounting for effects of substantial fluctuations in group size, as well as weather conditions and burrow characteristics. Moreover, emergence times were unaffected by group members' rates of foraging intake, suggesting that they were not related to food availability within territories. Groups appeared to mitigate the effects of emerging late in the morning by spending less time sunning, grooming and playing at the burrow before leaving to forage in the morning and after returning in the evening.
Second, emergence times were not driven by particular group members, and group differences were remarkably resilient to changes in group structure. All but one group saw a complete turnover in membership, such that none of the individuals alive in the latter years of the study had been present at the beginning. Different meerkats often emerged first on different days and the number of different individuals that were first up in a season did not affect seasonal emergence times, further precluding the possibility that particular individuals were responsible for differences in group emergence times. Moreover, emergence times did not change following immigration events, regardless of the emergence time in immigrants' original groups, suggesting that new members adopt the tradition of their hosts.
Finally, differences in group emergence times cannot be readily explained by genetic differences. Although genetic factors account for some of the individual variation in circadian rhythms and activity patterns in mammals (Viola et al. 2007; Cirelli 2009), they are unlikely to account for the persistent group differences reported here, given the high levels of gene flow between meerkat groups. As meerkats are fathered by immigrant males (Griffin et al. 2003; Spong et al. 2008), genetic differences between groups would erode unless the genes controlling emergence were maternally inherited, with philopatric females determining the time of emergence of the group from the burrow. Although genetic mechanisms cannot be definitively ruled out, the genetic determinants of mammalian circadian rhythms, involving multiple autosomal loci, render such strict sex-biased inheritance unlikely (Schwartz & Zimmerman 1990; Shimomura et al. 2001; Reppert & Weaver 2002).
The precise mechanisms by which group differences were maintained over multiple generations remain unclear. We suggest that differences in emergence times may be maintained as a result of informational cascades (Bikhchandani et al. 1998; Giraldeau et al. 2002), whereby new recruits base their decisions on the behaviour of others, leading to the transmission of behaviour patterns long after their originators have died. In contrast to foraging traditions, which tend to be eroded by information acquired through individual exploration (Thornton & Malapert 2009a,b), there may be strong pressure for individuals to remain within the safety of the group and thereby conform to the group norm (see Day et al. 2001 for similar effects in fish shoaling routes). As a result, groups exhibit distinctive behavioural phenotypes in the absence of environmental differences or genetic differentiation. Rather than focusing exclusively on variation between populations separated by large distances, future research on animal traditions may benefit from close examination of subtle differences in the social traits and activity patterns of neighbours.
Acknowledgements
We thank the Kotze family and Northern Cape Conservation for allowing us to work in the Kalahari, and M. Manser and the Mammal Research Institute for logistical support. D. Bell, T. Flower, N. Jordan, A. Malapert, K. McAuliffe, R. Sutcliffe, N. Tayar and the volunteers of the Kalahari Meerkat Project provided invaluable help in the field. We are grateful to A. Bateman, M. Bell, S. English, D. Lukas and S. Sharp for advice and discussion, and to the Natural Environment Research Council and Pembroke College, Cambridge for financial support. R. Kendal and an anonymous referee provided useful comments on an earlier draft of the paper.
References
- Beyer H. L.2004. Hawth's analysis tools for ArcGIS. See http://www.spatialecology.com/htools
- Bikhchandani S., Hirshleifer D., Welch I.1998Learning from the behavior of others: conformity, fads, and informational cascades. J. Econ. Perspect. 12, 151–170 [Google Scholar]
- Cirelli C.2009The genetic and molecular regulation of sleep: from fruit flies to humans. Nat. Rev. Neurosci. 10, 549–560 (doi:10.1038/nrn2683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Brotherton P. N. M., O'Riain M. J., Griffin A. S., Gaynor D., Kansky R., Sharpe L., McIlrath G. M.2001aContributions to cooperative rearing in meerkats. Anim. Behav. 61, 705–710 (doi:10.1006/anbe.2000.1631) [Google Scholar]
- Clutton-Brock T. H., et al. 2001bCooperation, control, and concession in meerkat groups. Science 291, 478–481 (doi:10.1126/science.291.5503.478) [DOI] [PubMed] [Google Scholar]
- Day R. L., MacDonald T., Brown C., Laland K. N., Reader S. M.2001Interactions between shoal size and conformity in guppy social foraging. Anim. Behav. 62, 917–925 (doi:10.1006/anbe.2001.1820) [Google Scholar]
- Freeberg T. M.2000Culture and courtship in vertebrates: a review of social learning and transmission of courtship systems and mating patterns. Behav. Proc. 51, 177–192 (doi:10.1016/S0376-6357(00)00127-3) [DOI] [PubMed] [Google Scholar]
- Galef B. G.1992The question of animal culture. Hum. Nat. 3, 157–178 (doi:10.1007/BF02692251) [DOI] [PubMed] [Google Scholar]
- Galef B. G., Giraldeau L. A.2001Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- Giraldeau L. A., Valone T. J., Templeton J. J.2002Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabek K. A., Jordan N. R., Clutton-Brock T. H.2008Radiocollars do not affect the survival or foraging behaviour of wild meerkats. J. Zool. 274, 248–253 (doi:10.1111/j.1469-7998.2007.00377.x) [Google Scholar]
- Griffin A. S., Pemberton J. M., Brotherton P. N. M., McIlrath G., Gaynor D., Kansky R., O'Riain J., Clutton-Brock T. H.2003A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta). Behav. Ecol. 14, 472–480 (doi:10.1093/beheco/arg040) [Google Scholar]
- Helfman G. S., Schultz E. T.1984Social transmission of behavioral traditions in a coral reef fish. Anim. Behav. 32, 379–384 (doi:10.1016/S0003-3472(84)80272-9) [Google Scholar]
- Hunt G. R., Gray R. D.2003Diversification and cumulative evolution in New Caledonian crow tool manufacture. Proc. R. Soc. Lond. B 270, 867–874 (doi:10.1098/rspb.2002.2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik V. M., Slater P. J. B.2000The different roles of social learning in vocal communication. Anim. Behav. 60, 1–11 (doi:10.1006/anbe.2000.1410) [DOI] [PubMed] [Google Scholar]
- Kirchner W. H.1987Tradition im bienenstaat: kommunikation zwichen den imagines und der brut der honigbiene durch vibrationssignale. PhD thesis, Julius-Maximilians Universität, Würzburg, Germany [Google Scholar]
- Laland K. N., Galef B. G.(eds)2009The question of animal culture. Cambridge, MA: Harvard University Press [Google Scholar]
- Laland K. N., Janik V. M.2006The animal cultures debate. Trends Ecol. Evol. 21, 542–547 (doi:10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- Laland K. N., Kendal J. R., Kendal R. L.2009Animal culture: problems and solutions. In The question of animal culture (eds Laland K. N., Galef B. G.), pp. 174–197 Cambridge, MA: Harvard University Press [Google Scholar]
- Langen T. A.1996Social learning of a novel foraging skill by white-throated magpie-jays (Calocitta formosa, Corvidae): a field experiment. Ethology 102, 157–166 (doi:10.1111/j.1439-0310.1996.tb01113.x) [Google Scholar]
- Leadbeater E., Chittka L.2007Social learning in insects: from miniature brains to consensus building. Curr. Biol. 17, R703–R713 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- Lefebvre L.1986Cultural diffusion of a novel food-finding behavior in urban pigeons: an experimental field test. Ethology 71, 295–304 (doi:10.1111/j.1439-0310.1986.tb00594.x) [Google Scholar]
- Midford P. E., Hailman J. P., Woolfenden G. E.2000Social learning of a novel foraging patch in families of free-living Florida scrub-jays. Anim. Behav. 59, 1199–1207 (doi:10.1006/anbe.1999.1419) [DOI] [PubMed] [Google Scholar]
- Perry S., et al. 2003Social conventions in wild white-faced capuchin monkeys: evidence for traditions in a neotropical primate. Curr. Anthropol. 44, 241–268 (doi:10.1086/345825) [Google Scholar]
- Reader S. M., Kendal J. R., Laland K. N.2003Social learning of foraging sites and escape routes in wild Trinidadian guppies. Anim. Behav. 66, 729–739 (doi:10.1006/anbe.2003.2252) [Google Scholar]
- Rendell L., Whitehead H.2001Culture in whales and dolphins. Behav. Brain Sci. 24, 309–324 [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R.2002Coordination of circadian timing in mammals. Nature 418, 935–941 (doi:10.1038/nature00965) [DOI] [PubMed] [Google Scholar]
- Schall R.1991Estimation in generalized linear models with random effects. Biometrika 78, 719–727 (doi:10.1093/biomet/78.4.719) [Google Scholar]
- Schwartz W. J., Zimmerman P.1990Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 10, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K., et al. 2001Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 11, 959–980 (doi:10.1101/gr.171601) [DOI] [PubMed] [Google Scholar]
- Spong G. F., Hodge S. J., Young A. J., Clutton-Brock T. H.2008Factors affecting reproductive success of dominant male meerkats. Mol. Ecol. 17, 2287–2299 (doi:10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- Thornton A., Malapert A.2009aExperimental evidence for social transmission of food acquisition techniques in wild meerkats. Anim. Behav. 78, 255–264 (doi:10.1016/j.anbehav.2009.04.021) [Google Scholar]
- Thornton A., Malapert A.2009bThe rise and fall of an arbitrary tradition: an experiment with wild meerkats. Proc. R. Soc. B 276, 1269–1276 (doi:10.1098/rspb.2008.1794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Waal E., Renevey N., Favre C. M., Bshary R.2010Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proc. R. Soc. B 277, 2105–2111 (doi:10.1098/rspb.2009.2260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik C. P., Ancrenaz M., Borgen G., Galdikas B., Knott C. D., Singleton I., Suzuki A., Utami S. S., Merrill M.2003Orangutan cultures and the evolution of material culture. Science 299, 102–105 (doi:10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- Viola A. U., Archer S. N., James L. M., Groeger J. A., Lo J. C. Y., Skene D. J., von Schantz M., Dijk D. J.2007PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 17, 613–618 (doi:10.1016/j.cub.2007.01.073) [DOI] [PubMed] [Google Scholar]
- Walden J., White K.1997Investigation of the controls on dune colour in the Namib Sand Sea using mineral magnetic analyses. Earth Planet. Sci. Lett. 152, 187–201 (doi:10.1016/S0012-821X(97)00154-4) [Google Scholar]
- Warner R. R.1988Traditionality of mating site preferences in a coral reef fish. Nature 335, 719–721 (doi:10.1038/335719a0) [Google Scholar]
- White K., Walden J., Gurney S. D.2007Spectral properties, iron oxide content and provenance of Namib dune sands. Geomorphology 86, 219–229 (doi:10.1016/j.geomorph.2006.08.014) [Google Scholar]
- Whiten A., van Schaik C. P.2007The evolution of animal ‘cultures' and social intelligence. Phil. Trans. R. Soc. B 362, 603–620 (doi:10.1098/rstb.2006.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C.1999Cultures in chimpanzees. Nature 399, 682–685 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]