Abstract
Aggression between species is a seldom-considered but potentially widespread mechanism of character displacement in secondary sexual characters. Based on previous research showing that similarity in wing coloration directly influences interspecific territorial aggression in Hetaerina damselflies, we predicted that wing coloration would show a pattern of character displacement (divergence in sympatry). A geographical survey of four Hetaerina damselfly species in Mexico and Texas showed evidence for character displacement in both species pairs that regularly occurs sympatrically. Hetaerina titia, a species that typically has large black wing spots and small red wing spots, shifted to having even larger black spots and smaller red wing spots at sites where a congener with large red wing spots is numerically dominant (Hetaerina americana or Hetaerina occisa). Hetaerina americana showed the reverse pattern, shifting towards larger red wing spots where H. titia is numerically dominant. This pattern is consistent with the process of agonistic character displacement, but the ontogenetic basis of the shift remains to be demonstrated.
Keywords: agonistic character displacement, interspecific aggression, heterospecific aggression, species recognition, competitor recognition
1. Introduction
Most studies of character displacement start with the observation of a geographical pattern (e.g. divergence in sympatry). When the displaced trait is a secondary sexual character, the pattern is usually hypothesized to result from selection against cross-species mating, i.e. reproductive character displacement (reviewed in Coyne & Orr 2004; Pfennig & Pfennig 2009). A seldom considered alternative is that such patterns may result from selection against interspecific aggression. Just as traits that play a role in mate choice within species are likely to become targets of selection for improved mate recognition when closely related species that still use similar sexual signals come into secondary contact, traits that play a role in intrasexual competition are likely to become targets of selection for improved competitor recognition when closely related species that still use similar agonistic signals come into secondary contact (Grether et al. 2009). Also just as selection against cross-species mating can cause species to diverge in both sexual signals and mate recognition functions (Coyne & Orr 2004; Lemmon et al. 2004), selection against interspecific aggression can cause species to diverge in both agonistic signals and competitor recognition functions. For lack of a suitable existing term, Grether et al. (2009) introduced the term agonistic character displacement for cases in which character displacement is driven by interspecific aggression. This is a distinctly different evolutionary process than either reproductive or ecological character displacement (reviewed in Grether et al. 2009). Empirically, however, reproductive and agonistic character displacement can be difficult to distinguish, because many secondary sexual characters are involved in both intrasexual competition and mate choice (Berglund et al. 1996).
Prior to documenting any geographical patterns, we identified Hetaerina damselflies as a system in which agonistic character displacement is likely to be operating in the absence of reproductive character displacement. Male wing coloration in Hetaerina is intrasexually selected and does not appear to be a target of female mate choice (Grether 1996; Cordoba-Aguilar et al. 2009b). Males of all species in the genus have conspicuous red wing coloration and compete to defend small mating territories in areas with flowing water; females visit these areas to mate and oviposit in submerged vegetation (Garrison 1990). Hetaerina territories are similar to the display courts of classic lekking species in that all activities besides mating (feeding, egg-laying, roosting, etc.) usually take place elsewhere (Grether & Grey 1996; Grether & Switzer 2000). Unlike classic lekking species, mating is semi-coercive; males do not perform courtship displays, and although female cooperation is required for copulation, males can clasp and detain resistant females (Grether 1996). Unlike damselflies with resource-based territories (e.g. Calopteryx spp.), males do not control access to oviposition sites within their territories and females only rarely oviposit inside their mate's territory (Alcock 1987; Weichsel 1987; Grether 1996; Cordoba-Aguilar et al. 2009b). Thus, a Hetaerina territory is best viewed as a defended air space within which the resident male has the priority of access to arriving females (Weichsel 1987; Grether 1996). In the defence of a territory, a damselfly may incur many costs, including energetic/physiological costs, injury costs, opportunity costs and predation costs (reviewed in Suhonen et al. 2008).
Logically, males of different Hetaerina species should not waste time and energy fighting over space. Nevertheless, interspecific territorial fights occur frequently between some sympatric species pairs. The proximate reason for this seemingly maladaptive behaviour might be that territory holders simply are unable to discriminate visually between conspecific and heterospecific intruders. Male wing coloration is the most conspicuous marker of species identity (Garrison 1990), and heterospecific fights occur more often between sympatric species that are similar in wing coloration (e.g. Hetaerina americana and Hetaerina occisa) than between species that are more divergent in wing coloration (e.g. Hetaerina titia and H. occisa) (Anderson & Grether 2010). In simulated territory intrusion tests with live tethered intruders, residents do not discriminate between conspecific and heterospecific intruders with similar wing coloration but show reduced aggression towards heterospecific intruders with divergent wing coloration (Anderson & Grether 2010). That wing coloration, per se, affects how territory residents respond to conspecific and heterospecific intruders has been demonstrated experimentally. Specifically, H. titia, a species with red and black wing spots, responds more aggressively to H. americana and H. occisa intruders with artificial black wings spots than to normal H. americana and H. occisa, which only have red wing spots. The same wing colour manipulation reduces aggression from H. americana and H. occisa territory holders, but only at sites where H. titia is present (Anderson & Grether 2010). The consistent difference in how males respond to wing colour-manipulated intruders at sympatric and allopatric sites provides clear evidence for agonistic character displacement because shifts in competitor recognition are not predicted under other character displacement processes (Anderson & Grether 2010). Here, we test for corresponding geographical shifts in male wing coloration across a broad swath of the range in which H. titia can be found in sympatry with H. americana and H. occisa.
Multiple geographical patterns have been presented as evidence for character displacement (reviewed in Schluter 2000; Pfennig & Pfennig 2009). The classic approach is to compare trait divergence in areas of sympatry with that in areas of allopatry (Dobzhansky 1937; Grant 1972; Waage 1979; Coyne & Orr 2004; Kirschel et al. 2009). An alternative approach is to compare sympatric populations that vary in the relative abundance of heterospecifics (Pfennig & Murphy 2002; Tynkkynen et al. 2004; Goldberg & Lande 2006; Fisher & Rosenthal 2010). The predicted pattern is that displacement of the trait in one species should be greater where the relative abundance of the other species is greater. One advantage of this approach is that it can be applied to species like H. titia that rarely, if ever, occur in allopatry. In general, when testing for geographical patterns indicative of character displacement, it is important to control for environmental factors other than the presence or abundance of heterospecifics (Goldberg & Lande 2006). A geographical pattern suggesting character displacement could merely reflect the outcome of species evolving independently to environmental changes (Endler 1986; Schluter 2000). Alternatively, adaptation along an environmental gradient could cause species to differ more in allopatry than in sympatry, even if character displacement is occurring (Goldberg & Lande 2006). If body size varies seasonally, as is often the case in damselflies (Grether 1995; Corbet 1999; Cordoba-Aguilar et al. 2009a), then character displacement could be obscured. With these considerations, we tested for geographical shifts in male Hetaerina wing coloration in relation to the presence or relative abundance of congeners while controlling for clinal and temporal variation.
2. Material and methods
(a). Study species and sites
Hetaerina is endemic to the Americas and reaches its highest diversity in the South American tropics (Garrison 1990). Males of most species in the genus have red spots at the base of all four wings. Hetaerina titia is unusual in having red spots only on the forewings and black (melanin) pigmentation on the hindwings and sometimes on the forewings. The black pigmentation is extremely variable in areal coverage, from only a fraction of the wing base to the entire hindwing and most of the forewing. The high level of variation in H. titia makes it a good candidate for character displacement, but this species rarely, if ever, occurs in allopatry. We therefore designed our study to make comparisons among sites where H. titia occurs with one or more congeners at different relative densities. We also measured these congeners at sites where H. titia is absent, to test for character shifts in them using the classic approach. Within sympatric sites, H. titia is syntopic (sensu Rivas 1964) with H. americana and H. occisa, that is, males of the different species defend territories in the same stretches of river. Between 2004 and 2008, we collected data at 45 sites where one or more of these three species was present (for locations and sampling dates, see electronic supplementary material, table S1). Hetaerina titia, H. americana and H. occisa were present at 18, 28 and 25 sites, respectively. It was uncommon for all three species to be found together (five sites). A fourth species, Hetaerina cruentata, was found at eight of the 45 sites, but never with H. titia. The distance between the closest sites ranged from 1.5 to 2105 km, with a median distance (± interquartile range) of 20.9 ± 13.5 km.
(b). Data collection
Species composition and estimates of relative abundance for each site were based on surveys conducted during May–September, which falls within the peak flight seasons of all Hetaerina species in the geographical region sampled (Gonzalez Soriano 1996; Abbott 2005). Each survey covered 200–500 m of suitable riparian habitat and was carried out under weather conditions favourable to damselfly territorial and mating behaviour (full sun or lightly clouded; air temperature in the shade greater than 21°C). During surveys, we caught damselflies using aerial nets, identified them to species based on clasper morphology (Garrison 1990) and photographed the right fore- and hindwings of mature males using a digital camera (Canon 10D) equipped with a 100 mm macro lens and a macro flash attachment (Canon MT-24EX). Total wing area and the areas covered with red and black pigment were measured manually on a computer screen using NIH Image. Wing spot areas were divided by wing area prior to analysis. Each wing measurement was assigned a Julian date corresponding to the first day of the corresponding survey.
A subset of sites were selected for detailed behavioural investigation reported elsewhere (Anderson & Grether 2010) and the relative abundance of each Hetaerina species from these surveys may be estimated robustly. Other sites included in the current dataset were visited on a single day and thus our estimates of species abundance from these surveys are necessarily less precise. To assess the reliability of these single-day surveys, we examined our records from the first day of surveys carried out at the more intensively studied sites. The rank order of relative species abundance was reliably determined on the first day, but it took several days to obtain reliable quantitative estimates of relative abundance. At sites that we visited repeatedly, the rank order of relative abundance did not change during the peak flight season (May–September). Therefore, for the analyses presented here, we use a binary measure of relative abundance, classifying one species at a site as ‘most common’ and the others as ‘less common’ during the peak flight season.
The effects of the relative abundance of heterospecifics on H. titia wing morphology were investigated in separate models with H. americana or H. occisa designated as the species of heterospecific. Furthermore, we used all of the H. titia sites by testing a model that allowed either H. americana or H. occisa to serve as a heterospecific and we pooled the relative abundances if both were present. At every site composed of all three species, we were able to unambiguously define a single species as most common, regardless of whether the relative abundance of heterospecifics was pooled or not.
(c). Data analysis
We constructed random-effects generalized least-squares regression models separately for each species and dependent variable, with site entered as a clustered variable (xtreg in STATA 10.0; StataCorp, College Station, TX, USA). The dependent variables were wing area, the relative area of red pigmentation or the relative area of black wing pigmentation (H. titia only). All models initially included the covariates Julian date, latitude and longitude. In cases where longitude and latitude were collinear, the longitude term was dropped. The effects of relative species abundance and sympatry versus allopatry were tested by including binary terms in the models. All final models had variance inflation factors less than five and thus did not suffer from multi-collinearity (Afifi et al. 2004). Normal probability plots showed the residuals to be approximately normal.
3. Results
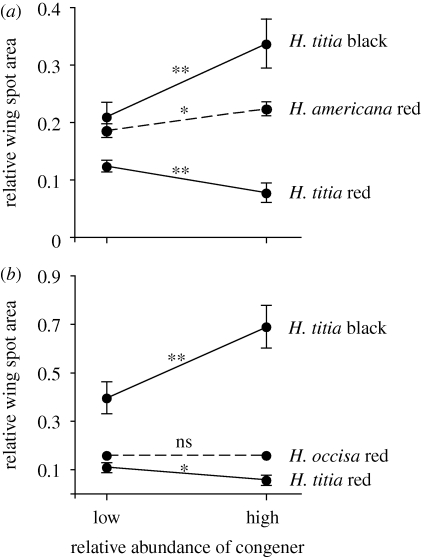
As predicted, H. titia had more black wing pigmentation, and less red wing pigmentation, at sites where H. americana or H. occisa was the most abundant species, compared with sites where H. titia was the most abundant species (table 1). These trends held and remained statistically significant when the analysis was restricted to the subset of sites where H. titia occurs with H. americana and relative abundance was based on just this species pair (figure 1 and table 1). The same was true when the analysis was restricted to the subset of sites where H. titia occurs with H. occisa, except that the effect of relative abundance on the red wing spots of H. titia was only marginally significant (table 1 and figure 1). None of these patterns can be attributed to body size variation because H. titia wing area was not correlated with the relative abundance of heterospecifics (table 1).
Table 1.
Evidence for character displacement in the wing coloration of H. titia. Analysis of geographical variation in (a) H. titia red pigmentation, (b) H. titia black pigmentation, and (c) H. titia wing area.
| Sympatric congener |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
H. americana |
H. occisa |
either species |
|||||||
| model term | B | z | p | B | z | p | B | z | p |
| (a) Sources of variation in H. titia red wing spot area | |||||||||
| relative abundance | −0.0461 | −2.80 | 0.005 | −0.0525 | −1.93 | 0.053 | −0.0257 | −2.07 | 0.039 |
| sampling date | 0.0006 | 17.84 | <0.001 | −0.0002 | −1.76 | 0.078 | 0.0006 | 20.66 | <0.001 |
| latitude | −0.0030 | −1.53 | 0.127 | 0.0146 | 1.03 | 0.303 | −0.0056 | −3.79 | <0.001 |
| longitude | −0.0089 | −3.59 | <0.001 | — | — | — | −0.0067 | −3.50 | <0.001 |
| R2 | 0.696 | 0.268 | 0.7292 | ||||||
| individuals, sites | 632, 13 | 619, 10 | 1000, 18 | ||||||
| (b) H. titia black wing spot area | |||||||||
| relative abundance | 0.1273 | 3.08 | 0.002 | 0.2929 | 2.70 | 0.007 | 0.0904 | 4.28 | <0.001 |
| sampling date | 0.0028 | 28.20 | <0.001 | 0.0039 | 3.17 | 0.002 | 0.0028 | 17.84 | <0.001 |
| latitude | −0.0102 | −2.09 | 0.036 | −0.1506 | −2.53 | 0.011 | −0.0107 | −6.60 | <0.001 |
| longitude | 0.0468 | 7.59 | <0.001 | — | — | 0.0495 | 41.79 | <0.001 | |
| R2 | 0.7609 | 0.1622 | 0.6691 | ||||||
| individuals, sites | 630, 13 | 618, 10 | 998, 18 | ||||||
| (c) H. titia wing area | |||||||||
| relative abundance | 15.6582 | 1.82 | 0.069 | 14.5040 | 0.82 | 0.409 | 7.4818 | 0.81 | 0.418 |
| sampling date | 0.2313 | 9.25 | <0.001 | 0.0246 | 0.15 | 0.878 | 0.2245 | 8.55 | <0.001 |
| latitude | −1.0851 | −1.10 | 0.273 | −4.0119 | −0.43 | 0.667 | −0.0475 | −0.04 | 0.965 |
| longitude | 5.3042 | 4.24 | <0.001 | — | — | 4.4142 | 3.16 | 0.002 | |
| R2 | 0.367 | 0.1224 | 0.5121 | ||||||
| individuals, sites | 634, 13 | 622, 10 | 1004, 18 | ||||||
Figure 1.
Character displacement patterns in Hetaerina wing coloration. (a) Character shifts in zones of sympatry between H. americana and H. titia; (b) character shifts in zones of sympatry between H. occisa and H. titia. LS means ± s.e. from the regression models of tables 1–3 are shown. *p < 0.05, **p < 0.01.
Allopatry versus sympatry comparisons revealed no evidence for character displacement in the wing coloration of H. americana or H. occisa (table 2). The only evidence for a sympatry effect is that H. americana had significantly larger wings where H. titia was present, compared with sites where H. titia was absent (electronic supplementary material, figure S1). Nevertheless, when the analysis was restricted to sympatric sites, a character displacement pattern emerged in the wing coloration of H. americana (figure 1). The red wing spots of H. americana were significantly larger at sites where H. titia was the most abundant species (p = 0.024; table 3). Hetaerina titia abundance was not a significant predictor of the size of the red wing spots on H. occisa or the wing areas of either species.
Table 2.
Analysis of geographical variation in H. americana and H. occisa red pigmentation and wing area from sites sympatric with H. titia and sites allopatric with H. titia.
| focal species |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
H. americana |
H. occisa |
|||||||||||
| red spot area |
wing area |
red spot area |
wing area |
|||||||||
| model term | B | z | p | B | z | p | B | z | p | B | z | p |
| sympatry w/ H.titia | 0.0125 | 1.08 | 0.282 | 13.1135 | 2.26 | 0.024 | 0.0021 | 1.16 | 0.246 | 1.3630 | 0.21 | 0.83 |
| sampling date | 0.0012 | 41.82 | <0.001 | 0.1423 | 5.49 | <0.001 | 0.0004 | 10.30 | <0.001 | 0.0833 | 1.30 | 0.194 |
| latitude | 0.0023 | 1.81 | 0.07 | 3.3952 | 5.56 | <0.001 | −0.0085 | −3.71 | <0.001 | 0.6568 | 0.13 | 0.897 |
| longitude | 0.0046 | 3.60 | <0.001 | 3.2608 | 5.41 | <0.001 | −0.0021 | −4.50 | <0.001 | −1.2361 | −0.96 | 0.339 |
| R2 | 0.7258 | 0.6139 | 0.3287 | 0.0541 | ||||||||
| individuals, sites | 874, 28 | 877,28 | 414,25 | 414,25 | ||||||||
Table 3.
Analysis of geographical variation in H. americana and H. occisa red pigmentation and wing area from sites sympatric with H. titia.
| focal species |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
H. americana |
H. occisa |
|||||||||||
| red spot area |
wing area |
red spot area |
wing area |
|||||||||
| model term | B | z | p | B | Z | p | B | z | p | B | z | p |
| relative abundance | 0.0378 | 2.26 | 0.024 | −6.7861 | −1.26 | 0.208 | −0.0003 | −0.06 | 0.954 | 7.3138 | 1.05 | 0.294 |
| sampling date | 0.0012 | 38.74 | <0.001 | 0.1316 | 5.28 | <0.001 | 0.0005 | 5.68 | <0.001 | 0.1320 | 1.51 | 0.132 |
| latitude | 0.0043 | 2.38 | 0.017 | 5.1090 | 9.16 | <0.001 | −0.0108 | −3.04 | 0.002 | 1.7674 | 0.42 | 0.674 |
| longitude | 0.0029 | 1.01 | 0.314 | −0.8022 | −0.81 | 0.417 | −0.0022 | −2.65 | 0.008 | −1.1850 | −1.06 | 0.289 |
| R2 | 0.6818 | 0.5671 | 0.3612 | 0.2113 | ||||||||
| individuals, sites | 523, 13 | 524,13 | 213,10 | 213, 10 | ||||||||
Independent of the presence/absence or relative abundance of heterospecifics, most wing characters showed significant clinal and/or temporal variation (tables 1–3; electronic supplementary material, table S2). The proportion of the wing covered with pigment increased with Julian date in all four species, including H. cruentata. Wing area also increased with Julian date in H. americana and H. titia, but not in the other two species.
4. Discussion
We tested for character displacement patterns in the wing coloration of the damselfly H. titia in relation to two of its sympatric congeners, H. americana and H. occisa, and vice versa. Hetaerina titia is a good candidate for character displacement because its black and red wing coloration is highly variable and some variants resemble the sympatric congeners, which only have red wing coloration, more closely than others. Our dataset for testing for character shifts in H. titia consisted of morphological measurements and estimates of relative species abundance from 18 sites where H. titia occurs with one or both congeners. We predicted that H. titia would shift towards having more black wing coloration and less red wing coloration at sites dominated by the congeners, in comparison to sites where H. titia is numerically dominant. Both predictions were upheld (figure 1). When the same approach was used to test for character shifts in the congeners in relation to the abundance of H. titia, H. americana showed the predicted character shift, i.e. larger red wing spots where H. titia is numerically dominant, but H. occisa did not (figure 1). Classic sympatry versus allopatry comparisons did not detect effects of the presence of H. titia on the wing coloration of either congener. Thus, we obtained consistent evidence for character displacement patterns in H. titia, no such evidence in H. occisa and mixed evidence in H. americana.
While it is not possible to robustly infer evolutionary mechanisms from geographical patterns, we have independent evidence for agonistic character displacement in this system. In simulated territory intrusion tests, putting black ink on the hindwings of H. americana or H. occisa intruders reduced the rate of mid-air attacks by territory holders of the same species. Furthermore, intruders with fully black hindwings were attacked less often than intruders with half-black hindwings. These graded responses to black wing coloration were found only at sites where H. titia is present, which suggests that they evolved in response to aggressive interference with H. titia (Anderson & Grether 2010). Thus, the observed shifts in the black wing coloration of H. titia are a predicted consequence of selection imposed by its congeners in an aggressive context. The effects of red wing spot size on heterospecific aggression have not been investigated experimentally, but the observed geographical shifts in the red wing spots of H. titia and H. americana are in the directions predicted by the agonistic character displacement hypothesis. It is possible that reproductive interference (i.e. reproductive character displacement) has also contributed to these geographical patterns, but we are not aware of any evidence that male coloration influences female choice/resistance in Hetaerina.
It is not clear why we did not also observe a character displacement pattern in H. occisa, but there are several possible reasons. Selection on H. occisa might be weak because this species is behaviourally dominant to H. titia, H. occisa wing spot size might be insufficiently heritable to respond to selection or gene flow might have obscured past responses to selection. We have no basis, as yet, for distinguishing among these alternatives. In general, there many possible reasons why character displacement patterns might not be observed (Goldberg & Lande 2006).
Common garden experiments will be required to firmly establish whether the observed geographical shifts in wing coloration shifts reflect genetic differentiation. Hetaerina wing coloration does not become developmentally fixed until about two weeks post-emergence (Grether 1996), so it is conceivable that the competitive environment experienced by males as larvae or as maturing adults influences wing spot development. Upon repeated visits to sites in the peak flight season, we consistently classified the same species as most common. We do not know, however, whether present-day measures of species' abundance reflect the relative abundance over evolutionary time. If population densities and relative abundance have varied over evolutionary time, this seems likely to promote a role for plasticity in generating this character displacement pattern (reviewed in Pfennig & Pfennig 2009). If the geographical shifts in wing coloration that we have documented here reflect a plastic response to the presence of other Hetaerina species, as opposed to genetic divergence in mean trait values, this could be an example of facultative character displacement (Pfennig & Murphy 2002). Demonstrating facultative character displacement would require showing that plasticity itself is displaced among populations. A final possibility is that a reaction norm common to all populations mediates the shift to the presence of the other species.
Ours is the second study to document a character displacement pattern in damselfly fighting colours. Tynkkynen et al. (2004) showed that the average size of the wing spots on male Calopteryx splendens decreased as the relative abundance of Calopteryx virgo increased across 23 sites in Finland. Interspecific territorial aggression (i.e. agonistic character displacement) was implicated as the mechanism responsible for this character displacement pattern because competitively dominant C. virgo males are most aggressive to C. splendens males with large wing spots (Tynkkynen et al. 2004, 2006). Selection against hybridization (i.e. reproductive character displacement) is a plausible alternative mechanism, however, because these species are known to hybridize and female C. splendens have been reported to choose mates on the basis of wing coloration (Siva-Jothy 1999; Cordoba-Aguilar 2002; Svensson et al. 2006, 2007).
We also found evidence for temporal and clinal variation in Hetaerina wing pigmentation, independent of the abundance of heterospecifics. The relationships between wing measurements and latitude/longitude were species specific, but relative wing spot sizes increased with Julian date in all four Hetaerina species investigated (tables 1–3; electronic supplementary material, table S2). This corroborates the work of Cordoba-Aguilar et al. (2009a), who also reported seasonal variation in spot expression of H. americana, and extends the finding to other Hetaerina species. In our study, the relationship between wing spot size and Julian date was approximately linear, but perhaps only because our samples were collected between late spring and late summer. Cordoba-Aguilar et al. (2009a) sampled throughout the year and found a peak in wing spot size in late summer, with smaller wing spots in spring and fall samples. Julian date probably correlates with, and therefore summarizes the influence of, multiple proximate environmental influences on wing spot size, such as temperature and the duration of larval development.
Our results suggest that, in the absence of interspecific aggression, the wing coloration of H. titia would be more similar to that of its congeners. An intriguing, albeit speculative, hypothesis is that melanin wing pigmentation was an evolutionary innovation that enabled H. titia to expand its range into regions where other Hetaerina species were already well established. This species has one of the broadest ranges of any Hetaerina spp. and coexists in microsympatry with several different congeners. Moreover, a territory mapping study revealed that H. titia territories were overdispersed in relation to the territories of sympatric congeners, while the reverse was observed in other species pairs (C. N. Anderson & G. F. Grether 2010, unpublished data). Although melanin wing pigmentation has been suggested to serve intraspecific, intrasexual signalling functions in H. titia (Cordoba-Aguilar et al. 2007), the intraspecific functions could have evolved secondarily. In any case, our inference that the observed wing colour shifts in H. titia are products of interspecific aggression does not depend on the evolutionary origins of melanin wing pigmentation.
Interest in the role of interspecific aggression in character displacement is rapidly growing (e.g. Adams 2004; Seehausen & Schluter 2004; Albert et al. 2007; reviewed in Grether et al. 2009). Many of these studies, like the one reported here, report patterns that are consistent with the process of agonistic character displacement. We have documented evidence for the process of agonistic character displacement in the Hetaerina system, by experimentally demonstrating the direct role that wing coloration plays in territorial responses to conspecific and heterospecific intruders (Anderson & Grether 2010). Future work ought to investigate the fitness consequences (e.g. reduced survival or lifetime reproductive success) of interspecific aggression (e.g. Tynkkynen et al. 2005). Furthermore, ruling out alternative processes that may generate similar patterns is a crucial direction for future research.
Acknowledgements
We thank Simon Alarcon, Gaby Besne, Veronica Campos, Jasmine Loveland, Eliot Miller, Erik Peñaloza and Nick Taft for assistance with fieldwork. John Abbott, Alex Córdoba-Aguilar, Enrique González-Soriano, Rodolfo Novelo Gutiérrez and Tony Gallucci provided support and advice for the identification of field sites. Enrique Gonzalez Soriano kindly granted us permission to collect Hetaerina in Mexico under his general collection permit from the Secretary of the Environment and Natural Resources. We also thank the Statistical Consulting group in the UCLA Academic Technology Services for statistical advice. We thank Howard Rundle, David Pfennig and one anonymous reviewer for helpful comments on the manuscript. This work was supported by grants from UCMEXUS and UCLA Council on Research (G.F.G.), the Edward W. Pauley Fellowship, an NSF Graduate Research Fellowship and a UCMEXUS dissertation improvement grant (C.N.A.).
References
- Abbott J. C.2005. Odonata central. See http://odonatacentral.bfl.utexas.edu/
- Adams D. C.2004Character displacement via aggressive interference in Appalachian salamanders. Ecology 85, 2664–2670 (doi:10.1890/04-0648) [Google Scholar]
- Afifi A. A., Clark V., May S.2004Computer-Aided Multivariate Analysis, 4th edn.Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- Albert A. Y. K., Millar N. P., Schluter D.2007Character displacement of male nuptial colour in threespine sticklebacks (Gasterosteus aculeatus). Biol. J. Linn. Soc. 91, 37–48 (doi:10.1111/j.1095-8312.2007.00777.x) [Google Scholar]
- Alcock J.1987The effects of experimental manipulation of resources on the behavior of two calopterygid damselflies that exhibit resource-defense polygyny. Can. J. Zool.-Rev. Canad. De Zool. 65, 2475–2482 (doi:10.1139/z87-374) [Google Scholar]
- Anderson C. N., Grether G. F.2010Interspecific aggression and character displacement of competitor recognition in Hetaerina damselflies. Proc. R. Soc. B 277, 549–555 (doi:10.1098/rspb.2009.1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund A., Bisazza A., Pilastro A.1996Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399 (doi:10.1006/bijl.1996.0043) [Google Scholar]
- Corbet P. S.1999Dragonflies: behavior and ecology of Odonata. Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- Cordoba-Aguilar A.2002Wing pigmentation in territorial male damselflies, Calopteryx haemorrhoidalis: a possible relation to sexual selection. Anim. Behav. 63, 759–766 (doi:10.1006/anbe.2001.1974) [Google Scholar]
- Cordoba-Aguilar A., Lesher-Trevino A. C., Anderson C. N.2007Sexual selection in Hetaerina titia males: a possible key species to understand the evolution of pigmentation in calopterygid damselflies (Odonata: Zygoptera). Behaviour 144, 931–952 (doi:10.1163/156853907781492672) [Google Scholar]
- Cordoba-Aguilar A., Jimenez-Cortes J. G., Lanz-Mendoza H.2009aSeasonal variation in ornament expression, body size, energetic reserves, immune response, and survival in males of a territorial insect. Ecol. Entomol. 34, 228–239 (doi:10.1111/j.1365-2311.2008.01061.x) [Google Scholar]
- Cordoba-Aguilar A., Raihani G., Serrano-Meneses M. A., Contreras-Garduno J.2009bThe lek mating system of Hetaerina damselflies (Insecta: Calopterygidae). Behaviour 146, 189–207 (doi:10.1163/156853909X410739) [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation. Sunderland, MA: Sinauer Associates [Google Scholar]
- Dobzhansky T.1937Genetics and the origin of species. New York, NY: Columbia University Press [Google Scholar]
- Endler J. A.1986Natural selection in the wild. Monographs in Population Biology, no. 21, 336p New York, NY: Princeton University Press [Google Scholar]
- Fisher H. S., Rosenthal G. G.2010Relative abundance of Xiphophorus fishes and its effect on sexual communication. Ethology 116, 32–38 (doi:10.1111/j.1439-0310.2009.01710.x) [Google Scholar]
- Garrison R. W.1990A synopsis of the genus Hetaerina with descriptions of 4 new species (Odonata, Calopterygidae). Trans. Am. Entomol. Soc. 116, 175–259 [Google Scholar]
- Goldberg E. E., Lande R.2006Ecological and reproductive character displacement on an environmental gradient. Evolution 60, 1344–1357 (doi:10.1554/05-696.1) [PubMed] [Google Scholar]
- Gonzalez Soriano E.1996Odonata. In Biodiversidad, taxonomia y biogeografia de artropodos de Mexico: hacia una sintesis de su conocimiento, vol. 1 (eds Llorente Bousquets J. E., García Aldrete A. N., González Soriano E.), pp. 148–167 México, DF: Universidad Nacional Autónoma de México [Google Scholar]
- Grant P. R.1972Convergent and divergent character displacement. Biol. J. Linn. Soc. 4, 39–68 (doi:10.1111/j.1095-8312.1972.tb00690.x) [Google Scholar]
- Grether G. F.1995Natural and sexual selection on wing coloration in the rubyspot damselfly in Hetaerina americana. PhD dissertation, University of California, Davis; [DOI] [PubMed] [Google Scholar]
- Grether G. F.1996Intrasexual competition alone favors a sexually dimorphic ornament in the rubyspot damselfly Hetaerina americana. Evolution 50, 1949–1957 (doi:10.2307/2410753) [DOI] [PubMed] [Google Scholar]
- Grether G. F., Grey R. M.1996Novel cost of a sexually selected trait in the rubyspot damselfly Hetaerina americana: conspicuousness to prey. Behav. Ecol. 7, 465–473 (doi:10.1093/beheco/7.4.465) [Google Scholar]
- Grether G. F., Switzer P. V.2000Mechanisms for the formation and maintenance of traditional night roost aggregations in a territorial damselfly. Anim. Behav. 60, 569–579 (doi:10.1006/anbe.2000.1511) [DOI] [PubMed] [Google Scholar]
- Grether G. F., Losin N., Anderson C. N., Okamoto K.2009The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 84, 617–635 (doi:10.1111/j.1469-185X.2009.00089.x) [DOI] [PubMed] [Google Scholar]
- Kirschel A. N. G., Blumstein D. T., Smith T. B.2009Character displacement of song and morphology in African tinkerbirds. Proc. Natl Acad. Sci. USA 106, 8256–8261 (doi:10.1073/pnas.0810124106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon A. R., Smadja C., Kirkpatrick M.2004Reproductive character displacement is not the only possible outcome of reinforcement. J. Evol. Biol. 17, 177–183 (doi:10.1046/j.1420-9101.2003.00643.x) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Murphy P. J.2002How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution 56, 1217–1228 (doi:10.1554/0014-3820.2002.056[1217:HFCAPP]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Pfennig K. S., Pfennig D. W.2009Character displacement: ecological and reproductive responses to a common evolutionary problem. Q. Rev. Biol. 84, 253–276 (doi:10.1086/605079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas L. R.1964A reinterpretation of the concepts sympatric and allopatric with proposal of the additional terms syntopic and allotopic. Syst. Zool. 13, 42–43 (doi:10.2307/2411436) [Google Scholar]
- Schluter D.2000Ecological character displacement in adaptive radiation. Am. Nat 156, S4–S16 (doi:10.1086/303412) [Google Scholar]
- Seehausen O., Schluter D.2004Male–male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc. R. Soc. Lond. B 271, 1345–1353 (doi:10.1098/rspb.2004.2737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva-Jothy M. T.1999Male wing pigmentation may affect reproductive success via female choice in a calopterygid damselfly (Zygoptera). Behaviour 136, 1365–1377 (doi:10.1163/156853999500776) [Google Scholar]
- Suhonen J., Rantala M. J., Honkavaara J.2008Territoriality in odonates. In Dragonflies: model organisms for ecological and evolutionary research, ch. 16 (ed. Cordoba-Aguilar A.), pp. 203–217 Oxford, UK: Oxford University Press [Google Scholar]
- Svensson E. I., Eroukhmanoff F., Friberg M.2006Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution 60, 1242–1253 (doi:10.1554/06-036.1) [PubMed] [Google Scholar]
- Svensson E. I., Karlsson K., Friberg M., Eroukhmanoff F.2007Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 17, 1943–1947 (doi:10.1016/j.cub.2007.09.038) [DOI] [PubMed] [Google Scholar]
- Tynkkynen K., Rantala M. J., Suhonen J.2004Interspecific aggression and character displacement in the damselfly Calopteryx splendens. J. Evol. Biol. 17, 759–767 (doi:10.1111/j.1420-9101.2004.00733.x) [DOI] [PubMed] [Google Scholar]
- Tynkkynen K., Kotiaho J. S., Luojumäki M., Suhonen J.2005Interspecific aggression causes negative selection on sexual characters. Evolution 59, 1838–1843 (doi:10.1554/04-716.1) [PubMed] [Google Scholar]
- Tynkkynen K., Kotiaho J. S., Luojumaki M., Suhonen J.2006Interspecific territoriality in Calopteryx damselflies: the role of secondary sexual characters. Anim. Behav. 71, 299–306 (doi:10.1016/j.anbehav.2005.03.042) [Google Scholar]
- Waage J. K.1979Reproductive character displacement in Calopteryx (Odonata, Calopterygidae). Evolution 33, 104–116 (doi:10.2307/2407369) [DOI] [PubMed] [Google Scholar]
- Weichsel J. I.1987The life history and behavior of Hetaerina americana (Fabricus) (Odonata: Calopterygidae). PhD dissertation, University of Michigan, Ann Arbor [Google Scholar]