Abstract
Anguillid freshwater eels show remarkable life histories. In the Atlantic, the European eel (Anguilla anguilla) and American eel (Anguilla rostrata) undertake extensive migrations to spawn in the oceanic Sargasso Sea, and subsequently the offspring drift to foraging areas in Europe and North America, first as leaf-like leptocephali larvae that later metamorphose into glass eels. Since recruitment of European and American glass eels has declined drastically during past decades, there is a strong demand for further understanding of the early, oceanic phase of their life cycle. Consequently, during a field expedition to the eel spawning sites in the Sargasso Sea, we carried out a wide range of dedicated bio-physical studies across areas of eel larval distribution. Our findings suggest a key role of oceanic frontal processes, retaining eel larvae within a zone of enhanced feeding conditions and steering their drift. The majority of the more westerly distributed American eel larvae are likely to follow a westerly/northerly drift route entrained in the Antilles/Florida Currents. European eel larvae are generally believed to initially follow the same route, but their more easterly distribution close to the eastward flowing Subtropical Counter Current indicates that these larvae could follow a shorter, eastward route towards the Azores and Europe. The findings emphasize the significance of oceanic physical–biological linkages in the life-cycle completion of Atlantic eels.
Keywords: European eel, American eel, larval drift, oceanic fronts, Sargasso Sea, Subtropical Counter Current
1. Introduction
Anguillid freshwater eels exhibit impressively long spawning migrations across oceanic environments (van Ginneken & Maes 2005; Aoyama 2009)—in the case of European eel, more than 5000 km (Schmidt 1922). The larvae, known as leptocephali, are subsequently transported back by ocean currents to continental waters, where the juvenile eels, known as glass eels, enter freshwater bodies or settle in coastal marine areas (Tesch 2003). Among freshwater eels, the European and American eels have attracted particular interest. It was not until the early twentieth century that Schmidt (1922) identified the southern Sargasso Sea as the spawning area of both species, a finding that has since been confirmed by several other studies (Schoth & Tesch 1982; McCleave 1993). Later findings suggested that the distribution of leptocephali is associated with thermal fronts within the subtropical convergence zone (STCZ; Kleckner et al. 1983; Kleckner & McCleave 1988), characterized by steep temperature and salinity gradients, which potentially act as sensory cues for spawning of eels. However, despite 100 years of research on eels, there is still a lack of knowledge about the specific hydrographic and biotic conditions governing species distribution and dispersal dynamics during the larval phase. Moreover, larval feeding biology, and dependence on planktonic production remain essentially unknown but important for the understanding of the oceanic early life of eel. Knowledge of the dependence of early life history on oceanographic features has recently become even more pertinent, as European eel has experienced severe declines in stock size and recruitment level (ICES 2009). The state of the stock has been ascribed in turn to overfishing and habitat destruction in continental regions and/or changes of oceanographic features and decreased productivity in the Sargasso Sea (van Ginneken & Maes 2005; Bonhommeau et al. 2008).
Despite the apparent overlap in spawning areas and spawning time, the offspring of European and American eel segregate and return to their respective continents, suggesting different larval drift routes, or drift via the same routes but with different duration of the larval stage. The latter hypothesis was initially proposed by Schmidt (1922) and has been further investigated by ocean modelling (Power & McCleave 1983; Kettle & Haines 2006; Bonhommeau et al. 2009). It assumes larval entrainment in the Antilles Current, Gulf Stream and North Atlantic drift and implies a shorter larval phase in American eel as opposed to European eel, causing an earlier deflection of American eel from the major currents. Under this scenario, the larval drift of American eel would last for about 1 year while the duration for European eel larvae would be at least 2 years. However, in the southern Sargasso Sea, small European eel larvae (less than 10 mm) have been found in high abundance as far east as 52° W (Schoth & Tesch 1982; Boëtius & Harding 1985), and if larvae of such easterly distribution should follow the route described above, this would involve initial westward drift for up to 2000 km before entering the Antilles Current/Gulf Stream and being advected eastward. Further, otolith analyses of glass eels suggest little age difference between the two species at the glass eel stage; for both species, the age is estimated to be of the order of 1 year (Lecomte-Finiger 1994; Arai et al. 2000). Although this age determination is controversial owing to inherent uncertainties in the interpretation of otolith rings (McCleave 2008; Bonhommeau et al. in press), these contradictory observations led us to reconsider the larval drift routes and the possibilities for an additional/alternative eastward-oriented drift of European eel larvae. While most of the currents in this region have a westward trend, the wind and hydrography in the STCZ produce an eastward flowing so-called Subtropical Counter Current (SCC; Ullman et al. 2007; Bischof et al. 2009). This current prevails in the southern Sargasso Sea where eels spawn, and has been proposed as an alternative or supplementary drift route for European eel larvae (McCleave 1993).
Hence, in order to analyse relationships between frontal hydrography and the early life of eel while providing a basis for assessing potential drift routes of larvae, we prepared a multidisciplinary research programme at the eel spawning sites in the southern Sargasso Sea. Field investigations were carried out from the vessel ‘Vaedderen’ in the Sargasso Sea during early spring 2007. Our primary aims were to assess the retention of eel larvae by hydrographic frontal structures, and to examine the linkage between advection of European eel larvae and the flow related to the fronts and the SCC.
2. Material and methods
The cruise took place from 28 March to 11 April 2007, and measurements and sampling were carried out day and night at 33 stations on three transects, along the longitudes 64° W, 67° W and 70° W, respectively (figure 1). Station-to-station distances were decided according to measures of sea surface temperature (SST) obtained before and during the period of investigation, from the Operational Sea Surface Temperature and Sea Ice Analysis project (OSTIA, http://ghrsst-pp.metoffice.com). This project uses satellite data provided by the GODAE High Resolution Sea Surface Temperature Project, together with in situ observations to determine the SST. The analysis is performed daily and produce data at a resolution of 1/20° (approx. 5 km) by interpolation of information. We used SST data from 27 March 2007 for planning station locations while the analysis of 4 April 2007 was used in our comparison to findings from the cruise period.
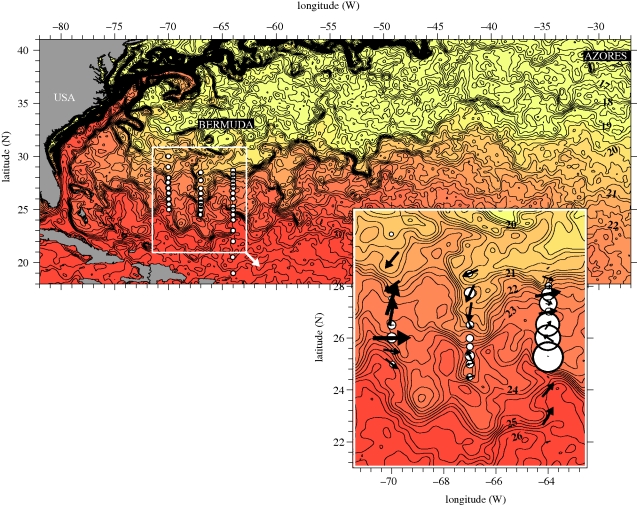
Figure 1.
Satellite image of sea surface temperature, SST, in the western North Atlantic on 4 April 2007. SST are contoured at 0.25°C intervals, closely spaced isotherms indicate frontal bands. Sampling positions are shown by white circles. Inserted enlargement of the study area illustrates relative abundances of A. Anguilla according to circle area, as well as current velocities at 50 m depth according to the size and direction of arrows.
Direction and velocity of currents were measured by two (600 and 75 kHz) ship-mounted Acoustic Doppler Current Profilers (RD Instruments). Measurements were averaged every 5 min within depth intervals of 2 or 16 m (for the 600 and 75 kHz, respectively). Ship speed influenced data quality, and we only used data where internal quality estimates exceeded 90 per cent. This was the case at stations where ship speed was less than 2 knots, and these measurements were averaged for the entire period at the station. Water from an inlet at 5 m depth was continuously pumped onboard the ship and the temperature and salinity were measured by a Seabird SBE21 every 0.5 min along the entire cruise track.
At each station, temperature and salinity were profiled vertically in 0.5 m intervals to 400 m using a Seabird 9/11 CTD equipped with a 12 Niskin bottle (30 l) rosette sampler. This was followed by a vertical haul for zooplankton. An opening–closing net (Multinet, HydroBios) that had an opening of 0.25 m2 and was equipped with five nets of 50 µm mesh was lowered to 400 m depth, and retrieved at a speed of 10 m min−1. The nets were opened and closed at different depth strata, though for the present purpose samples were integrated to present zooplankton abundance in the 50–200 m interval. Samples were preserved in 4 per cent formaldehyde, and zooplankton was later identified, enumerated and length measured.
The last procedure at the station was to haul for fish larvae, using a ring net of diameter 3.5 m equipped with a 25 m long net of 560 µm mesh. This gear was lowered to 250 m in an oblique haul, at a ship speed of 2.5 knots and a wire pay-out and retrieval of 25 and 15 m min−1, respectively. A flowmeter in the net opening measured water entering the net, and based on the estimated volume filtered and the depth of haul, the catch was estimated to number per square metre. Coverage of the full vertical distribution of eel larvae during the cruise was verified by a series of double hauls at seven of the stations, sequentially to 150 and to 250 m. The catch from each haul was brought to the laboratory, kept cold and within 1 h, the Anguilla-like larvae were sorted, photographed, length measured and transferred to a vial containing RNALATER (QIAGEN, Hilden, Germany). The remaining sample was preserved in 96 per cent ethanol, and after the cruise, this sample was sorted for all fish larvae, and additional Anguilla-like larvae were photographed, length measured and transferred to vials containing 96 per cent ethanol.
The two North Atlantic Anguilla species are morphologically very similar, and the traditional technique for species identification at the larval stage, based on myomere counting, holds some uncertainty (Boëtius & Harding 1985). We therefore systematically used molecular species identification to distinguish the two species. DNA was extracted from each larva using the E.Z.N.A.TM kit (OMEGA BIOTEK, Norcross, GA, USA). Subsequently, in order to distinguish between Anguilla anguilla and Anguilla rostrata, we used a molecular species identification approach developed by Trautner (2006). The assay is based on four species-specific primers that amplify fragments of the maternally inherited mitochondrial cytochrome b region using polymerase chain reaction (PCR). PCR product length differs between A. anguilla (789 bp) and A. rostrata (589 bp). Fragments were separated by electrophoresis in 1 per cent agarose gels. For further independent verification of species identity, we used the approach of Pichiri et al. (2006). This molecular species identification assay is based on PCR amplification of the nuclear 5S rDNA region, which yields fragments approximately 1200 bp in A. rostrata and approximately 600 bp in A. anguilla.
Statistical analyses were carried out using the procedure GLM in the program SAS (SAS Institute Inc., Cary, NC, USA).
3. Results
In our interpretation of the remotely sensed information on SSTs across the North Atlantic, we focused on the variability in the area west of 60° W between latitudes 20° N and 35° N where high concentrations of larvae of A. anguilla and A. rostrata have previously been documented (Boëtius & Harding 1985). The satellite pictures established the gradual climatological decline in temperature from south to north, but fronts of steeper temperature changes were also evident (figure 1). Two frontal bands dominated in the southern Sargasso Sea between 70° W and 60° W, whereas the frontal bands dissolved or merged east and west of this area.
Based on the pre-cruise observations of SST, we prepared a sampling scheme along three cross-frontal transects that would enable examination of the hydrography and biology related to the double frontal structure. The first series of hydrographic profiles from CTD casts along 64° W showed the vertical extension of the warm (greater than 28°C), mixed subtropical water mass separated from a deeper, colder (less than 20°C) water mass by a thermocline covering the layer from approximately 100–200 m water depth (figure 2a). Further to the north along this transect, at approximately 24° N, the thermocline inclined and isotherms from the 75–125 m depth layer rose and formed a surface front. After a distance of another approximately 280 km, a new surfacing of isotherms was evident, now from the 125–175 m depth layer, leading to a second front. North of this second front, the water column was only slightly stratified. The vertical sections obtained at the two other transects were not of the same coverage, and did not fully cross the two fronts. However, comparison with satellite-observed SSTs from the same period and the temperature changes along transects made it possible to evaluate coverage and interpret hydrographical connections between transects. The range of temperature between the sharp changes along 64° W (21.5°C and 25.5°C, from south and north, respectively) indicates to what extent the STCZ is covered by the other transects (figure 2b). There was generally good correspondence between the remotely sensed SSTs and our ship-based observations (figures 1 and 2a,b).
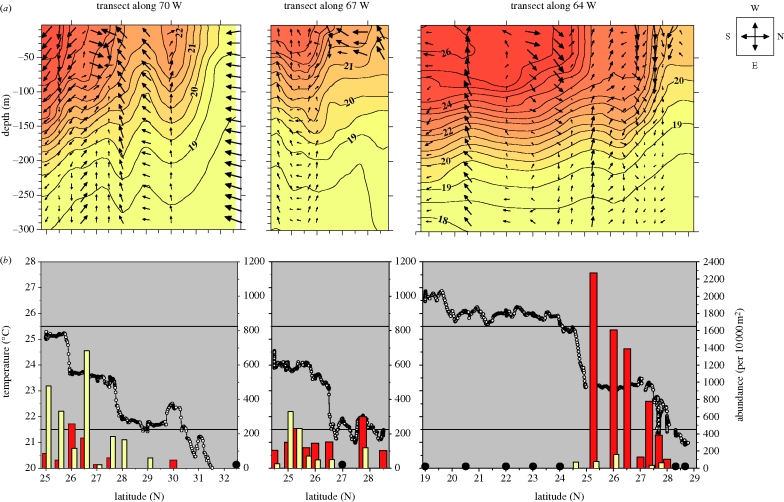
Figure 2.
Observations of hydrography and abundances of eel larvae along transects. (a) Vertical transect profiles of temperature as measured by CTD casts are contoured using 0.5°C isotherms. Direction and velocity of horizontal currents at given depths are shown by arrows. Cardinal directions and an arrow size representing 0.3 m s−1 are illustrated by legend figure to the right of panel; note that along-transect currents (north/south) are illustrated by left/right directed arrows. (b) Underway measurements of SST are indicated by small connected circles. Temperature range from 21.5°C to 25.5°C is indicated by full lines. Abundances of eel larvae are shown by height of bars; note scale differences between transects. Estimates of zero abundance are indicated by large, black circles. Red bar, A. Anguilla; yellow bar, A. rostrata.
The ship-mounted current profilers (ADCP) supplied us with estimates of current directions and velocities at each sampling station (figures 1 and 2a, arrows). The estimates showed a high variability, which is to be expected in this dynamic area where the hydrography is influenced by eddies and undulations of the frontal bands (Voorhis 1969; Eriksen et al. 1991). However, when crossing the frontal structures, we observed distinctive changes in current patterns. Strong along-frontal flow (up to 0.3 m s−1) in an easterly direction was apparent at transects along 64° W and 70° W when passing the southernmost frontal band, and an eastward-oriented current was also apparent at the northernmost frontal band along 64° W. Current directions in the vicinity of the northernmost frontal band were less consistent at transects along 67° W and 70° W, albeit generally southeastward. Vertically, the current velocity tended to decline with depth, especially across the thermocline, whereas the direction showed only little change with depth. Hence, general trends in current directions and velocities were apparent, showing a strong eastward flow related to thermal fronts in the upper 150 m of the water column.
Our ring net hauls assembled a wide range of larval species, and most of the hauls contained larvae of the genus Anguilla, which were subsequently assigned to species using molecular markers. Of the comparative hauls to maximum depths of 150 and 250 m, respectively, the abundance estimate of Anguilla larvae (per unit area) was not systematically larger for the haul to 250 m. This suggests that during our study almost all Anguilla larvae resided above 150 m, well within reach of our standard sampling depth of 250 m. Leptocephali larvae are known to show considerable net avoidance during daytime; however, this behaviour is of less importance when larvae are in their smallest stages and they are sampled with a relatively large gear such as our 3.5 m wide ring net (Castonguay & McCleave 1987). While net avoidance is influenced by larval size and light, a significant relationship between larval mean length in catches and light intensity would point to an effect in our case. However, variability in mean lengths at stations could also be due to larval drift away from sites of initial spawning, i.e. there might be a spatial trend in larval age and hence, mean lengths from a given site. We investigated these two potential causes of larval mean length variability (day/night difference and spatial trend across the frontal zone) by an analysis of covariance for each species. The analyses showed non-significant influence of day/night variability for both species (ANCOVA, p > 0.3 for both). No significant trend in mean length variation across the frontal zone was seen for A. rostrata (ANCOVA, p > 0.7), while mean lengths of A. anguilla showed a significant northward increase (ANCOVA, p < 0.03).
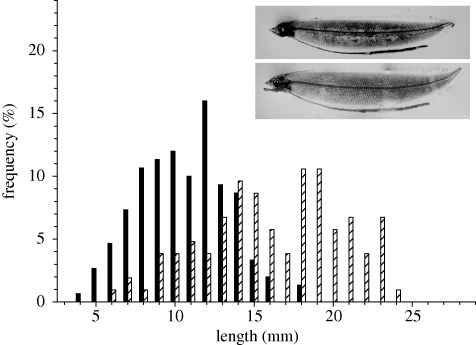
We compared the respective spatial distributions of A. anguilla and A. rostrata, and their relation to hydrographic features (figure 2b). Two characteristic patterns emerged. Firstly, larvae of both species appeared confined by the two frontal bands, with highest abundances within the bands or in the transitional zone between these. This indicates a northern and southern limit of larval distributions. Secondly, we observed marked changes in larval abundances and species composition in the east–west direction. Of the two species, A. anguilla dominated at the easternmost transect (95%), whereas A. rostrata dominated at the westernmost transect (75%). Larvae varied significantly in size, and A. anguilla were generally shorter than A. rostrata (figure 3). Beside these major groups of larvae, obviously originating from the spawning in 2007, we caught four A. anguilla of a much larger size (37–42 mm). Such larvae of a deviating size have commonly been observed during cruises targeting eel larvae in the area, and these larvae are believed to be individuals from the previous year's spawning (Boëtius & Harding 1985).
Figure 3.
Length distributions of A. anguilla and A. rostrata. Based on all sampled larvae, bars for each species accumulate to 100%. Inserted are pictures of two sampled letocephali larvae, A. angullia (upper picture; black bars) and A. rostrata (lower picture; striped bars) illustrated specimens are 10 and 12 mm respectively.
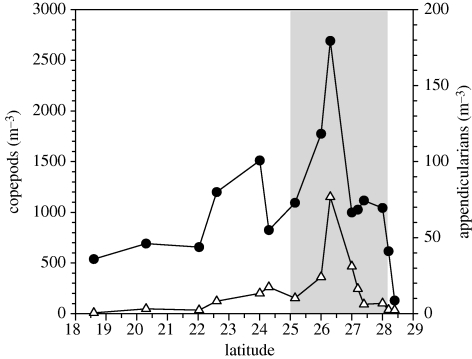
When finding Anguilla larvae confined within the convergence zone (STCZ), we investigated whether this zone of larval retention provides superior feeding conditions for the larvae. Except for a study that shows the presence of appendicularian remains (Mochioka & Iwamizu 1996), it has not been possible to identify the stomach contents of leptocephali larvae microscopically. However, molecular analyses of the stomach contents of eel larvae carried out during the present study indicate that the diet covers a wide range of plankton taxa, including copepods (Riemann et al. in press). It is unknown to what extent the larvae have eaten these as live organisms or as their degraded remains, for example, as part of appendicularian houses or faecal pellets. The sequence of plankton tows along longitude 64° W covered the STCZ and larval distributions in full, and for this transect, we estimated the mean density of appendicularians and copepods in the 50–200 m depth stratum where most eel larvae reside (Castonguay & McCleave 1987). The densities of these plankton organisms peaked in the convergence zone, and declined towards the more stratified waters in the south, and towards the less stratified waters in the north (figure 4). These findings suggest that Anguilla larvae in general would find higher food concentrations within as compared with outside fronts associated with the STCZ.
Figure 4.
Densities of potential prey to the eel larvae. Densities of appendicularians (triangles) and copepods (circles) in the 50–200 depth stratum. All species and stages are combined for each group. Shaded area illustrates the frontal zone, as it is indicated by the temperature range in figure 2b.
4. Discussion
We found a characteristic frontal hydrography in the areas of eel larval distributions. When supplemented with SST information from remote sensing, our hydrographic profiles along the three transects clearly illustrated the characteristics of the multiple frontal structure in the STCZ. This is seen as an inclination of the thermocline in two sharp fronts with a broad mixing zone in between. Such a large-scale pattern of interconnected frontal bands is a recurrent phenomenon of the STCZ, and the position and strength of the thermal fronts correspond with earlier observations of interconnected, undulating frontal bands that cross the southern Sargasso Sea and extend towards the east/northeast (Voorhis 1969; Eriksen et al. 1991; Ullman et al. 2007). Furthermore, the latitudes of the observed frontal bands are within the range of earlier findings, e.g. other cruises for eel larvae from February to April also found the northern band (and the northern limit of larval eel distributions) between 27° N and 28° N (Schoth & Tesch 1982; Kleckner & McCleave 1988).
Our observations across the STCZ along the first of the transects revealed both a southern and a northern limit of eel larval distributions set by the two frontal bands. Patterns were less apparent at the two other transects, but overall the findings suggest retention of eel larvae within the frontal structure. Such retention has important implications for understanding the early life of eels in the Sargasso Sea. First, as shown for several other fish species (Munk et al. 2009), eel spawning is presumably directed towards hydrographic structures that both allow larval drift towards nursery areas and diminish their dispersal away from drift routes. Such conditions are provided by hydrographic fronts, in this case the subtropical oceanic fronts and the associated currents. Second, larvae are retained in areas of specific physical/biological conditions to which they are probably adapted and which may offer them superior feeding conditions. Our understanding of eel larval feeding is still limited, but our findings of enhanced abundances of plankton organisms in the mixing zone of the STCZ strongly suggest that retention of larvae within this specific zone is to their advantage.
While they both appeared linked to hydrographical patterns, the two Anguilla species differed in distributional characteristics. We sampled the overlap region where the distributions of A. anguilla and A. rostrata larvae meet (Schmidt 1922), and while differences between peak abundances on the south–north axis were small, our sampling showed a marked change in relative dominance of each species on the west–east axis. This change in relative abundance of the two species is more apparent than seen during earlier studies in the area, e.g. observations from the 1980s (McCleave et al. 1987). The small spatial overlap implies that a large proportion of larvae of each species could be influenced differently by prevailing hydrography and currents. West of the area, where A. rostrata dominates, the SCC weakens (Bischof et al. 2009) and larval A. rostrata would predominantly be influenced by westerly currents and follow a westerly/northerly route entrained in the Antilles/Florida Currents. Conversely, a significant proportion of the more easterly distributed A. anguilla larval population could be entrained in the easterly frontal currents and advected in the SSC towards the Azores and Europe, while the other would be entrained in the Gulf Stream system. An easterly drift would lead to a first arrival of larvae (i.e. smaller larvae/juveniles) in the southern part of the European continent as is commonly observed (Bertin 1956).
Whether or not such eastward drift to Europe could be accomplished within a year remains to be determined. Our measured velocities of frontal currents indicate that organisms entrained in these currents could be advected substantial distances. We measured eastward frontal flows of 0.2–0.3 m s−1 (approx. 6000–9000 km yr−1), which is to be compared with a direct distance to Europe from this area of the order of 5500 km. Equally strong frontal currents, up to 0.5–1.0 m s−1, have been found during other studies of the same subtropical fronts (Eriksen et al. 1991; Fernández & Pingree 1996). However, such high velocities are probably not sustained for the entire migration because frontal characteristics change with seasons (Ullman et al. 2007), and the meandering of frontal bands leads to a route that is significantly longer than a direct distance. Notwithstanding, the estimates of frontal current velocities in the SSC illustrate that this current has the potential for advecting larvae to Europe considerably faster than the approximately 2 years estimated by modelling studies of drift in the Gulf Stream current system (Kettle & Haines 2006; Bonhommeau et al. 2009).
The oceanic influence on the recruitment of eel has been investigated in a number of recent studies, and these find significant correlation between large-scale ocean–atmospheric changes at spawning grounds and time-lagged indices of glass-eel recruitment (e.g. Friedland et al. 2007; Bonhommeau et al. 2008). The bio-physical linkages of key importance in the early life of fishes are, however, poorly resolved by the large-scale measures, integrating over hundreds of kilometres. There is increasing recognition of the need for more detailed in situ studies that cover the relationships on finer scales. In the present study, we gain new insight into the variability in physical and biological measures at the eel spawning grounds in the Sargasso Sea. The findings highlight a key role of frontal processes for eel larval retention, feeding and drift. Hence, we propose that further emphasis should be given to the frontal dynamics in the STCZ and the flow of the SCC in the interpretation of the life-cycle completion of North Atlantic eels and in the search for possible causes of their declining recruitment.
Acknowledgements
We thank the captain, the crew and other participants for excellent assistance during the cruise. J. L. Høyer provided great support on the SST analysis. Two anonymous reviewers provided helpful comments on an earlier version of the manuscript. G.E.M. acknowledges a post-doctoral and travel grant funded by the Research Foundation-Flanders (FWO-Vlaanderen). The project was supported by grants from Villum Kann Rasmussen Foundation, Elisabeth and Knud Petersens Foundation, Knud Højgaards Foundation and the Danish Natural Science Research Council. The present work was carried out as part of the Galathea 3 expedition under the auspices of Dansk Expeditions Fond. This is contribution no. P61 of the Galathea 3 expedition.
References
- Aoyama J.2009Life history and evolution of migration in catadromous eels (Genus Anguilla). Aqua BioSci. Monogr. 2, 1–42 [Google Scholar]
- Arai T., Otake T., Tsukamoto K.2000Timing of metamorphosis and larval segregation of the Atlantic eels Anguilla rostrata and A. anguilla, as revealed by otolith microstructure and microchemistry. Mar. Biol 137, 39–45 (doi:10.1007/s002270000326) [Google Scholar]
- Bertin L.1956Eels—a biological study. London, UK: Cleaver-Hume Press Ltd [Google Scholar]
- Bischof B., Mariano A. J., Ryan E. H.2009The Subtropical Counter Current. See http://oceancurrents.rsmas.miami.edu/atlantic/subtropical-cc.html
- Boëtius J., Harding E. F.1985A re-examination of Johs Schmidt's eel investigations. Dana 4, 129–162 [Google Scholar]
- Bonhommeau S., Chassot E., Rivot E.2008Fluctuations in European eel (Anguilla anguilla) recruitment resulting from environmental changes in the Sargasso Sea. Fish. Oceanogr. 17, 32–44 [Google Scholar]
- Bonhommeau S., Le Pape O., Gascuel D., Blanke B., Tréguier A.-M., Grima N., Vermard Y., Castonguay M., Rivot E.2009Estimates of the mortality and the duration of the trans-Atlantic migration of European eel Anguilla anguilla leptocephali using a particle tracking model. J. Fish Biol. 74, 1891–1914 (doi:10.1111/j.1095-8649.2009.02298.x) [DOI] [PubMed] [Google Scholar]
- Bonhommeau S., Castonguay M., Rivot E., Sabatié R., Le Pape O.In press The duration of migration of Atlantic Anguilla larvae. Fish Fish. (doi:10.1111/j.1467-2979.2010.00362.x) [DOI] [PubMed] [Google Scholar]
- Castonguay M., McCleave J. D.1987Vertical distributions, diel and ontogenetic vertical migrations and net avoidance of Anguilla and other common leptocephali in the Sargasso Sea. J. Plankton Res. 9, 195–214 (doi:10.1093/plankt/9.1.195) [Google Scholar]
- Eriksen C. C., Weller R. A., Rudnick D. L., Pollard R. T., Regier L. A.1991Ocean frontal variability in the frontal air–sea interaction experiment. J. Geophys. Res. 96, 8569–8591 (doi:10.1029/90JC02531) [Google Scholar]
- Fernández E., Pingree R. D.1996Coupling between physical and biological fields in the North Atlantic subtropical front southeast of the Azores. Deep Sea Res. I 43, 1369–1393 [Google Scholar]
- Friedland K. D., Miller M. J., Knights B.2007Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES J. Mar. Sci. 64, 519–530 (doi:10.1093/icesjms/fsm022) [Google Scholar]
- ICES. 2009. International Council for Exploration of the Sea. Report of the ICES Advisory Committee 2009, ICES Advice, Book 9. [Google Scholar]
- Kettle A. J., Haines K.2006How does the European eel (Anguilla anguilla) retain its population structure during its larval migration across the North Atlantic Ocean? Can. J. Fish. Aquat. Sci. 63, 90–106 (doi:10.1139/f05-198) [Google Scholar]
- Kleckner R. C., McCleave J. D.1988The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to thermal fronts and surface water masses. J. Mar. Res. 46, 647–667 (doi:10.1357/002224088785113469) [Google Scholar]
- Kleckner R. C., McCleave J. D., Wippelhauser G. S.1983Spawning of American eel, Anguilla rostrata, relative to thermal fronts in the Sargasso Sea. Environ. Biol. Fish. 9, 289–293 (doi:10.1007/BF00692377) [Google Scholar]
- Lecomte-Finiger R.1994The early life of the European eel. Nature 370, 424 (doi:10.1038/370424a0) [Google Scholar]
- McCleave J. D.1993Physical and behavioural controls on the oceanic distribution and migration of leptocephali. J. Fish Biol. 43(Suppl. A), 243–273 [Google Scholar]
- McCleave J. D.2008Contrasts between sampling times of Anguilla species estimated from larval sampling at sea and from otolith analysis of recruiting glass eels. Mar. Biol. 155, 249–262 (doi:10.1007/s00227-008-1026-8) [Google Scholar]
- McCleave J. D., Kleckner R. C., Castonguay M.1987Reproductive sympatry of American and European eels and implications for migration and taxonomy. Am. Fish. Soc. Symp. 1, 286–297 [Google Scholar]
- Mochioka N., Iwamizu M.1996Diet of anguilloid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Mar. Biol. 125, 447–452 [Google Scholar]
- Munk P., Fox C. J., Bolle L. J., Van Damme C. J. G., Fossum P., Kraus G.2009Spawning of North Sea fishes linked to hydrographic features. Fish. Oceanogr. 18, 458–469 (doi:10.1111/j.1365-2419.2009.00525.x) [Google Scholar]
- Pichiri G., Nieddu M., Manconi S., Casu C., Coni P., Salvadori S., Mezzanotte R.2006Isolation and characterization of two different 5S rDNA in Anguilla anguilla and in Anguilla rostrata: possible markers of evolutionary divergence. Mol. Ecol. Not. 12, 1471–8278 [Google Scholar]
- Power J., McCleave J. D.1983Simulation of the North Atlantic drift of Anguilla leptocephali. Fish. Bull. USA 81, 483–500 [Google Scholar]
- Riemann, et al. In press Qualitative assessment of the diet of European eel larvae in the Sargasso Sea resolved by DNA barcoding. Biol. Lett. (doi:10.1098/rsbl.2010.0411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.1922The breeding places of the eel. Phil. Trans. R. Soc. Lond. B 211, 179–208 (doi:10.1098/rstb.1923.0004) [Google Scholar]
- Schoth M., Tesch F.-W.1982Spatial distribution of 0-group eel larvae (Anguilla sp.) in the Sargasso Sea. Helgoländer Meer Esun. 35, 309–320 (doi:10.1007/BF02006139) [Google Scholar]
- Tesch F.2003The eel. Oxford, UK: Blackwell Science Ltd [Google Scholar]
- Trautner J.2006Rapid identification of European (Anguilla anguilla) and North American eel (Anguilla rostrata) by polymerase chain reaction. Informationen aus der Fischereiforschung 53, 49–51 [Google Scholar]
- Ullman D. S., Cornillon P. C., Shan Z.2007On the characteristics of subtropical fronts in the North Atlantic. J. Geophys. Res. 112, C01010 (doi:10.1029/2006JC003601) [Google Scholar]
- van Ginneken V. J. T., Maes G. E.2005The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev. Fish Biol. Fish. 15, 367–398 [Google Scholar]
- Voorhis A. D.1969The horizontal extent and persistence of thermal fronts in the Sargasso Sea. Deep Sea Res. 16(Suppl.), 331–337 [Google Scholar]