Abstract
Anolis lizards communicate with displays consisting of motion of the head and body. Early portions of long-distance displays require movements that are effective at eliciting the attention of potential receivers. We studied signal-motion efficacy using a two-dimensional visual-motion detection (2DMD) model consisting of a grid of correlation-type elementary motion detectors. This 2DMD model has been shown to accurately predict Anolis lizard behavioural response. We tested different patterns of artificially generated motion and found that an abrupt 0.3° shift of position in less than 100 ms is optimal. We quantified motion in displays of 25 individuals from five species. Four species employ near-optimal movement patterns. We tested displays of these species using the 2DMD model on scenes with and without moderate wind. Display movements can easily be detected, even in the presence of windblown vegetation. The fifth species does not typically use the most effective display movements and display movements cannot be discerned by the 2DMD model in the presence of windblown vegetation. A number of Anolis species use abrupt up-and-down head movements approximately 10 mm in amplitude in displays, and these movements appear to be extremely effective for stimulating the receiver visual system.
Keywords: vision, motion, signal, design, lizard, Anolis
1. Introduction
A major goal of the scientific study of animal communication is to develop an understanding of the selective forces and constraints that result in the evolution of the physical properties of animal signals. An effective signal must stimulate the sensory system of the intended receiver, making sensory response patterns an important selective force. Numerous examples have been reported, from nearly every signalling modality, where signal properties are well matched to the tuning of the sensory receptors of signal receivers (reviewed in Bradbury & Vehrencamp 1998). Motion is a critical feature of many visual signals (Hailman 1977; Fleishman 1992; Bradbury & Vehrencamp 1998; Eckert & Zeil 2001; Rosenthal 2007). It plays an especially important role in eliciting the attention of potential signal viewers (Fleishman 1992; Dukas 2002; Peters & Evans 2003a; Ord & Stamps 2008). In this paper, we explore the relationship between visual-motion perception and visual-signal design in anoline lizards.
Lizards have become one of the most important model systems for the study of motion-based signalling. Previous studies have proceeded by quantifying the physical characteristics of the moving stimuli, such as temporal pattern, amplitude, duration, velocity and/or acceleration, directly (Jenssen 1970, 1977; Fleishman 1992), or with a computer-based optical flow analysis that extracts velocity vectors from moving images (Peters et al. 2002; Peters & Evans 2003a,b; Ord et al. 2007) and then testing lizard behavioural response to variations of these characteristics. These approaches have lead to very important insights, but they have some limitations. Visual systems do not encode physical properties of motion directly. Rather, they are extracted through neural processing from the output of simple neural circuits, called elementary motion detectors (EMDs), that respond to localized correlated changes of image intensity on the retina over time (Borst & Egelhaaf 1989). Because they capture and encode all aspects of a moving signal that can be used for further neural processing, EMD response properties are likely to be an important factor in the evolution of the design of moving signals (Eckert & Zeil 2001). The most widely used model of EMD response is known as a ‘correlation detector’ (Reichardt 1961; Borst & Egelhaaf 1993), which has been used successfully to predict motion detection responses in a variety of invertebrate and vertebrate animals (Borst & Egelhaaf 1989, 1993; Dror et al. 2001; Borst 2007; Meso & Zanker 2009). This approach has been expanded to a model consisting of a two-dimensional grid of interconnected EMDs, referred to as a two-dimensional visual-motion detection (2DMD) model, used to study the perception of the motion patterns embedded in complex natural scenes (Zeil & Zanker 1997; Eckert & Zeil 2001; Zanker & Zeil 2005).
Pallus et al. (2010) used a computational 2DMD model (based on Zanker & Zeil 2005) to study motion perception in the lizard Anolis sagrei. Behaviour experiments were used to determine appropriate spatial and temporal parameters for the model. The 2DMD model accurately predicted a number of important visually guided attention behaviours in A. sagrei and other Anolis species reported in the literature.
In this paper, we apply this model to the study of motion patterns used in Anolis visual communication. Anolis lizards use visual displays in a variety of social contexts. In the most frequently used display, referred to as an ‘assertion’ display (Carpenter 1967; Stamps 1977; Fleishman 1992), territorial males move throughout their home range and signal spontaneously every few minutes. These displays are not directed at a particular individual, and are believed to attract females to, and repel males from, territories and may play a role in stimulating the female reproductive system (Stamps 1977; Fleishman 1992; Ord et al. 2007). Since these displays are often directed at distant, inattentive viewers, it has been hypothesized that the early display movements function to draw visual attention (Fleishman 1988; Ord & Stamps 2008), and in this study we focus on the first 10 s of the displays.
Anolis lizard display movements mainly consist of up-and-down motion patterns of the head, body and a throat fan (called the dewlap) in a direction perpendicular to the body axis (Jenssen 1977). In order to identify the patterns of motion that most effectively stimulate the motion detection system, we created a series of artificial up-and-down patterns and tested their relative effectiveness in stimulating our lizard-visual-system-based 2DMD model. We then compared the optimal patterns of artificial movement with patterns of motion found in the displays of five species of Puerto Rican Anolis lizards. We also applied the 2DMD model to the field-recorded displays to see whether the display movements effectively stimulated the 2DMD model and whether the 2DMD model could easily distinguish display movements from natural motion caused by windblown vegetation in the habitat.
2. Material and methods
(a). The motion detection model
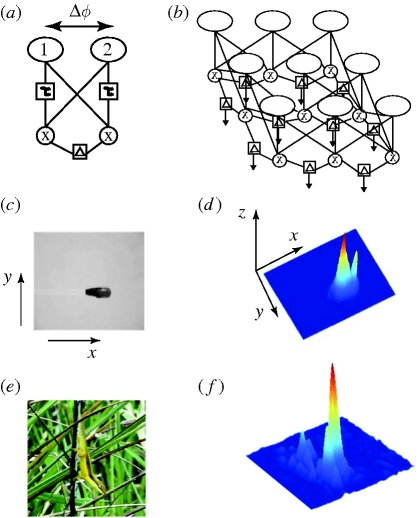
The model is described and illustrated in detail elsewhere (Pallus & Fleishman 2008; Pallus et al. 2010). The 2DMD model is a Matlab implementation of the model described in Zanker & Zeil (2005). The basic features are summarized in figure 1. The model parameters Δϕ (distance between adjacent receptors) = 0.3° visual angle, and τ (time constant of the low-pass filter) = 0.1 s, were determined for the Anolis attention response through behavioural experiments (Pallus et al. 2010). Input to the model consisted of a series of single frames of monochrome video (converted from colour with the Matlab Image Processing Toolbox function ‘rgb2gray’) recorded at a rate of 30 s−1. Prior to analysis, each input frame was processed with a ‘difference of Gaussians’ spatial filter, which mimics the effect of a centre-surround receptive field (e.g. Ibbotson & Clifford 2001). The extent of the spatial filter is matched to Δϕ in order to eliminate spatial aliasing and to spread prominent image features out over the distance between detectors, allowing detection of motion that is smaller in magnitude than Δϕ (Pallus et al. 2010). Video sequences ranging from 2 to 10 s in duration were entered into the model to create an x- and y-axis output vector at every pixel for every new frame, which were used to calculate the direction and magnitude of motion response at every point for each frame. In this paper, only output magnitude (not direction) is reported. Input videos were cropped to a size of approximately 120 × 120 pixels. Figure 1c–f illustrates typical input and output frames. For each video, we plotted maximum 2DMD output per frame against frame number and the maximum value for the entire sequence. Output from the 2DMD model is in arbitrary units that depend both on the nature of the motion and on the magnitude of local contrast variation between any moving object and the background.
Figure 1.
(a) A correlation-type elementary motion detector (EMD) consists of two brightness-sensitive receptors (labelled 1 and 2) separated by a distance equal to the spacing constant, Δϕ. Output from detector 1 is time delayed by passage through a low-pass temporal filter with time constant = τ, and multiplied by the temporally unfiltered output from detector 2. The operation is performed reciprocally and the two results are subtracted to yield an output magnitude in arbitrary units. (b) EMDs are interconnected in a grid to form a two-dimensional motion detector (2DMD) that can be used to model motion in two-dimensional scenes captured on video. (c) Artificial motion stimuli were created with a dark-tipped glass rod connected to an oscillograph pen motor. (d) One frame of output from the 2DMD model is depicted as a three-dimensional graph. The x–y-coordinates correspond to the position in the video and the z-coordinate indicates output magnitude. (e) An example of a frame of video of A. pulchellus. (f) One frame of 2DMD output of the A. pulchellus in response to a display movement in the presence of wind.
(b). Artificial stimuli
A 100 mm clear glass rod, with the tip covered with dark wax, was attached to an oscillograph pen motor and oriented to create motion predominately in the y-axis against a white background and illuminated with a non-flickering tungsten source (figure 1c). Sine, square and triangle wave motion ranging in frequency from 1 to 10 Hz was driven by a function generator. Amplitudes were set to equal 0.5, 1.0 or 2.0 times Δϕ, with a peak-to-peak movement of 8 mm = Δϕ (=0.3° at 1.5 m). We also tested single linear movements (ramp functions) of the three different amplitudes with rise times ranging from 2.0 to 1000 ms. Motion was recorded with a Sony DCR TRV900 digital video camera positioned 1.5 m from the stimulus. Exposure time was set to 1/30 s, which allowed rapid motion to blur and eliminated sampling artefacts for high-frequency motion. This mimics the effects of the retinal integration time of the anoline retina (Fleishman et al. 1995). Videos sequences were downloaded to a computer and converted to AVI format using the software Premiere Pro 1.5. Videos were originally recorded as two interlaced fields. The de-interlace function was used to remove every odd field to eliminate double images resulting from rapid motion. Each video sequence was processed with the 2DMD program. An example of one frame of output is shown in figure 1d.
(c). Lizard displays
Lizard displays were recorded over a 10-day period in April 2005 using the same methods as above, except that shorter exposure times (which varied with light intensity) were used. Five species of Anolis were observed on the eastern half of the island of Puerto Rico at five distinctly different habitat locations. Four of the species (Anolis pulchellus, Anolis cristatellus, Anolis krugi and Anolis gundlachi) are normally found on brush or tree trunks within 2 m of the ground. The fifth species, Anolis stratulus, is found primarily in forest canopy tree crowns, and was recorded from the top of a canopy tower structure at the El Verde research station. Individual lizards were spotted and observed with the camera running until they spontaneously displayed. The camera height was positioned at approximately the same height as the lizard. Immediately after each display, we determined the distance to the displaying lizard using a Leica Disto laser range finder. We used this information and camera magnification to calibrate display movements to real distance units. Before and after each display, we recorded wind speed with a hand-held anemometer, and used these data to estimate wind speed during the display. Following Pallus et al. (2010), we divided wind speed into three categories: no/low (0–1.5 km h−1), moderate (1.5–4 km h−1) and strong (greater than 4 km h−1). No displays were observed to occur during strong wind. In the videos with moderate wind, there was always some vegetation motion of a magnitude in the video that exceeded the largest lizard head movements. In the no/low wind cases, the lizard head movements were always the greatest movement on the video.
We limited analysis to a single assertion display by each individual. Since Anolis males are highly territorial, we assumed that lizards recorded from locations greater than 30 m apart were different individuals. We concluded that displays were assertion if (i) during and after recording we observed no conspecifics within 2 m of the displaying individual, (ii) the lizard exhibited no aggressive postural modifiers (as described in Jenssen 1977), and (iii) the lizard did not move directly towards another individual after the display was completed. After completion of the fieldwork, we examined our videos and found the first high-quality assertion display recorded from each individual and used that in the analysis.
The early parts of the displays consist almost entirely of movements of the head and body, with only occasional extension of the dewlap. We therefore measured display movements by digitizing the position of the lizard's nose along the single axis of maximum motion over the first 10 s of the display in each video field (60 fields s−1). We used the first 10 s because we expected the early portion of the display to contain movements designed to attract attention (Fleishman 1992; Peters & Evans 2003a). We recorded the amplitude of each major head movement and recorded the number of video fields taken to complete each movement, (temporal precision = 1 video field or 17 ms). The displays consisted of a series of large-amplitude movements, and some much smaller movements. We defined ‘major movements’ as any motion equal to at least half the amplitude of the largest movement within the display, and limited our analysis of amplitude and time to these. We determined an average value of amplitude and rise time for each individual and averaged these values to characterize motion in each species. Prior to processing with the 2DMD model, we resized videos using the Matlab image processing toolbox function ‘imresize’ to make them equivalent to a viewing distance of 1.5 m at lowest camera magnification and then cropped the videos to a size of approximately 120 × 120 pixels.
We were interested in determining whether display movements could be easily detected by the 2DMD model in the presence of windblown vegetation. The output of the 2DMD model is highly dependent on local contrast within each scene (Pallus et al. 2010), so that it was necessary to compare display and vegetation movements under identical conditions. We therefore examined the 12 displays (by five different species) from our sample in which there was moderate wind blowing while the display occurred to see whether the greatest response from the 2DMD model occurred in response to display movements.
3. Results
(a). Artificial stimuli
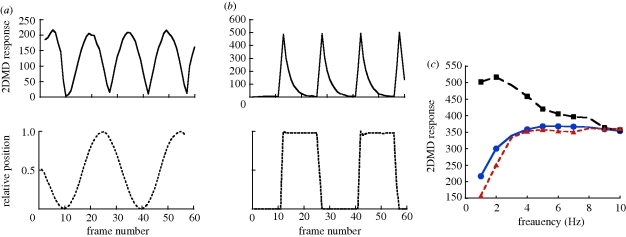
Examples of the 2DMD model output to sinusoidal and square-wave motion are shown in figure 2a,b. Figure 2c summarizes the maximum response as a function of frequency. For sine and triangle waves, response gradually increased with frequency to a plateau at approximately 5 Hz. The square-wave response was highest at the low frequencies and dropped steadily as frequency increased beyond 2 Hz. The highest response to square waves was approximately 1.5 × the highest response to sine or triangle waves.
Figure 2.
Examples of 2DMD output in response to different motion patterns. Response magnitude versus frame number for video of a (a) 1 Hz sine wave and (b) a 1 Hz square wave. (c) Maximum amplitude (from 3 s of stimulus and response for a single sample) for sine, square and triangle wave stimuli. For all three waveforms, amplitude was equal to Δϕ. Filled blue circles, sine wave; filled black squares, square wave; filled red triangles, triangle wave.
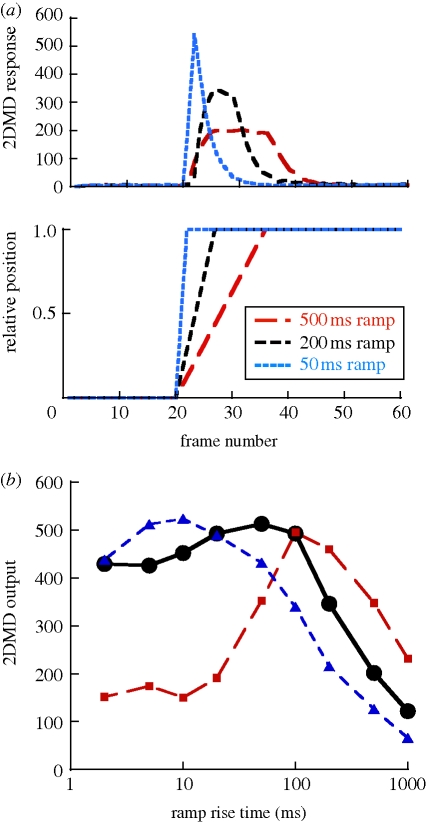
The great effectiveness of low-frequency square waves lead us to test ramp functions. Examples of ramp function with different rise times and their 2DMD outputs are shown in figure 3a. Figure 3b summarizes response maxima as a function of stimulus rise time for three different stimulus amplitudes (relative to Δϕ). When the motion was equal to 2 × Δϕ, the response exhibited linear velocity tuning. For the motion amplitude equal to Δϕ, the response was elevated for the shorter rise times and remained elevated up to a rise time of 100 ms and then dropped steadily for longer rise times. For the amplitude = 1/2 Δϕ, the response was elevated at short rise times and fell steadily for rise times longer than 50 ms. The optimal motion pattern appeared to be an abrupt shift of position (taking 100 ms or less) from a stationary position to a new stationary position approximately equal to Δϕ away.
Figure 3.
(a) Examples of stimuli and 2DMD response for linear ramp movements with different rise times. (b) Maximum 2DMD response (average of four samples) versus rise time for ramps of three different amplitudes: 1/2 Δϕ (filled blue triangles), 1 × Δϕ (filled black circles) and 2 × Δϕ (filled red squares).
(b). Lizard displays
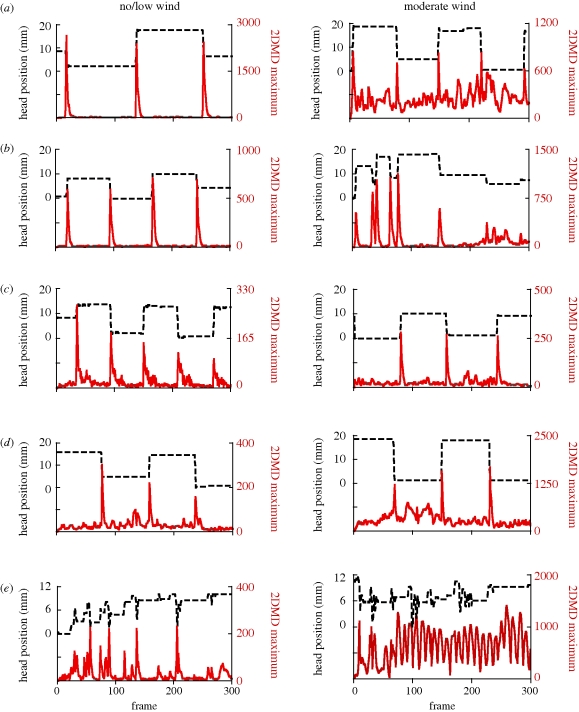
Figure 4 shows typical examples of assertion displays in no/low and moderate wind from all five species. The first 10 s of displays of the non-canopy species (figure 4a–d) are dominated by abrupt up or down head shifts with delays in between. Only the canopy-dwelling species A. stratulus differs from this pattern (figure 4e). Its typical display consists of a series of vollies of rapidly repeated up and down movements.
Figure 4.
Typical examples of the first 10 s of assertion display head movements (shown with a black dashed line) and maximum 2DMD output per frame (solid red line) for five different species. The left-hand displays were recorded in no/low wind and the right-hand displays were recorded while moderate wind was blowing. (a) Anolis gundlachi; (b) A. cristatellus; (c) A. pulchellus; (d) A. krugi; and (e) A. stratulus, which was recorded from the top of a forest canopy tower.
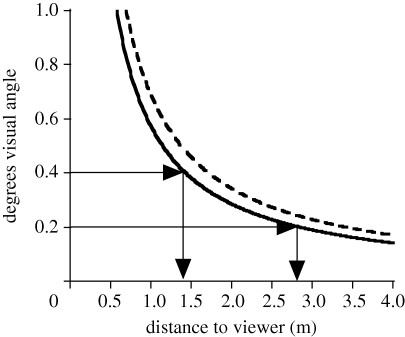
Amplitude and rise time for major movements are summarized in table 1. Our modelling predicted that abrupt shifts of less than 100 ms would be optimally effective (50 ms for amplitude of motion less than the receptor spacing). All major movements were completed in less than 50 ms. Average motion amplitude ranged from 8 to 13 mm, depending on species. Figure 5 illustrates the relationship between distance to the viewer and visual angle for movements within this range.
Table 1.
Amplitude and rise time of major movements in assertion displays. (Major movements are movements within each display that are greater than half the amplitude of the largest movement. Rise time precision is limited to 1 video field, or 17 ms.)
| species | n | mean rise time (ms (s.d.)) | mean motion amplitude (mm (s.d.)) | range of number of major movements per display |
|---|---|---|---|---|
| cristatellus | 5 | 37 (11) | 11 (3) | 3–7 |
| gundlachi | 5 | 43 (6) | 12 (4) | 2–5 |
| pulchellus | 6 | 28 (7) | 9 (1) | 3–5 |
| stratulus | 7 | 37 (10) | 8 (2) | 3–9 |
| krugi | 2 | 34 (0) | 13 (3) | 4 |
Figure 5.
Distance to the viewer versus degrees of visual angle for motion patterns of 10 mm (solid line) and 12 mm (dashed line).
Maximum output per frame for the 2DMD model is shown in red under each plot of head position. Note that the large differences between the displays in 2DMD magnitude are mainly due to differences in the local contrast conditions under which the displays were videotaped. Abrupt motion caused large peaks in the 2DMD output. For the four non-canopy species, in every case (n = 9), the display movements caused 2DMD output peaks (identifiable by their timing and position within the frame) that exceeded peaks produced by windblown vegetation. The canopy species, A. stratulus, produced a different result. A typical example is shown in figure 4e. We recorded three A. stratulus displays in the presence of wind, and in all three cases, the 2DMD response to the display was completely masked by the response to the windblown vegetation.
4. Discussion
There is strong evidence that the early stages of visual-motion processing involve localized EMD circuits that can be modelled as correlation detectors (see, e.g. Borst 2007; Meso & Zanker 2009), and Pallus et al. (2010) showed that visual attention responses by A. sagrei to motion in the visual periphery can be predicted with this model. We have shown here that correlation detectors tuned to the lizard visual system are optimally stimulated by an abrupt shift of an image over a distance approximately equal to the EMD space constant in less than 100 ms. The initial portion of the assertion displays of four species of Puerto Rican Anolis use precisely this pattern (see also Ord et al. 2007; Ord & Stamps 2008). The five Puerto Rican species used in this study are part of a single evolutionary radiation, and it is thus possible that this behaviour evolved only once. This use of square-wave-like patterns at the beginning of long-distance displays has also been reported for two distantly related species: A. aeneus (Stamps & Barlow 1973) and A. auratus (Fleishman 1992).
The results of this study appear to explain some seemingly contradictory conclusions from earlier studies. Fleishman (1986) studied motion detection in A. auratus, by presenting them with simple up-and-down motion patterns in the visual periphery that gradually increased in amplitude, and testing the probability of a gaze shift towards the stimulus. Low-frequency (1.5 Hz) square waves consistently produced the highest responses, and it was concluded that stimulus motion that combined high acceleration and high velocity was most effective. However, this conclusion seemed to be contradicted by two other results. First, high-frequency sine waves, which have high acceleration and velocity, were less effective than low- and middle-frequency sine waves. Second, maximum response always occurred at motion amplitudes of approximately 0.2–0.4° visual angle. Higher amplitude motion, which has higher acceleration and velocity, was significantly less likely to elicit response. Similarly, working with A. sagrei, Pallus et al. (2010) found that optimal motion amplitude for eliciting attention was in the 0.2–0.4° range, with higher amplitudes producing lower responses. It was also shown that in response to small targets moving linearly across a computer screen, A. sagrei detection probability was highest at medium velocity and dropped off at higher speeds (i.e. it exhibited velocity tuning). Thus, in some cases, high acceleration and velocity produced high responses, but in others it did not.
The current study appears to resolve these contradictions. The key factor that makes square-wave-like movements optimally effective is not their velocity or acceleration per se. Optimum response occurs when the image sits over one set of receptors and then is rapidly displaced to sit over another set of receptors at a distance roughly equal to one EMD spacing constant away and stops. This occurs because the EMD multiplier briefly receives input from both sets of paired detectors until the low-pass-filtered output from the first set of receptors decays (figure 1a). It is not important how the image moves between the locations, as long as it does so in a short period of time relative to the EMD time constant. When the image of an object moves more than the distance between the receptive fields of the two interconnected detectors, the 2DMD model predicts, and living animals exhibit, velocity tuning, with middle-range velocities being the most effective (figure 3b; Meso & Zanker 2009; Pallus et al. 2010).
The fact that the response of the 2DMD model to artificial stimuli was strikingly similar to responses of live animals to analogue versions of similar stimuli (Fleishman 1986; Pallus et al. 2010) gave us confidence in applying the model to analysis of motion in natural scenes. However, we offer a note of caution. The modelling introduces a number of simplifying assumptions about motion processing. For example, when an animal views a natural scene, visual-motion stimuli are continuous and they are processed continuously by the visual system. The 2DMD model samples single still frames of video in discrete steps, and in some cases, discrete analysis can introduce artefacts in motion perception (e.g. Sekuler et al. 1990; Straw 2003). We reduced these artefacts by blurring our stimulus images over a time period equivalent to retinal integration time. Nevertheless, it is possible that for some stimuli, a discrete-sampling motion model and continuous-sampling motion process will not produce the same response (Zanker & Zeil 2005; Brinkworth & O'Carroll 2009).
We found that under moderate wind conditions, the display movements of four of five of our species always produced maximum 2DMD output of higher magnitude than the maximum produced simultaneously by windblown vegetation. Further visual processing by the nervous system may enhance the effectiveness of these movements in the presence of wind. For example, a process that removes the strong confounding effects of local contrast would make display movements much easier to detect in conditions where some moving vegetation forms a very strong contrast against the visual background.
Ord et al. (2007) reported that A. cristatellus and A. gundlachi increase the ‘speed’ of their displays when the speed of background motion (owing to windblown vegetation) increases. An examination of the examples presented in their paper suggests that in both low and high wind, individual movements were abrupt and completed within one frame of video. The reported increase in display speed appears to result from a small increase in the amplitude of the movements and an increase in the number of up-and-down movements during the display. Our dataset for each species was too small to test statistically for differences in display form in wind versus no/low wind conditions. We did not observe consistent differences in display amplitude in our small samples. However, in our examples of gundlachi and cristatellus displays (but not for the other species), we observed a greater number of up-and-down movements in the first 10 s of the display in the presence of wind (figure 4a,b). This makes sense. If these abrupt start-and-stop movements are highly effective at stimulating motion detectors, and if windblown vegetation tends to make motion more difficult to see, then the lizards can compensate by increasing the number of these high-visibility movements per unit time. In a different variation of this adaptation, Peters et al. (2007) found that in its display, the Jacky lizard, Amphibolurus muricatus, does not increase the frequency of introductory tail movements in the presence of windblown vegetation, but rather increases the period of time during which they present these movements. Peters et al. (2008) suggested that the different strategies may relate to differences in the typical position of the lizard relative to the nearest vegetation. It appears that in response to windblown vegetation, some lizard species increase the number of high-visibility introductory movements either by presenting them more frequently or by presenting them for a longer period of time.
The forest canopy species A. stratulus did not produce movements that caused 2DMD output that was visible in the presence of moderate wind. The rapid multiple up-and-down display movements do not optimally stimulate the 2DMD model. Further, A. stratulus occupy a habitat that poses particular problems for a simple motion detection system. In sunny conditions, the forest canopy produces extremely high contrasts between shadowed leaves and branches and clear sky seen through the trees, which causes even modest vegetation motion to produce strong output from the 2DMD model. Moreover, in the canopy, even moderate wind produces large vegetation movements. It appears that in this environment A. stratulus has been forced to use some different strategy for making its display motion visible, although it is not immediately obvious to us what this strategy is.
In behavioural experiments, Pallus et al. (2010) determined that the peak attention response of A. sagrei occurred in response to motion over the range 0.2–0.4° visual angle. The average amplitude of square-wave-like introductory movements by the four species from this study that use them ranged from 8 to 13 mm. Figure 5 shows a plot of visual angle versus distance for movements of 10 and 12 mm. These movements fall within the range of high detectability over distances of approximately 1.5–3.5 m, which suggests that they are well designed for drawing the attention of conspecifics at the edges of their territories. Figure 5 also suggests that a display directed at a very close-range receiver should use reduced amplitude for maximum effectiveness. This has never been tested directly in Anolis. However, it has been shown that A. auratus use lower amplitude displays to a nearby conspecific than it uses in its assertion display (Fleishman 1988), and Stamps & Barlow (1973) showed that A. aeneus adds abrupt higher amplitude movements when presented with a more distant conspecific. Ord & Stamps (2008) demonstrated that A. gundlachi is more likely to use high-amplitude motion patterns at the beginning of their display when the receiver is several metres away, particularly in viewing conditions where detection is more difficult. On the other hand, in the only direct experimental test of this idea on a lizard, Peters & Allen (2009) found that Jacky lizards (A. muricatus) make no change in threat display amplitude based on the distance to an intruder. Clearly, the influence of receiver distance on display amplitude merits further study.
In summary, we have found that by analysing motion detection in anoline lizards using a biologically based motion detector model, we have come up with a compelling explanation for the pattern of movements used in the early portions of display of a number of species. The most effective motion stimulus is an abrupt shift in position approximately equal to the spacing between interacting receptors of the basic EMD that processes the early stages of motion. These motion patterns form a prominent feature of the introductory component of the displays of a number of species of Anolis lizards. This is a dramatic example of how the basic ‘wiring’ of a simple neural circuit that is responsible for the earliest stages of sensory detection and processing can influence the evolution of the design of a communication signal.
Acknowledgement
We thank Jochen Zeil, Johannes Zanker and Michael Rudko for assistance in developing the 2DMD program and two anonymous reviewers for suggested improvements in the manuscript. This work was supported by an Undergraduate Science Education grant from the Howard Hughes Medical Institute and a visiting scientist fellowship to L.J.F. (hosted by J. Zeil) from the Center for Visual Sciences at the Australian National University.
References
- Borst A.2007Correlation versus gradient type motion detectors: the pros and cons. Phil. Trans. R. Soc. B 362, 369–374 (doi:10.1098/rstb.2006.1964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A., Egelhaaf M.1989Principles of visual motion detection. Trends Neurosci. 12, 297–306 (doi:10.1016/0166-2236(89)90010-6) [DOI] [PubMed] [Google Scholar]
- Borst A., Egelhaaf M.1993Detecting visual motion: theory and models. In Visual motion and its role in stabilization of gaze (eds Miles F. A., Wallman J.), pp. 3–27 London, UK: Elsevier; [PubMed] [Google Scholar]
- Bradbury J. W., Vehrencamp S. L.1998Principles of animal communication. Sunderland, MA: Sinauer Associates [Google Scholar]
- Brinkworth R. S. A., O'Carroll D. C.2009Robust models for optic flow coding in natural scenes inspired by insect biology. PLoS Comput. Biol. 5, e1000555 (doi:10.1371/journal.pcbi.1000555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C.1967Aggression and social structure in iguanid lizards. In Lizard ecology, a symposium (ed. Milstead W. W.), pp. 87–106 Columbia, MO: U. Missouri Press [Google Scholar]
- Dror R. O., O'Carroll D. C., Laughlin S. B.2001Accuracy of velocity estimation by Reichardt correlators. J. Opt. Soc. Am. A 18, 241–252 (doi:0740-3232/2001/020241-12) [DOI] [PubMed] [Google Scholar]
- Dukas R.2002Behavioural and ecological consequences of limited attention. Phil. Trans. R. Soc. Lond. B 357, 1539–1547 (doi:10.1098/rtsb.2002.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. P., Zeil J.2001Toward an ecology of motion vision. In Motion vision: computational, neural, and ecological constraints (eds Zanker J. M., Zeil J.), pp. 333–369 Berlin, Germany: Springer [Google Scholar]
- Fleishman L. J.1986Motion detection in the presence and absence of background motion in an Anolis lizard. J. Comp. Physiol. A 159, 711–720 (doi:10.1007/BF00612043) [DOI] [PubMed] [Google Scholar]
- Fleishman L. J.1988Sensory influences on the physical design of a visual display. Anim. Behav. 36, 1420–1424 (doi:10.1016/S0003-3472(88)80212-4) [Google Scholar]
- Fleishman L. J.1992The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am. Nat. 139, S36–S61 (doi:10.1086/285304) [Google Scholar]
- Fleishman L. J., Marshall C. J., Hertz P. E.1995Comparative study of temporal response properties of the visual system of three species of anoline lizards. Copeia 1995, 422–431 (doi:10.2307/1446905) [Google Scholar]
- Hailman J. P.1977Optical signals. Bloomington, IN: Indiana University Press [Google Scholar]
- Ibbotson M. R., Clifford C. W. G.2001Characterizing temporal delay filters in biological motion detectors. Vis. Res. 41, 2311–2323 (doi:10.1016/S0042-6989(01)00126-2) [DOI] [PubMed] [Google Scholar]
- Jenssen T. A.1970Female response to filmed displays of Anolis nebulosus (Sauria, Iguanidae). Anim. Behav. 18, 640–647 (doi:10.1016/0003-3472(70)90007-2) [Google Scholar]
- Jenssen T. A.1977Evolution of anoline lizard display behavior. Am. Zool. 17, 203–215 [Google Scholar]
- Meso A. I., Zanker J. M.2009Speed encoding in correlation detectors as a consequence of spatial structure. Biol. Cybern. 100, 361–370 (doi:10.1007/s00422-009-0307-8) [DOI] [PubMed] [Google Scholar]
- Ord T., Stamps J. A.2008Alert signals enhance animal communication in ‘noisy’ environments. PNAS 105, 18 830–18 835 (doi:10.10.1073/pnas.0807657105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord T., Peters R., Clucas B., Stamps J. A.2007Lizards speed up visual displays in noisy motion habitats. Proc. R. Soc. B 274, 1057–1062 (doi:10.1098/rspb.2006.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallus A. C., Fleishman L. J.2008A two-dimensional motion detector based on biological principles. See http://www.union.edu/academic_depts/bioengineering/visual_motion/index.php [Google Scholar]
- Pallus A. C., Fleishman L. J., Castonguay P. M.2010Modeling and measuring the visual detection of ecologically relevant motion by an Anolis lizard. J. Comp. Physiol. A 196, 1–13 (doi:10.1007/s00359-009-0487-7) [DOI] [PubMed] [Google Scholar]
- Peters R. A., Allen S. J.2009Movement signal choreography unaffected by receiver distance in the Australian Jacky lizard, Amphibolurus muricatus. Behav. Ecol. Sociobiol. 63, 1593–1602 (doi:10.1007/s00265-009-0754-1) [Google Scholar]
- Peters R. A., Evans C. S.2003aIntroductory tail-flick of the jacky dragon visual display: signal efficacy depends on duration. J. Exp. Biol. 206, 4293–4307 (doi:10.1242/jeb00664) [DOI] [PubMed] [Google Scholar]
- Peters R. A., Evans C. S.2003bDesign of the jacky dragon visual display: signal and noise characteristics in a complex moving environment. J. Comp. Physiol. A 189, 447–459 (doi:10.1007/s00359-003-0423-1) [DOI] [PubMed] [Google Scholar]
- Peters R. A., Clifford C. W. G., Evans C. S.2002Measuring the structure of dynamic visual signals. Anim. Behav. 64, 131–146 (doi:10.1006/anbe.2002.3015) [Google Scholar]
- Peters R. A., Hemmi J. M., Zeil J.2007Signaling against the wind: modifying motion-signal structure in response to increased noise. Curr. Biol. 17, 1231–1234 (doi:10.1016/j.cub.2007.06.035) [DOI] [PubMed] [Google Scholar]
- Peters R. A., Hemmi J. M., Zeil J.2008Image motion environments: background noise for movement-based animal signals. J. Comp. Physiol. A 194, 441–456 (doi:10.1007/s00359-008-0317-3) [DOI] [PubMed] [Google Scholar]
- Reichardt W.1961Autocorrelation, a principle for the evaluation of sensory information by movement detectors. In Sensory communication (ed. Rosenblith W. A.), pp. 303–317 New York, NY; London, UK: MIT Press; Wiley [Google Scholar]
- Rosenthal G. G.2007Spatiotemporal dimensions of visual signals in animal communication. Annu. Rev. Syst. 38, 155–178 (doi:10.1146/annurevecolsys.38.091206.095745) [Google Scholar]
- Sekuler R., Anstis S., Braddick O. J., Brandt T., Movshon J. A., Orban G.1990The perception of motion. In Visual perception: the neurophysiological foundations. (eds Spillman L., Werner J. S.), pp. 205–230 San Diego, CA: Academic Press [Google Scholar]
- Stamps J. A.1977Social behavior and spacing patterns in lizards. In Biology of the Reptilia: ecology and behavior (eds Gans C., Tinkle D. W.), pp. 265–334 New York, NY: Academic Press [Google Scholar]
- Stamps J. A., Barlow G. W.1973Variation and stereotypy in the displays of Anolis aeneus (Sauria, Iguanidae). Behaviour 47, 67–94 (doi:10.1163/156853973X00283) [Google Scholar]
- Straw A. D.2003Neural responses to moving natural scenes. PhD thesis, University of Adelaide, Adelaide [Google Scholar]
- Zanker J. M., Zeil J.2005Movement-induced motion signal distributions in outdoor scenes. Netw. Comp. Neur. Syst. 16, 357–376 (doi:10:1080/09548980500497758) [DOI] [PubMed] [Google Scholar]
- Zeil J., Zanker J. M.1997A glimpse into crabworld. Vis. Res. 37, 3417–3426 (doi:10.1016/S0042-6989(97)00106-5) [DOI] [PubMed] [Google Scholar]