Abstract
Anthropogenic disturbances such as fishing, mining, oil drilling, bioprospecting, warming, and acidification in the deep sea are increasing, yet generalities about deep-sea biogeography remain elusive. Owing to the lack of perceived environmental variability and geographical barriers, ranges of deep-sea species were traditionally assumed to be exceedingly large. In contrast, seamount and chemosynthetic habitats with reported high endemicity challenge the broad applicability of a single biogeographic paradigm for the deep sea. New research benefiting from higher resolution sampling, molecular methods and public databases can now more rigorously examine dispersal distances and species ranges on the vast ocean floor. Here, we explore the major outstanding questions in deep-sea biogeography. Based on current evidence, many taxa appear broadly distributed across the deep sea, a pattern replicated in both the abyssal plains and specialized environments such as hydrothermal vents. Cold waters may slow larval metabolism and development augmenting the great intrinsic ability for dispersal among many deep-sea species. Currents, environmental shifts, and topography can prove to be dispersal barriers but are often semipermeable. Evidence of historical events such as points of faunal origin and climatic fluctuations are also evident in contemporary biogeographic ranges. Continued synthetic analysis, database construction, theoretical advancement and field sampling will be required to further refine hypotheses regarding deep-sea biogeography.
Keywords: biogeography, deep sea, dispersal, range, vicariance, establishment
1. Introduction
In the last 130 years, our view of the deep ocean floor that extends beyond the continental shelves (less than 200 m depth) radically changed (reviewed in Koslow (2007)). The deep sea was once considered a dark, homogeneous wasteland, unchanging through time and devoid of biodiversity. Exploration of this vast ecosystem continues to reveal a multitude of novel, high-productivity habitats, including chemosynthetic environments (Sibuet & Olu 1998; Van Dover 2000) and large organic food falls (reviewed in Smith & Baco-Taylor (2003)). Intricate canyons, expansive ridge systems and submerged mountains, as well as distinct suboxic environments provide habitat complexity (e.g. Wilson & Kaufmann 1987; Vetter & Dayton 1998; Van Dover 2000; Levin 2003; Cordes et al. 2009). Even the expansive mud bottom—the backdrop for this variation—experiences episodic storms and currents, patchy organic input and disturbance regimes linked to surface ocean processes (e.g. Tyler 1988; Gooday 2002).
Our perceptions of the deep sea as a ‘biological desert’ were transformed by findings of spectacularly high biodiversity (Sanders 1968; Grassle 1989) and remarkable in situ evolutionary radiations (Little & Vrijenhoek 2003). These findings stimulated much discussion regarding the maintenance of diversity in this food-poor habitat, leading to the idea that micro-habitat structure may create niche diversity and enhance species turnover on local scales (reviewed in Snelgrove & Smith (2002a)). High species turnover on the deep-sea floor, estimated at 45–80% over hundreds to thousands of kilometres (Grassle & Maciolek 1992; Glover et al. 2002; Brandt et al. 2005), is often cited as evidence of restricted species ranges. However, these turnover rates are similar to other systems (vascular plants Nekola & White (1999), e.g. intertidal invertebrates Nakaoka et al. (2006)). Moreover, high turnover does not necessarily preclude extensive geographical ranges if species are patchily distributed within those ranges.
These more recent claims of restricted ranges thus represent a radical departure from earlier studies, which suggested that the lack of perceived environmental variability and geographical barriers to dispersal would lead to exceedingly large ranges of deep-sea species. The biogeography of deep-sea organisms has historically been a black box characterized more by inference than data—unsurprising given the challenges of sampling this remote environment. However, modern research techniques, higher resolution sampling efforts and expansion of public databases now allow for a re-examination of existing hypotheses. In this review, we examine major outstanding questions concerning the biogeography of deep-sea invertebrates on the seafloor (figure 1). We ask: (i) do deep-sea species exhibit broad geographical distributions? (ii) how has the history of the deep sea through the Phanaerozoic shaped biogeography on the seafloor? (iii) what contemporary processes constrain ranges in the deep sea? In considering these questions, we strive to construct a new framework reflecting modern scientific developments, and identify future research needs.
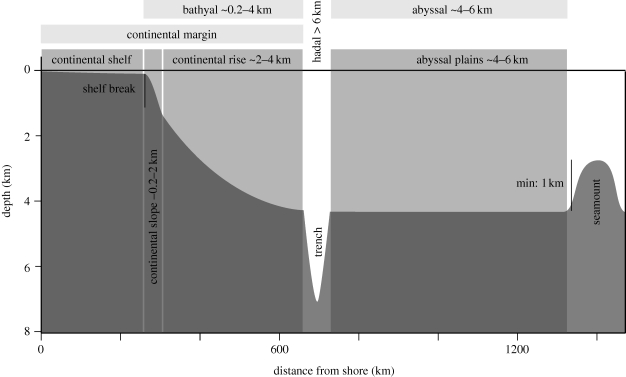
Figure 1.
Profile of typical continental margin and deep-sea system. Major regions are labelled. Adapted from Gage & Tyler (Gage & Tyler 1991).
(a). Species distributions in the contemporary deep sea
Severe undersampling of the deep sea, estimated to be much less than 1 per cent of the total seafloor McClain (2007), prevents full characterization of species ranges. For many species, we can only conservatively estimate the maximum linear range extent with any accuracy. However, the use of singular linear dimensions to characterize range is not without precedent as terrestrial and shallow-water biogeographers often employ these metrics to convey useful information, e.g. Rapoport's Rule (e.g. Fortes & Absalao (2004)). Based on such observations we can thus begin to describe some general patterns in species distributions.
Studies employing morphospecies as basic taxonomic units largely support earlier theories of cosmopolitanism in many groups inhabiting the soft-bottom seafloor, notably nematodes, foraminifera, mollusca and echinodermata (e.g. Madsen 1961; Sibuet 1979; Tyler 1980; Rex 2002; Gooday et al. 2004; Vanreusel et al. 2010). For example, approximately 25 per cent of known bivalve mollusc species occur in multiple ocean basins, most with ranges covering more than 20° latitude (Allen (2008), C. R. McClain & T. G. Gullett 2009, unpublished data). Many elasipod holothurians also appear widely distributed on the abyssal plains, with only a few bathyal taxa confined to a single region (Hansen 1975; Young et al. 1997). Patterns in amphipod crustaceans are somewhat unclear, but several widely distributed abyssal species are known, and many others possess at least basin-wide distributions (Barnard 1961; Thurston 1990).
While chemosynthetic habitats such as hydrothermal vents, cold methane seeps and large food falls share many common taxa (reviewed in Smith et al. (2003)), regional and/or global distribution of this specialized fauna remains poorly resolved. At the generic level, several distinct groups appear broadly distributed, including Osedax, a group of bone-eating worms Glover et al. (2005), the wood-boring bivalve genus Xylophaga Voight (2009) and numerous vent and seep taxa (Van Dover 2000; Cordes et al. 2007). Even geographically isolated western Pacific vents share 50 per cent of genera with eastern sites (Hessler & Lonsdale 1991). However, at the species level, seep species may be restricted to individual or adjacent sites Sibuet & Olu (1998), whereas vent communities share 10–15% of species between regions or basins Desbruyeres et al. (2006), and are often broadly distributed within continuous ridge systems (e.g. Hessler & Lonsdale 1991; Tunnicliffe & Fowler 1996).
Like chemosynthetic systems, seamounts may represent island-like habitats potentially isolated by hydrography or sheer distance, leading to predictions of high endemicity (McClain 2007; Brewin et al. 2009; Clark et al. 2010). Early studies reported that up to 36 per cent of taxa on seamounts were endemic, albeit with the caveat of severe undersampling of the deep sea (reviewed in Wilson & Kaufmann 1987; Rogers 1994; McClain 2007). Accumulating species-level data now indicate that seamount communities are comprised of non-endemic species drawn from a regional species pool, often with broad geographical distributions of hundreds to thousands of kilometres (Brewin et al. (2009), reviewed in Samadi et al. 2006; O'Hara 2007; O'Hara et al. 2008; Thoma et al. 2009; Clark et al. 2010; Howell et al. 2010). For example, 79 per cent of the species on a well-studied California seamount possess ranges greater than 1000 km (McClain et al. 2009a). Thus, early hypotheses of large deep-sea species ranges appear to hold true for seamount fauna.
More recent biogeographic studies employing genetic tools are helping to unravel taxonomic problems that can complicate range-size determinations. These methods have identified cryptic species complexes misdiagnosed as single morphospecies, as well as distinctive morphotypes or life-history stages of a single species given synonymous species names (Raupach et al. 2007; Vrijenhoek 2008). Molecular studies also indicate cosmopolitanism in many groups, revealing surprising genetic homogeneity over large distances in vent (Won et al. 2003; Hurtado et al. 2004; Johnson et al. 2006), seamount (Bucklin et al. 1987; Smith et al. 2004; Jones et al. 2006; Samadi et al. 2006) and abyssal plain Bisol et al. (1984) populations separated by thousands of kilometres. Genetic analyses verified reportedly widespread distributions of a common polychaete worm throughout the abyssal Pacific, Atlantic and Southern Oceans Mincks et al. (2009), and in three abyssal foraminifera species found in the Arctic and Antarctic, separated by 17 000 km (Pawlowski et al. 2007).
In contrast to this evidence of extensive horizontal gene flow, multiple soft-bottom taxa show significant genetic differentiation along bathymetric gradients of only a few kilometres (France 1994; France & Kocher 1996; Howell et al. 2004; Zardus et al. 2006). Thus, biogeographic patterns are likely to vary considerably between the abyssal seafloor and the continental slopes, which experience considerable heterogeneity in carbon flux, topography, sediment type and current regime (Carney 2005; Zardus et al. 2006; Levin & Dayton 2009). Indeed, earlier studies suggested geographical ranges may increase (Allen & Sanders 1996; Wilson & Kaufmann 1987) with depth as environmental conditions become more homogeneous. More recent molecular work supports this idea, demonstrating genetic homogeneity among abyssal populations and restricted gene flow along depth gradients in Atlantic molluscs (Etter et al. 2005; Zardus et al. 2006).
A preponderance of evidence now suggests that deep-sea genera, and often species, are broadly distributed, regardless of habitat type. Interestingly, patchy habitats like seamounts and vents do not appear to differ from the surrounding abyssal plains in this respect. Seep species may prove an exception to the rule, but more comprehensive sampling will be required. In contrast, bathymetric gradients may pose significant constraints on species ranges relative to horizontal distances, indicating broad geographical distributions may be more frequent at greater depths.
(b). Processes governing species distributions in the deep sea
Variation in range size results from a complex interaction of physical, biological and historical factors (Brown et al. 1996; Holt 2003; Holt & Keitt 2005; Pineda et al. 2009). Taxon-specific physiological adaptations and life-history strategies arise under historical selection pressures, and govern the intrinsic ability to move between habitat patches. Extrinsic factors in turn impact propagule survival during dispersal, provide physical barriers to movement and influence successful colonization of new patches. Overlain on these processes are species-specific niche requirements within the environmental landscape combined with biological interactions permitting populations to persist (Holt & Keitt 2005; Soberon 2007; Soberon & Nakumura 2009). Here, we examine the contribution of historical contingencies, intrinsic dispersal abilities and extrinsic factors that interact with both dispersal and the adult niche to determine geographical distributions of deep-sea species.
(c). History of the deep-sea environment and fauna
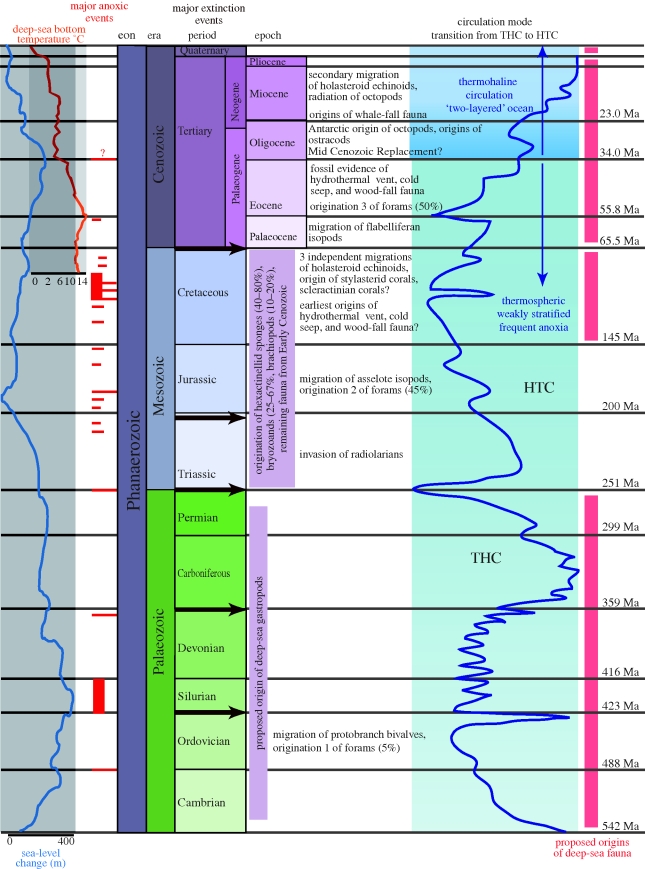
Extreme variability in temperature, oxygen and circulation characterize the deep-sea palaeoceanographic history (figure 2). Since the Eocene/Palaeocene boundary (approx. 55 Ma), deep water has cooled approximately 15°C, following minor warming in the Late Cretaceous Waelbroeck et al. (2001), and a similar cool period at the Eocene/Oligocene boundary (approx. 34 Ma). Deep-ocean circulation has alternated between two historical ocean types one driven by high-latitude deep-water formation (thermohaline, THC), resulting in cold, oxygenated deep water, and one driven by salinity-induced stratification at low latitudes (halothermal, HTC) resulting in warm, saline deep water and reduced global circulation (Jeppsson 1990; Horne 1999; Rogers 2000). THC conditions have prevailed since the Eocene/Oligocene transition, but a warmer, low-oxygen HTC phase dated back to the Triassic. During this period, deep-water anoxia was frequent and widespread (Jacobs & Lindberg 1998; Rogers 2000; Waelbroeck et al. 2001), with the most severe events associated with rapid THC–HTC transitions in the mid-Cretaceous, and at the Permian/Triassic and Ordovician/Silurian boundaries (Horne 1999).
Figure 2.
The geological, biological, chemical and physical history of the deep sea. Figure shows from left to right sea-level change in metres (light blue line); deep-sea bottom temperate in degrees celsius (orange line); major anoxic events (dark red blocks, width denotes regional vs. global); major extinction events (grey arrows within timescale), major migrations of fauna into the deep (text); circulation mode (dark blue line) and proposed origins of deep-sea fauna (light red blocks). Data for figure come from (Menzies & Imbrie 1958; Clarke 1962; Bensen 1975; Allen 1978; Lipps & Hickman 1982; Jeppsson 1990; Jacobs & Lindberg 1998; Horne 1999; Wilson 1999; Rogers 2000; Waelbroeck et al. 2001; Little & Vrijenhoek 2003; Smith & Stockley 2005; Kiel & Goedert 2006; Linder et al. 2008; Strugnell et al. 2008).
Most current hypotheses for the origins of the deep-sea fauna centre on ‘extinction and replacement’—a near-complete extinction of deep-sea fauna triggered by catastrophic anoxic events, with subsequent invasion and radiation of shallow-water species into the deep (Rogers (2000), reviewed in Horne 1999; Wilson 1999). Thus, the deep-sea fauna may be relatively young, dating back to the last major anoxic event at the Eocene/Oligocene boundary (reviewed in Wilson (1999)). However, many clades appear to have survived these anoxic periods (Clarke 1962; Wilson 1999; Raupach et al. 2009). The specialized fauna of contemporary oxygen minimum zones (reviewed in Levin (2003)) suggests the potential for evolutionary adaptation (Wilson 1999). Anoxic events may have eliminated vulnerable taxa producing a more resistant deep-sea fauna through time evidence by hypoxia tolerances among deep-sea fauna (Levin 2003). Anoxia may have also encouraged speciation rather than extinction, with low-oxygen waters posing barriers to gene flow, and promoting allopatric speciation in localized well-oxygenated refugia (e.g. along particular isobaths or in isolated basins) (Wilson 1999; Rogers 2000).
Adaptation, filtering and refugia may explain the apparent lack of common origin point for contemporary deep-sea fauna, which appear to be comprised of clades originating throughout the Phanaerozoic, rather than a fauna of uniform age (demonstrated early by Menzies & Imbrie 1958; Wilson 1999; figure 2). For example, only approximately 5 per cent of the extant deep-sea foraminiferan genera date to the early Palaeozoic, while the rest are younger (Lipps & Hickman 1982). Asellote isopod crustaceans, abundant in the contemporary deep sea, date back to the Jurassic with evidence of impressive deep-sea radiations, whereas flabelliferan isopods have more recent Cenozoic origins (Wilson 1999; Raupach et al. 2004, 2007, 2009). Bivalve molluscs, early inhabitants of the deep sea, date to the late Ordovician but show evidence of more recent radiations (Allen 1978). In some groups, deep-sea invasions are reflected in onshore–offshore trends in fauna age, with older clades inhabiting bathyal depths and younger clades in the abyss (Menzies & Imbrie 1958; Jablonski et al. 1983; Jablonski & Bottjer 1991). Numerous contemporary taxa ranging from corals to echinoderms appear to have invaded the deep sea from shallower waters—some multiple times (Menzies & Imbrie 1958; Smith & Stockley 2005; Linder et al. 2008).
Molecular and fossil evidence places the origins of the hydrothermal vent fauna relatively recently (approx. 100 Ma; reviewed in Little & Vrijenhoek (2003)). The divergence of vesicomyid clams, found in all the chemosynthetic habitats, is coincident with those of large whales, leading some to posit whale-falls as evolutionary steps from shallow water to seeps and vents Baco et al. (1999), although more recent work suggests early Cenozoic whale-fall communities lacked vesicomyids and were compositionally more similar to wood-falls of the time (Kiel & Goedert 2006). Colonization of chemosynthetic habitats probably occurred in the order these habitats appeared: seeps to wood to whale (Shank et al. 1998; Kiel & Goedert 2006). These combined findings cast doubt on theories of a uniformly ‘young’ or ‘old’ deep-sea fauna of common origin.
With multiple points of origin and radiations, contemporary deep-sea clades bear unique biogeographic signatures. For example, the North Atlantic Ocean is dominated by asellote isopods originating in the Jurassic, whereas the South Atlantic contains more recent (Tertiary) flabelliferan isopods (Wilson 1999). Contemporary ranges reflect sites of invasion from the continental shelves, because physiological tolerance to conditions at these sites may dictate the routes by which invasions occurred, e.g. through an isothermal water column. Suggested invasion sites include the Antarctic continental shelf and the Mediterranean Ocean (reviewed in Gage & Tyler (1991)), but at times during the Phanaerozoic more isothermal conditions may have permitted multiple invasions at multiple sites (Wilson 1999). In particular, molluscs are tied to multiple origin points Clarke (1962) while many crustaceans are linked to Antarctic origns Wilson (1999), which may explain the differences often observed in the biogeography of the two groups (Rex et al. 2005; Brandt et al. 2007). Contemporary deep-sea biogeography thus includes many taxon-specific patterns requiring historical context, highlighting the potential pitfalls of extrapolating single-taxon studies to the global deep-sea fauna.
(d). Migration and larval dispersal
Shallow-water marine environments, once considered open systems with free exchange of planktonic larvae, have transitioned to a view that local recruitment and small-scale population genetic structure commonly occur despite a lack of obvious physical barriers (Cowen & Sponaugle 2009). These notions have yet to be revisited in the deep sea, where many key physiologically limiting variables such as light, oxygen, temperature and pressure are relatively constant over great distances. As we have shown, cosmopolitan distributions are common, yet many taxa show significant population structure, suggesting extrinsic factors may limit dispersal potential.
Most marine invertebrates are relatively non-motile as adults, but migrations do occur in response to episodic food inputs (Jones et al. 1998; Billett et al. 2001), implying rapid movement over large spatial scales is possible. Shallow-water echinoids move at rates of about 1–4 cm min−1 Domenici et al. (2003), indicating potential spatial scales for movement greater than 200 km (assuming lifespans of approx. 10 years; Ebert & Southon (2003)). Longer-lived deep-sea organisms may move even greater distances (Cailliet et al. 2001). However, significantly greater dispersal potential exists during the planktonic larval stage (Young et al. 1997).
The majority of marine invertebrates produce intermediate larval forms, although some diverse and particularly abundant deep-sea groups are direct-developing (e.g. nematodes, isopods). Larval forms vary widely in development time owing to nutritional mode (planktotrophic versus lecithotrophic), temperature and phylogenetic constraints. These variations in planktonic larval duration (PLD) are thought to translate into variations in dispersal distance (e.g. Kinlan & Gaines 2003; Bradbury et al. 2008; Shanks 2009). Larval type may thus provide some indication of a species' dispersal potential, but the larval modes of most deep-sea invertebrates are unknown, and a ‘typical’ deep-sea strategy does not appear to exist (cf. Tyler & Young 1999; Young 2004). Lecithotrophic larvae are commonly observed in abyssal echinoderms and polychaetes, and in molluscs from a variety of habitats, but planktotrophic and brooding taxa also occur in significant numbers. Peracarid crustaceans, one of the most speciose groups in the deep, are entirely brooders.
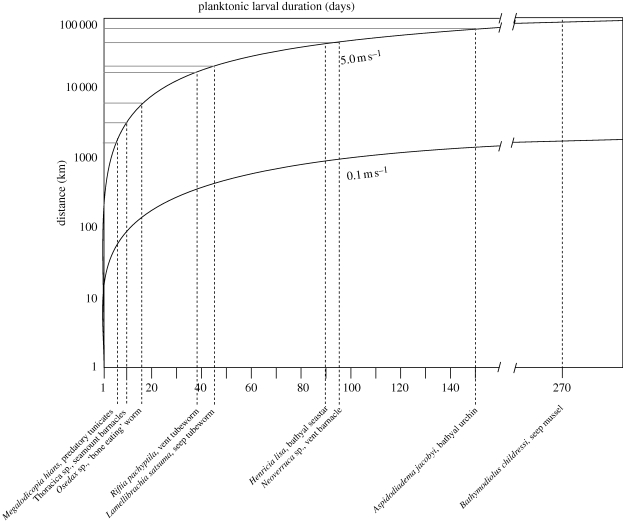
In the absence of general patterns in reproductive strategy, we compiled direct measurements of PLD for various deep-sea taxa in order to estimate the potential dispersal distances given typical deep-sea current speeds and ideal conditions (figure 3). If currents are both linear and constant (we concede this is an unrealistic assumption), dispersal distances on the order of 102–105 km could occur, suggesting amazing intrinsic potential for dispersal among deep-sea invertebrates and the overwhelming effect of extrinsic factors that ultimately limit range size. Although dispersal potential is often assumed to influence range size (Thorson 1950; Jablonski & Lutz 1983; Scheltema 1986), quantitative tests provide little empirical support for this relationship, in both shallow and deep seas (Emlet 1995; Young et al. 1997; Paulay & Meyer 2006; Lester et al. 2007).
Figure 3.
Potential larval dispersal distance of deep-sea fauna based on current speed, and published estimates of planktonic larval duration (Young & George 2000; Marsh et al. 2001; Watanabe et al. 2004; Havenhand et al. 2005; Buhl-Mortensen & Hoeg 2006; Miyake et al. 2006; Mercier & Hamel 2008; Arellano & Young 2009; Rouse et al. 2009). The two trajectories assume constant and unidirectional currents, and represent published estimates for lowest and highest velocity currents associated with surface waters or benthic storms (Havenhand et al. 2005). Note that currents demonstrating periodic changes in direction would greatly limit dispersal (Marsh et al. 2001).
In cold-water habitats like the deep sea and poles (e.g. Pearse & Lockhart (2004)), metabolic constraints may significantly lengthen PLD (Brown et al. 2004; Bradbury et al. 2008). O'Connor et al. (2007) clearly demonstrated this temperature dependence in larval development across multiple vertebrate and invertebrate taxa. Thus, deep-sea taxa may exhibit greater dispersal distances than their shallow-water counterparts (cf. Bradbury et al. (2008)). Temperature effects may in part explain the discrepancy between dispersal distance and range size.
Pressure may also lengthen larval development times in the deep sea (Somero 1998). Some echinoderm larvae exhibit broader pressure tolerances than adults of the same species (Tyler et al. 2000; Villalobos et al. 2006; Pradillon & Gaill 2007), although hydrothermal vent tubeworms exhibit very narrow pressure and temperature tolerances. Adults of vent tubeworms live near vent fluids at 50°C at hundreds of atmospheres, while embryos develop best at approximately 5–10°C at ambient pressures. Thus, embryos are likely to leave the native vent site and develop in the water column (Pradillon & Gaill 2007).
(e). Dispersal barriers
Numerous shallow-water studies have shown evidence of restricted realized dispersal and local recruitment even in taxa with long PLD (e.g. Banks et al. 2007; Piggott et al. 2008; Zulliger et al. 2009), suggesting behavioural and/or environmental factors play an important role in limiting dispersal. For example, currents connect suitable habitat patches in order for propagules to colonize new areas and minimizing dilution of larval cohorts along their dispersal trajectory (e.g. Van Dover et al. 2001; Tokuda et al. 2006; Young et al. 2008). Hydrographic features may trap planktonic larvae over seamounts or other topography (McClain 2007; Brewin et al. 2009), or pose wall-like barriers to dispersal between basins (e.g. Antarctic polar front, (Rogers et al. 2006; Hunter & Halanych 2008), Pacific equatorial currents Won et al. (2003)). Currents transiting the equatorial Pacific hamper dispersal between southern and northern vents along the East Pacific Rise (EPR) (Won et al. 2003). Oxygen minimum zones may represent a similar barrier, isolating communities above and below inhospitable waters (Rogers 2000). Currents also influence directionality of dispersal, potentially linking isolated habitat patches separated by thousands of kilometres, and minimizing dilution of larval cohorts along their dispersal trajectory (e.g. Van Dover et al. 2001; Tokuda et al. 2006; Young et al. 2008).
Topography also impedes exchange between deep-sea populations and figures prominently in attempts to classify deep-sea biogeographic provinces (electronic supplementary material, appendix S1). The Gibraltar sill segregates the Atlantic and Mediterranean deep-water masses and prevents Atlantic fauna from colonizing the relatively species-poor Mediterranean (reviewed in Sardà et al. (2004)). Disruption of currents by seamounts or fracture zones, particularly along oceanic ridge axes, may limit dispersal and generate genetic diversity among hydrothermal vent systems (Van Dover et al. 2002; Tyler & Young 2003). These discontinuities interrupt the ‘ridge highway’ between vent sites, producing species with smaller ranges (e.g. Won et al. 2003; Johnson et al. 2006; Young et al. 2008), although exceptions do occur (Hurtado et al. 2004). Mid-oceanic ridges may also prevent dispersal on the abyssal plains. Half of the known species of deep-sea bivalves are restricted to either the eastern or western Atlantic McClain et al. (2009b), suggesting a role of the Mid-Atlantic Ridge. Similarly, the Walvis Ridge may isolate peracarid crustaceans in the Angola Basin (Brandt et al. 2005). Landmasses also provide obvious physical barriers, but approximately 15–20% of species are shared between the Pacific and Atlantic (Vinogradova 1997). Recent studies provide evidence of genetic homogeneity between major ocean basins Zardus et al. (2006) and shared fauna between ocean basins. Inconsistencies in the role of topographic features as barriers to dispersal lends support for the filtering concept presented by Monnoit & Monniot (1978), who noted these features were less barriers and more zones of faunal mixing.
The sheer distance between suitable habitat patches can form an insurmountable barrier (i.e. ‘isolation by distance’; Wright (1943)), as demonstrated in shallow-water systems where genetic clines exist in some taxa with high dispersal potential (Sotka & Palumbi 2006). Indeed, distance-decay relationships, in which similarity decreases with distance, are common features of ecological systems (Nekola & White 1999; Soininen et al. 2007). Thus, even in relatively homogeneous habitats like the abyssal plains, distance effects on dispersal may lead to population differentiation and faunal turnover on regional scales. Few studies explicitly examine deep-sea species turnover or gene flow over large spatial scales between seamounts McClain (2007) or between oceanic basins Allen (2008), but the role of distance has been invoked frequently for chemosynthetic environments (Vrijenhoek 1997; Won et al. 2003; Shank & Halanych 2007; Audzijonyte & Vrijenhoek 2010). In the ‘stepping stone’ model, gene flow occurs only among neighbouring vent populations and decreases with increasing distance. Tests of this model yield mixed results possibly owing to differences in distance between vent sites at fast and slow spreading centres Van Dover (1995) or sampling issues (Audzijonyte & Vrijenhoek 2010). Dispersal distances that exceed between-site distances at fast spreading centres may generate homogeneous populations over larger distances, whereas greater spacing of vent sites at slow spreading centres could prevent dispersal between sites (Desbruyères et al. 2001).
(f). Niche requirements
The role of niche-specificity, including habitat requirements and species-level interactions (e.g. competition, predation), in governing deep-sea biogeographic ranges is very poorly studied. Nonetheless, specific niche requirements will of course govern species' abilities to establish a population at a given location even if dispersal to that location is possible. Phenotypic and faunal patterning in deep-sea habitats provide some evidence of niche effects. For example, oxygen concentration appears to drive intra- and interspecific clines in invertebrate phenotypes McClain & Rex (2001), with oxygen minimum zones contain distinct suites of tolerant species (Levin 2003). Similarly, calcium carbonate dissolution is known to restrict depth distributions in taxa with exoskeletons (e.g. McClain et al. (2004)). Trophic requirements will also play an important role, not only for chemosynthetic fauna restricted to specialized and patchily distributed habitats, but for heterotrophic fauna as well.
A sizeable literature explores the unique adaptations of deep-sea deposit-feeders that enable them to subsist in the food-poor soft-sediment environment (e.g. Jumars et al. (1990)). Diversity of deposit-feeding strategies corresponds to a high degree of niche diversity that actually allows surprisingly large numbers of species to coexist in an extremely food-limited environment (Snelgrove & Smith 2002b). Organic carbon flux also influences biodiversity and standing stock over large spatial scales (Rex et al. 2006; Smith et al. 2008; McClain et al. 2009b), and generates bathymetric and latitudinal gradients in alpha diversity (Rex et al. 1993; Levin et al. 2001). However, effects of flux on biogeography are only explored in the context of bathymetric zonation (Carney 2005). The relationship between species ranges and the considerable horizontal variation in vertical flux (Lampitt & Anita 1997; Lutz et al. 2007) requires additional attention. For example, an abyssal source-sink system may exist in which extremely oligotrophic areas cannot sustain viable population sizes without input from eutrophic areas (Rex et al. 2005). Under such a scenario, little faunal turnover would be expected along horizontal gradients in flux extending into oligotrophic abyssal regions.
A promising approach to uncovering relationships between environmental factors and species ranges in the deep sea has recently been demonstrated using ecological niche models (ENMs), which linked specific niche requirements of stony corals with spatial gradients in abiotic factors (Tittensor et al. 2009). Applied in terrestrial settings for a decade (reviewed in Hirzel et al. 2002), ENMs and similar models have only recently been employed in the deep sea (Metaxas & Bryan 2007). Tittensor et al. (2009) are the first to examine deep-sea communities at a global scale using ENMs to disentangle important environmental variables influencing coral distributions on seamounts and predict locations of suitable habitat.
2. Conclusions
The sheer size of the deep sea continues to confound attempts to ascertain broad-scale patterns. However, a clear articulation of biogeographic patterns and processes is vital to advancing conservation efforts and mitigating increasing anthropogenic threats (Glover & Smith 2003; Orr et al. 2005; Robison 2009). Although, generalizations remain tentative owing to gross undersampling and incomplete taxonomic information, many taxa appear broadly distributed across the deep-sea floor. Surprisingly, this trend is apparent not only among soft-bottom taxa inhabiting the vast abyssal plain, but also in highly patchy environments such as hydrothermal vents and seamounts. However, though maximum linear extent of abyssal seafloor and vent species ranges are similar, areal extent is likely to be smaller in vent species confined to long but narrow ridges. Methane seep and large food-fall assemblages may have more restricted ranges, but the extent to which this pattern reflects undersampling, as opposed to limited gene flow in spatially and temporally patchy habitats, remains unclear. At the generic level, overwhelming evidence indicates high levels of cosmopolitanism across all habitats.
Many taxa probably possess an amazing intrinsic ability for larval dispersal, which may be further augmented by extended larval development in cold deep waters. Dispersal potential is thus expected to increase with depth and exceed that of shallow marine taxa. However, barriers to dispersal such as currents, landmasses and topographic features can of course curtail this potential. These barriers clearly define general patterns of biogeography in the deep (electronic supplementary material, appendix 1), although many dispersal barriers appear semipermeable across habitat types, affecting taxa differently. Deep-sea biogeographic patterns also clearly bear the signature of historical events, as evidenced by multiple points of evolutionary origin, and the apparent role of variable deep-water oxygen concentrations. All current evidence points to a deep-sea fauna composed of taxa, neither entirely old nor young, filtered through many climatic fluctuations (figure 2).
Sampling remote deep-sea systems is fraught with difficulty. Acquiring faunal samples requires considerably financial and technological input and years of forward planning. Rough seas, either owing to inclement weather or increased winds frequent at higher latitudes, or equipment and technological issues amplified by the isolation of being at sea often limit ship-based sampling even further. Far less than 1 per cent of the deep sea Stuart et al. (2009) has been sampled and our conclusions hinge on this fact. The small area and uneven distribution of deep-sea floor sampling, concentrated in the Northern Hemisphere in proximity to American and European oceanographic institutions, impedes more comprehensive biogeographic synthesis (Stuart et al. 2009). Recent modelling work Tittensor et al. (2010) suggests that in some instances up to an order of magnitude more sampling may be required to detect true endemicity in the deep sea. Rare species Rabinowitz (1981), especially those narrowly distributed and locally rare, may be exceptionally difficult to sample in the deep sea, leading to overestimation of the contribution of broadly distributed species to deep-sea biodiversity. However, work on gastropods suggests that local rarity may not equate to small geographical range (Rex 2002).
The way forward for deep-sea biogeography includes maximizing the yields on data already collected, continued and dedicated financial support by funding agencies with understanding of the risk, development of technologies that decrease risk and expenditure, and continued sampling. Areas of particular need include:
— Detailed phylogenies in a geographical context, which will clarify timing and points of origin for deep-sea invasions, and identify possible triggers for such events (e.g. Linder et al. 2008; Raupach et al. 2009).
— Population genetics and phylogeography for all deep-sea habitats and especially for the abyssal plains. Genetic data—severely lacking in habitats other than seamounts and hydrothermal vents—would elucidate scales of connectivity and identify environmental gradients that may be generating biogeographic breaks in a system traditionally perceived as relatively homogeneous. Across all deep-sea habitats, a clearer understanding is needed of the relationship between population and species genetic differentiation, i.e. ‘concordance of intraspecific and interspecifc’ phylobiogeographic patterning Palumbi (1996), with a recognition of the continuum that represents gene flow and structure (Palumbi 1994; Bohonak 1999; Taylor et al. 2000).
— An integrated biogeographic framework of hard-substrate areas of the deep sea. The current paradigm views individual seamounts or hydrothermal vent areas in isolation and should be broadened in scope to consider other hard-substrate habitats like deep-water reefs, ridge lines, slopes and canyons. Recent work indicates many shared species between these various habitats (Carney 1994; Van Dover 2000; O'Hara et al. 2008; McClain et al. 2009a), and such an approach would thus yield more realistic estimates of endemism/cosmopolitanism.
— Assessment of the effects of productivity patterns on biogeography, in the contemporary ocean as well as over geological timescales. Carbon flux shapes patterns in biodiversity and the evolution of deep-sea faunas (Gooday 2002; Smith et al. 2008; McClain et al. 2009b) and will probably prove important in deep-sea biogeography.
— Reproductive patterns and estimates of larval dispersal ability in deep-sea fauna, which are largely lacking for groups other than echinoderms and vent species (e.g. Young et al. 1997; Tyler & Young 2003). Realistic scales of dispersal must be quantified if we are to understand the processes that generate contemporary biogeographic patterns and, more importantly, to develop effective conservation strategies for deep-sea habitats.
— Improved biogeographic schemes and habitat suitability models using state of the art analyses to guide conservation (e.g. Bachraty et al. 2009; Tittensor et al. 2009). The unprecedented amount of environmental data publicly available (e.g. National Oceanographic Database, NODC, http://www.nodc.noaa.gov/), efforts to compile and synthesize data from deep-sea habitats in online databases (e.g. Ocean Biogeographic Information System, OBIS, http://www.iobis.org/;, Census of Marine Life, CoML, http://www.coml.org/) and methodological advances (see Elith et al. 2006; Nogues-Bravo 2009) will facilitate additional such efforts. Environmental conditions may successfully characterize a species distribution, but understanding the role of biological interactions in defining the biogeographic range or interact with environmental parameters will be key to understanding deep-sea biodiversity (Soberon 2007; Soberon & Nakumura 2009).
— Explicit analyses of faunal turnover (beta-diversity) and biogeographic ranges in relation to both dispersal limits, environmental heterogeneity (e.g. distance decay Nekola & White (1999)), and each other. Large-scale environmental and biological datasets are becoming sufficiently comprehensive to provide the necessary tools for such analyses.
— Examination of whether deep-sea biota follow macroecological and biogeographic ‘rules’. Conformity or deviation will provide valuable insights into structuring forces in the deep sea as well as in other environments (McClain et al. 2009b).
— Explicitly linking knowledge of deep-sea biogeography to future climate change scenarios. Species will either move (range shifts), acclimatize (phenotypic plasticity), adapt (evolution) or die (extinction) (e.g. Hofmann & Todgham (2010)). What scope do deep-sea taxa possess for acclimatization and adaptation? How will ranges be affected? Answers will require continued initiatives to forge collaborations among disparate disciplines (e.g. Evolution and Climate Change in the Oceans programme).
— As ever, more sampling, more sampling and even more sampling…combined with the continued development of databases (e.g. OBIS, Seamounts Online (http://seamounts.sdsc.edu/), and Dryad (www.dryad.org)) and the continued promotion of data sharing, synthesis and collaboration (e.g. CoML, National Evolutionary Synthesis Center, and the National Center for Ecological Analysis and Synthesis; see also Sidlauskas et al. (2009)).
If we have learned anything over the last few decades of deep-sea exploration, it is that we still have much to learn. Historically, two different positions are taken with regard to biogeographic research. The first focuses on individual clades and seeks to identify ‘diverse ways that biotas have developed, both spatially and temporally though global history’ McDowall (2004) (e.g. Ebach et al. (2003)). The second emphasizes identifying ‘overarching biotic relationships’ (McDowall 2004). We advocate proceeding with both as new data and exploration on both fronts will consistently force us to re-evaluate existing paradigms. We must be equally open to the idea that generalities and departures from them may exist, but exceptions to ‘rules’ present valuable opportunities for new insight.
Acknowledgements
This manuscript benefited from lively discussions and informal reviews by C.L. Van Dover, A. Glover, J. Payne, C. Mah, K. Zelnio, T. Roberts, M. Rex, K. Halanych, A. Boyer and E. Schuettplez, as well as the insightful comments of two anonymous reviewers. C.R.M. wishes to thank his loving wife, Michelle, for patience and support. C.R.M. is supported by the National Evolutionary Synthesis Center, NSF no. EF-0423641.
References
- Allen J. A.1978Evolution of the deep sea protobranch bivalves. Phil. Trans. R. Soc. Lond. B 284, 387–401 (doi:10.1098/rstb.1978.0076) [Google Scholar]
- Allen J. A.2008Bivalvia of the deep Atlantic. Malacologia 50, 57–173 (doi:10.4002/0076-2997-50.1.57) [Google Scholar]
- Allen J. A., Sanders H. L.1996The zoogeography, diversity and origin of the deep-sea protobranch bivalves of the Atlantic: the epilogue. Prog. Oceanogr. 38, 95–153 (doi:10.1016/S0079-6611(96)00011-0) [Google Scholar]
- Arellano S. M., Young C. M.2009Spawning, development, and the duration of larval life in a deep-sea cold-seep mussel. Biol. Bull. 216, 149–162 (doi:10.2307/25470737) [DOI] [PubMed] [Google Scholar]
- Audzijonyte A., Vrijenhoek R. C.2010When gaps really are gaps: statistical phylogeography of hydrothermal vent invertebrates. Evolution. http://www3.interscience.wiley.com/journal/123314127/abstract. [DOI] [PubMed] [Google Scholar]
- Bachraty C., Legendre P., Desbruyeres D.2009Biogeographic relationships among deep-sea hydrothermal vent faunas at global scale. Deep-Sea Res. I 56, 1371–1378 (doi:10.1016/j.dsr.2009.01.009) [Google Scholar]
- Baco A. R., Smith C. R., Peek A. S., Roderick G. K., Vrijenhoek R. C.1999The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial COI DNA sequences. Mar. Ecol. Prog. Ser. 182, 137–147 (doi:10.3354/meps182137) [Google Scholar]
- Banks S. C., Piggott M. P., Williamson J. E., Bové U., Holbrook N. J., Beheregaray L. B.2007Oceanic variability and coastal topography shape genetic structure in a long-dispersing sea urchin. Ecology 88, 3055–3064 (doi:10.1890/07-0091.1) [DOI] [PubMed] [Google Scholar]
- Barnard J. L.1961Gammaridean Amphipoda from depth of 400 to 6000 m. Galathea Rep. 5, 23–128 [Google Scholar]
- Bensen R. H.1975The origin of the psychrosphere as recorded in changes of deep-sea ostracode assemblages. Lethaia 8, 69–83 (doi:10.1111/j.1502-3931.1975.tb00919.x) [Google Scholar]
- Billett D. S. M., Bett B. J., Rice A. L., Thurston M. H., Galéron J., Sibuet M., Wolff G. A.2001Long-term changes in the megabenthos of the Porcuping Abyssal Plain (NE Atlantic). Prog. Oceanogr. 50, 325–348 (doi:10.1016/S0079-6611(01)00060-X) [Google Scholar]
- Bisol P. M., Costa R., Sibuet M.1984Ecological and genetical survey of two deep-sea holothurians: Benthogone rosea and Benthodytes typica. Mar. Ecol. Prog. Ser. 15, 275–281 (doi:10.3354/meps015275) [Google Scholar]
- Bohonak A. J.1999Dispersal, gene flow, and population structure. Quat. Rev. Biol. 74, 21–45 (doi:10.1086/392950) [DOI] [PubMed] [Google Scholar]
- Bradbury I. R., Laurel B., Snelgrove P. V. R., Bentzen P., Campana S. E.2008Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B 275, 1803–1809 (doi:10.1098/rspb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A., et al. 2005Diversity of peracarid crustaceans (Malacostraca) from the abyssal plain of the Angola Basin. Organ. Divers. Evol. 5(Suppl. 1), 105–112 [Google Scholar]
- Brandt A., Brix S., Brökeland W., Choudhury M., Kaiser S., Malyutina M.2007Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean—results from the ANDEEP I-III expeditions. Deep-Sea Res. II 54, 1760–1775 (doi:10.1016/j.dsr2.2007.07.015) [Google Scholar]
- Brewin P. E., Stocks K. I., Haidvogel D. B., Condit C., Gupta A.2009Effects of oceanographic retention on decapod and gastropod community diversity on seamounts. Mar. Ecol. Prog. Ser. 383, 225–237 (doi:10.3354/meps07987) [Google Scholar]
- Brown J. H., Stevens G. C., Kaufman D. M.1996The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 27, 597–623 (doi:10.1146/annurev.ecolsys.27.1.597) [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Bucklin A., Wilson R. R., Jr, Smith K. L., Jr1987Genetic differentiation of seamount and basin populations of the deep-sea amphipod Eurythenes gryllus. Deep-Sea Res. 34, 1795–1810 (doi:10.1016/0198-0149(87)90054-9) [Google Scholar]
- Buhl-Mortensen L., Hoeg J. T.2006Reproduction and larval development in three scalpellid barnacles, Scalpellum scalpellum (Linnaeus 1767), Ornatoscalpellum stroemii (M. Sars 1859) and Arcoscalpellum michelottianum (Seguenza 1876), Crustacea: Cirripedia: Thoracica): implications for reproduction and dispersal in the deep sea. Mar. Biol. 149, 829–844 (doi:10.1007/s00227-006-0263-y) [Google Scholar]
- Cailliet G. M., Andrews A. H., Burton E. J., Watters D. L., Kline D. E., Ferry-Graham L. A.2001Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 36, 739–764 (doi:10.1016/S0531-5565(00)00239-4) [DOI] [PubMed] [Google Scholar]
- Carney R. S.1994Consideration of the oasis analogy for chemosynthetic communities at the Gulf of Mexico hydrocarbon vents. Geo-Mar. Lett. 14, 149–159 (doi:10.1007/BF01203726) [Google Scholar]
- Carney R. S.2005Zonation of deep biota on continental margins. Oceanogr. Mar. Biol. Annu. Rev. 43, 211–278 [Google Scholar]
- Clark M. R., et al. 2010The ecology of seamounts: structure, function, and human impacts. Annu. Rev. Mar. Sci. 2, 253–278 (doi:10.1146/annurev-marine-120308-081109) [DOI] [PubMed] [Google Scholar]
- Clarke A. H., Jr1962On the composition, zoogeography, origin and age of the deep-sea mollusc fauna. Deep-Sea Res. 9, 229–306 [Google Scholar]
- Cordes E. E., Carney S. L., Hourdez S., Carney R. S., Brooks J. M., Fisher C. R.2007Cold seeps of the deep Gulf of Mexico: community structure and biogeographic comparisons to Atlantic equatorial belt seep communities. Deep-Sea Res. I 54, 637–653 (doi:10.1016/j.dsr.2007.01.001) [Google Scholar]
- Cordes E. E., Cunha M. R., Galéron J., Mora C., Olu-Le Roy K., Sibuet M., Van Gaever S., Vanreusel A., Levin L. A.2009The influence of geological, geochemical, and biogeneic habitat heterogeneity on seep biodiversity. Mar. Ecol. 31, 51–65 [Google Scholar]
- Cowen R. K., Sponaugle S.2009Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 1, 443–466 (doi:10.1146/annurev.marine.010908.163757) [DOI] [PubMed] [Google Scholar]
- Desbruyeres D., Hashimoto J., Fabri M.2006Composition and biogeography of hydrothermal vent communities in western Pacific back-arc basins. In Back-arc spreading systems: geological, biological, chemical, and physical interactions, pp. 215–234 Washington, DC: American Geophysical Union [Google Scholar]
- Desbruyères D., et al. 2001Variations in deep-sea hydrothermal vent communities on the Mid-Atlantic Ridge near the Azores plateau. Deep-Sea Res. I 48, 1325–1346 (doi:10.1016/S0967-0637(00)00083-2) [Google Scholar]
- Domenici P., Gonzalez-Calderon D., Ferrari R. S.2003Locomotor performance in the sea urchin Paracentrotus lividus. J. Mar. Biol. Assoc. UK 83 (doi:10.1017/S0025315403007094h) [Google Scholar]
- Ebach M., Humphries C. J., Williams D. M.2003Phylogenetic biogeography deconstructed. J. Biogeogr. 30, 1285–1296 [Google Scholar]
- Ebert T. A., Southon J. R.2003Red sea urchins (Strongylocentrotus franciscanus) can live over 100 years: confirmation with A-bomb 14Carbon. Fishery Bull. 101, 915–922 [Google Scholar]
- Elith J., et al. 2006Novel methods improve prediction of species' distributions from occurrence data. Ecography 29, 129–151 (doi:10.1111/j.2006.0906-7590.04596.x) [Google Scholar]
- Emlet R. B.1995Developmental mode and species geographic range in regular sea urchins (Echinodermata: Echinoidea). Evolution 49, 476–489 (doi:10.2307/2410272) [DOI] [PubMed] [Google Scholar]
- Etter R. J., Rex M. A., Chase M. R., Quattro J. M.2005Population differentiation decreases with depth in deep-sea bivalves. Evolution 59, 1479–1491 [PubMed] [Google Scholar]
- Fortes R. R., Absalao R. S.2004The applicability of Rapoport's rule to the marine molluscs of the Americas. J. Biogeogr. 31, 1909–1916 (doi:10.1111/j.1365-2699.2004.01117.x) [Google Scholar]
- France S. C.1994Genetic population structure and gene flow among deep-sea amphipods, Abyssorchomene spp., from six California continental borderland basins. Mar. Biol. 118, 67–77 (doi:10.1007/BF00699220) [Google Scholar]
- France S. C., Kocher T. D.1996Geographic and bathymetric patterns of mitochondrial 16S rRNA sequence divergence among deep-sea amphipods, Eurythenes gryllus. Mar. Biol. 126, 633–643 (doi:10.1007/BF00351330) [Google Scholar]
- Gage J. D., Tyler P. A.1991Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge, UK: Cambridge University Press [Google Scholar]
- Glover A. G., Smith C. R.2003The deep-sea floor ecosystem: current status and prospects of anthropogenic change by the year 2025. Environ. Conserv. 30, 219–241 (doi:10.1017/S0376892903000225) [Google Scholar]
- Glover A. G., Smith C. R., Paterson G. J. L., Wilson G. D. F., Hawkins L., Sheader M.2002Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Mar. Ecol. Prog. Ser. 240, 157–170 (doi:10.3354/meps240157) [Google Scholar]
- Glover A. G., Källström B., Smith C. R., Dahlgren T. G.2005World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc. R. Soc. B 272, 2587–2592 (doi:10.1098/rspb.2005.3275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooday A. J.2002Biological responses to seasonally varying fluxes of organic matter to the ocean floor: a review. J. Oceanogr. 58, 305–332 (doi:10.1023/A:1015865826379) [Google Scholar]
- Gooday A. J., Hori S., Todo Y., Okamoto T., Kitazato H., Sabbatini A.2004Soft-walled, monothalamous benthic foraminiferans in the Pacific, Indian and Atlantic Oceans: aspects of biodiversity and biogeography. Deep Sea Res. Part I Oceanogr. Res. Pap. 51, 33–53 (doi:10.1016/j.dsr.2003.07.002) [Google Scholar]
- Grassle J. F.1989Species diversity in deep-sea communities. Trends Ecol. Evol. 4, 12–15 (doi:10.1016/0169-5347(89)90007-4) [DOI] [PubMed] [Google Scholar]
- Grassle J. F., Maciolek N. J.1992Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am. Nat. 139, 313–341 (doi:10.1086/285329) [Google Scholar]
- Hansen B.1975Systematics and biology of the deep-sea holothurians. Part I. Elasipoda. Galathea Rep. 13, 1–262 [Google Scholar]
- Havenhand J. N., Matsumoto G. I., Siedel E.2005Megalodicopia hians in the Monterey submarine canyon: distribution, larval development, and culture. Deep-Sea Res. I 53, 215–222 (doi:10.1016/j.dsr.2005.11.005) [Google Scholar]
- Hessler R. R., Lonsdale P. F.1991Biogeography of mariana trough hydrothermal vent communities. Deep Sea Res. 38, 185–199 (doi:10.1016/0198-0149(91)90079-U) [Google Scholar]
- Hirzel A. H., et al. 2002Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83, 2027–2036 (doi:10.1890/0012-9658(2002)083[2027:ENFAHT]2.0.CO;2) [Google Scholar]
- Hofmann G. E., Todgham A. E.2010Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Physiol. 72, 127–145 (doi:10.1146/annurev-physiol-021909-135900) [DOI] [PubMed] [Google Scholar]
- Holt R. D.2003On the evolutionary ecology of species' ranges. Evol. Ecol. Res. 5, 159–178 [Google Scholar]
- Holt R. D., Keitt T.2005Species' borders: a unifying theme in ecology. Oikos 108, 3–6 (doi:10.1111/j.0030-1299.2005.13145.x) [Google Scholar]
- Horne D. J.1999Ocean circulation modes of the Phanaerozoic: implications for the antiquity of deep-sea bnethonic invertebrates. Crustaceana 72, 999–1018 (doi:10.1163/156854099503906) [Google Scholar]
- Howell K. L., Rogers A. D., Tyler P. A., Billett D. S. M.2004Reproductive isolation among morphotypes of the Atlantic seastar species Zoroaster fulgens (Asteroidea: Echinodermata). Mar. Biol. 144, 977–984 (doi:10.1007/s00227-003-1248-8) [Google Scholar]
- Howell K. L., Mowles S. L., Foggo A.2010Mounting evidence: near-slope seamounts are faunally indistinct from an adjacent bank. Mar. Ecol. http://www3.interscience.wiley.com/journal/123428893/abstract [Google Scholar]
- Hunter R. L., Halanych K. M.2008Evaluating connectivity in the brooding brittle star Astrotoma agassizii across the Drake Passage in the Southern Ocean. J. Heredity 99, 137–148 (doi:10.1093/jhered/esm119) [DOI] [PubMed] [Google Scholar]
- Hurtado L. A., Lutz R. A., Vrijenhoek R. C.2004Distinct patterns of genetic differentiation among annelids of eastern Pacific hydrothermal vents. Mol. Ecol. 13, 2603–2615 (doi:10.1111/j.1365-294X.2004.02287.x) [DOI] [PubMed] [Google Scholar]
- Jablonski D., Bottjer D. J.1991Environmental patterns in the origins of higher taxa: the post-Palaeozoic fossil record. Science 252, 251–253 (doi:10.1126/science.252.5014.1831) [DOI] [PubMed] [Google Scholar]
- Jablonski D., Lutz R. A.1983Larval ecology of marine benthic invertebrates: palaeobiological implications. Biol. Rev. Camb. Phil. Soc. 58, 21–89 (doi:10.1111/j.1469-185X.1983.tb00380.x) [Google Scholar]
- Jablonski D., Sepkoski J. J., Jr, Bottjer D. J., Sheehan P. M.1983Onshore-offshore patterns in evolution of Phanaerozoic shelf communities. Science 22, 1123–1125 (doi:10.1126/science.222.4628.1123) [DOI] [PubMed] [Google Scholar]
- Jacobs D. K., Lindberg D. R.1998Oxygen and evolutionary patterns in the sea: onshore/offshore trends and recent recruitment of deep-sea faunas. Proc. Natl Acad. Sci. USA 95, 9396–9401 (doi:10.1073/pnas.95.16.9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson L.1990An oceanic mode for lithological and faunal changes tested on the Silurian record. J. Geol. Soc. Lond. 147, 663–674 (doi:10.1144/gsjgs.147.4.0663) [Google Scholar]
- Johnson S. B., Young C. R., Jones W. J., Waren A., Vrijenhoek R. C.2006Migration, isolation, and speciation of hydrothermal vent limpets (Gastropoda; Lepetodrilidae) across the Blanco Transform Fault. Biol. Bull. 210, 140–157 (doi:10.2307/4134603) [DOI] [PubMed] [Google Scholar]
- Jones E. G., Collins M. A., Bagley P. M., Addison S., Priede I. G.1998The fate of cetacean carcasses in the deep sea: observations on consumption rates and succession of scavenging species in the abyssal north-east Atlantic Ocean. Proc. R. Soc. Lond. B 265, 1119–1127 (doi:10.1098/rspb.1998.0407) [Google Scholar]
- Jones W. J., Clague D., Vrijenhoek R.2006Population genetics and ecology of seamount clam (Limidae: Acesta) populations in the northeastern Pacific Ocean. Semount biogeosciences network. La Jolla, CA: Scripps Institution of Oceanography [Google Scholar]
- Jumars P. A., Mayer L. M., Deming J. W., Baross J. A., Wheatcroft R. A.1990Deep-sea deposit-feeding strategies suggested by environmental and feeding constraints. Phil. Trans. R. Soc. Lond. B 331A, 85–101 [Google Scholar]
- Kiel S., Goedert J. L.2006Deep-sea food bonanzas: early Cenozoic whale-fall communities resemble wood-fall rather than seep communities. Proc. R. Soc. B 273, 2625–2631 (doi:10.1098/rspb.2006.3620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlan B. P., Gaines S. D.2003Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020 (doi:10.1890/01-0622) [Google Scholar]
- Koslow T.2007The silent deep: the discovery, ecology, and conservation of the deep sea. Chicago, IL: University of Chicago Press [Google Scholar]
- Lampitt R. S., Anita A. N.1997Particle flux in the deep seas: regional characteristics and temporal variability. Deep-Sea Res. I 44, 1377–1403 (doi:10.1016/S0967-0637(97)00020-4) [Google Scholar]
- Lester S. E., Ruttenberg B. I., Gaines S. D., Kinlan B. P.2007The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758 (doi:10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]
- Levin L. A.2003Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr. Mar. Biol. Annu. Rev. 41, 1–45 [Google Scholar]
- Levin L. A., Dayton P. K.2009Ecological theory and continental margins: where shallow meets deep. Trends Ecol. Evol. 24, 606–617 (doi:10.1016/j.tree.2009.04.012) [DOI] [PubMed] [Google Scholar]
- Levin L. A., Etter R. J., Rex M. A., Gooday A. J., Smith C. R., Pineda J., Stuart C. T., Hessler R. R., Pawson D.2001Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 32, 51–93 (doi:10.1146/annurev.ecolsys.32.081501.114002) [Google Scholar]
- Linder A., Cairns S. D., Cunningham C. W.2008From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE 3, e2429 1–5 (doi:10.1371/journal.pone.0002429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps J. H., Hickman C. S.1982Origin, age, and evolution of antarctic and deep-sea faunas. In The environment of the deep sea (eds Ernst W. G., Morin J. G.), pp. 324–356 Englewood Cliffs, NJ: Prentice-Hall, Inc [Google Scholar]
- Little C. T. S., Vrijenhoek R. C.2003Are hydrothermal vent animals living fossils? Trends Ecol. Evol. 18, 582–588 (doi:10.1016/j.tree.2003.08.009) [Google Scholar]
- Lutz M. J., Caldeira K., Dunbar R. B., Behrenfeld M. J.2007Seasonal rhythms of net primary production and particulate organic carbon flux describe biological pump efficiency in the global ocean. J. Geophys. Res. C. Oceans 112, C10011 (doi:10.1029/2006JC003706) [Google Scholar]
- Madsen F. J.1961The Porcellanasteridae: a monographic revision of an abyssal group of seastars. Galathea Rep. 4, 33–173 [Google Scholar]
- Marsh A. G., Mullineaux L. S., Young C. M., Manahan D. T.2001Larval dispersal potential of the tubeworm Riftia pachyptila at deep-sea hydrothermal vents. Nature 411, 77–80 (doi:10.1038/35075063) [DOI] [PubMed] [Google Scholar]
- McClain C. R.2007Guest editorial: seamounts: identity crisis or split personality? J. Biogeogr. 34, 2001–2008 (doi:10.1111/j.1365-2699.2007.01783.x) [Google Scholar]
- McClain C. R., Rex M. A.2001The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar. Biol. 139, 681–685 (doi:10.1007/s002270100617) [Google Scholar]
- McClain C. R., Johnson N. A., Rex M. A.2004Morphological disparity as a biodiversity metric in lower bathyal and abyssal gastropod assemblages. Evolution 58, 338–348 [DOI] [PubMed] [Google Scholar]
- McClain C. R., Lundsten L., Ream M., Barry J., DeVogelaere A.2009aEndemicity, biogeography, composition, and community structure on a northeast Pacific seamount. PLoS ONE 4, e4141 (doi:10.1371/journal.pone.0004141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C. R., Rex M. A., Etter R. J.2009bDeep-sea macroecology. In Marine macroecology (eds Witman J. D., Roy K.), pp. 65–100 Chicago, IL: University of Chicago Press [Google Scholar]
- McDowall R. M.2004What biogeography is: a place for process. J. Biogeogr. 31, 345–351 (doi:10.1046/j.0305-0270.2003.01020.x) [Google Scholar]
- Menzies R. J., Imbrie J.1958On the antiquity of the deep-sea bottom fauna. Oikos 9, 192–210 (doi:10.2307/3564764) [Google Scholar]
- Mercier A., Hamel J. F.2008Depth-related shift in life history strategies of a brooding and broadcasting deep-sea asteroid. Mar. Biol. 156, 205–223 (doi:10.1007/s00227-008-1077-x) [Google Scholar]
- Metaxas A., Bryan T.2007Predictive habitat model for deep gorgonians needs better resolution: reply to Etnoyer and Morgan. Mar. Ecol. Prog. Ser. 339, 313–314 (doi:10.3354/meps339313) [Google Scholar]
- Mincks S. L., Dyal P. L., Paterson G. L. J., Smith C. R., Glover A. G.2009A new species of Aurospio (Polychaeta, Spionidae) from the Antarctic shelf, with analysis of its ecology, reproductive biology and evolutionary history. Mar. Ecol. 30, 181–197 (doi:10.1111/j.1439-0485.2008.00265.x) [Google Scholar]
- Miyake A. G., et al. 2006Rearing and observation methods of vestimentiferan tubeworms and its early development at atmospheric pressure. Cah. Biol. Mar. 47, 471–475 [Google Scholar]
- Monnoit C., Monniot F.1978Recent work on the deep-sea tunicates. Oceanogr. Mar. Biol. Annu. Rev. 16, 181–228 [Google Scholar]
- Nakaoka M., Ito N., Yamamoto T., Okuda T., Noda T.2006Similarity of rocky intertidal assemblages along the Pacific coast of Japan: effects of spatial and geographic distance. Ecol. Res. 21, 425–435 (doi:10.1007/s11284-005-0138-6) [Google Scholar]
- Nekola J. C., White P. S.1999The distance decay of similarity in biogeography and ecology. J. Biogeogr. 26, 867–878 (doi:10.1046/j.1365-2699.1999.00305.x) [Google Scholar]
- Nogues-Bravo D.2009Prediction the past distribution of species climatic niches. Global Ecol. Biogeogr. 18, 521–531 (doi:10.1111/j.1466-8238.2009.00476.x) [Google Scholar]
- O'Connor M. I., Bruno J. F., Gaines S. D., Halpern B. S., Lester S. E., Kinlan B. P., Weiss J. M.2007Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl Acad. Sci. USA 104, 1266–1271 (doi:10.1073/pnas.0603422104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara T. D.2007Seamounts: centres of endemism or species richness for ophiuroids. Global Ecol. Biogeogr. 16, 720–732 (doi:10.1111/j.1466-8238.2007.00329.x) [Google Scholar]
- O'Hara T. D., Rowden A. A., Williams A.2008Cold-water coral habitats on seamounts: do they have a specialist fauna? Divers. Distrib. 14, 925–934 (doi:10.1111/j.1472-4642.2008.00495.x) [Google Scholar]
- Orr J. C., et al. 2005Anthropogenic ocean acidification over the twenty-first century and its impacts on calcifying organisms. Nature 437, 681–686 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- Palumbi S. R.1994Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 25, 547–572 (doi:10.1146/annurev.es.25.110194.002555) [Google Scholar]
- Palumbi S. R.1996What can molecular genetics contribute to marine biogeography? An urchin's tale. J. Exp. Mar. Biol. Ecol. 203, 75–92 (doi:10.1016/0022-0981(96)02571-3) [Google Scholar]
- Paulay G., Meyer C.2006Dispersal and divergence across the greatest ocean region: do larvae matter? Integr. Comp. Biol. 46, 269–281 (doi:10.1093/icb/icj027) [DOI] [PubMed] [Google Scholar]
- Pawlowski J., Fahrni J., Lecroq B., Longet D., Cornelius N., Excoffier L., Cedhagen T., Gooday A. J.2007Bipolar gene flow in deep-sea benthic foraminifera. Mol. Ecol. 16, 4089–4096 (doi:10.1111/j.1365-294X.2007.03465.x) [DOI] [PubMed] [Google Scholar]
- Pearse J. S., Lockhart S. J.2004Reproduction in cold water: paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep-Sea Res. II 51, 1533–1549 (doi:10.1016/j.dsr2.2004.06.023) [Google Scholar]
- Piggott M. P., Banks S. C., Tung P., Beheregaray L. B.2008Genetic evidence for different scales of connectivity in a marine mollusc. Mar. Ecol. Prog. Ser. 365, 127–136 (doi:10.3354/meps07478) [Google Scholar]
- Pineda J., Reyns N. B., Starczak V. R.2009Complexity and simplification in understanding recruitment in benthic populations. Popul. Ecol. 51, 17–32 (doi:10.1007/s10144-008-0118-0) [Google Scholar]
- Pradillon F., Gaill F.2007Pressure and life: some biological strategies. Rev. Environ. Sci. Biotechnol. 6, 181–195 (doi:10.1007/s11157-006-9111-2) [Google Scholar]
- Rabinowitz D.1981Seven forms of rarity. In The biological aspects of rare plant conservation (ed. Synge H.), pp. 205–217 New York, NY: Wiley [Google Scholar]
- Raupach M. J., Held C., Wagele J.-W.2004Multiple colonization of the deep sea by the Asellota (Crustacea: Peracarida: Isopoda). Deep-Sea Res. II 51, 1787–1795 (doi:10.1016/j.dsr2.2004.06.035) [Google Scholar]
- Raupach M. J., Malyutina M., Brandt A., Wägele J.-W.2007Molecular data reveal a highly diverse species flock within the munnopsoid deep-sea isopod Betamorpha fusiformis (Barnard, 1920) (Crustacea: Isopoda: Asellota) in the Southern Ocean. Deep-Sea Res. II 54, 1820–1830 (doi:10.1016/j.dsr2.2007.07.009) [Google Scholar]
- Raupach M. J., Mayer C., Malyutina M., Wägele J.-W.2009Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proc. R. Soc. B 276, 799–808 (doi:10.1098/rspb.2008.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M. A.2002Biogeography of the deep-sea gastropod Palazzia planorbis (Dall, 1927): an uncommon form of rarity. Nautilus 116, 36–38 [Google Scholar]
- Rex M. A., Stuart C. T., Hessler R. R., Allen J. A., Sanders H. L., Wilson G. D. F.1993Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature 365, 636–639 (doi:10.1038/365636a0) [Google Scholar]
- Rex M. A., McClain C. R., Johnson N. A., Etter R. J., Allen J. A., Bouchet P., Warén A.2005A source-sink hypothesis for abyssal biodiversity. Am. Nat. 165, 163–178 (doi:10.1086/427226) [DOI] [PubMed] [Google Scholar]
- Rex M. A., et al. 2006Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar. Ecol. Prog. Ser. 317, 1–8 (doi:10.3354/meps317001) [Google Scholar]
- Robison B.2009Conservation of deep pelagic biodiversity. Conserv. Biol. 23, 847–858 (doi:10.1111/j.1523-1739.2009.01219.x) [DOI] [PubMed] [Google Scholar]
- Rogers A. D.1994The biology of seamounts. Adv. Mar. Biol. 30, 304–360 [DOI] [PubMed] [Google Scholar]
- Rogers A. D.2000The role of the oceanic oxygen minima in generating biodiversity in the deep sea. Deep-Sea Res. II 47, 119–148 (doi:10.1016/S0967-0645(99)00107-1) [Google Scholar]
- Rogers A. D., Morley S., Fitzcharles E., Jarvis K., Belchier M.2006Genetic structure of Patagonian toothfish (Dissostichus eleginoides) populations on the Patagonian Shelf and Atlantic and western Indian Ocean sectors of the Southern Ocean. Mar. Biol. 149, 915–924 (doi:10.1007/s00227-006-0256-x) [Google Scholar]
- Rouse G. W., Wilson N. G., Goffredi S. K., Johnson S. B., Smart T., Widmer C., Young C. M., Vrijenhoek R. C.2009Spawning and development in Osedax boneworms (Siboglinidae, Annelida). Mar. Biol. 156, 395–405 (doi:10.1007/s00227-008-1091-z) [Google Scholar]
- Samadi S., Bottan L., Macpherson E., De Forges B. R., Boisselier M.-C.2006Seamount endemism questionedby the geographic distribution and population genetic structure of marine invertebrates. Mar. Biol. 149, 1463–1475 (doi:10.1007/s00227-006-0306-4) [Google Scholar]
- Sanders H. L.1968Marine benthic diversity: a comparative study. Am. Nat. 102, 243–282 (doi:10.1086/282541) [Google Scholar]
- Sardà F., Calafat A., Flexas M. M., Tselepides A., Canals M., Espino M., Tursi A.2004An introduction to Mediterranean deep-sea biology. Sci. Marina 68, 7–38 [Google Scholar]
- Scheltema R. S.1986On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bull. Mar. Sci. 39, 290–322 [Google Scholar]
- Shank T. M., Halanych K. M.2007Toward a mechanistic understanding of larval dispersal: insights from genomic fingerprinting of the deep-sea hydrothermal vent tubeworm Riftia pachyptila. Mar. Ecol. Evol. Perspect. 28, 25–35 [Google Scholar]
- Shank T. M., et al. 1998Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): evidence from mitochondrial cytochrome oxidase subunit I. Mol. Phylogenet. Evol. 13, 244–254 (doi:10.1006/mpev.1999.0642) [DOI] [PubMed] [Google Scholar]
- Shanks A. L.2009Pelagic larval duration and dispersal distance revisited. Biol. Bull. 216, 373–385 (doi:10.2307/25548167) [DOI] [PubMed] [Google Scholar]
- Sibuet M.1979Distribution and diversity of asteroids in Atlantic abyssal basins. Sarsia 64, 85–91 [Google Scholar]
- Sibuet M., Olu K.1998Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive marigns. Deep-Sea Res. II 45, 517–567 (doi:10.1016/S0967-0645(97)00074-X) [Google Scholar]
- Sidlauskas B., et al. 2009Linking big: the continuing promise of evolutionary synthesis. Evolution 64, 871–880 (doi:10.1111/j.1558-5646.2009.00892.x) [DOI] [PubMed] [Google Scholar]
- Smith C. R., Baco-Taylor A. R.2003Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 41, 311–354 [Google Scholar]
- Smith C. R., Baco A. R., Hannides A., Ruplinger D.2003Chemosynthetic habitats on the California slope: whale-, wood- and kelp-falls compared to vents and seeps. In Biogeography and biodiversity of chemosynthetic ecosystems: planning for the future. Southampton, UK: Southampton Oceanography Centre [Google Scholar]
- Smith C. R., et al. 2008Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 23, 518–528 (doi:10.1016/j.tree.2008.05.002) [DOI] [PubMed] [Google Scholar]
- Smith A. B., Stockley B.2005The geological history of deep-sea colonization by echinoids: roles of surface productivity and deep-water ventilation. Proc. R. Soc. B 272, 865–869 (doi:10.1098/rspb.2004.2996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., McVeagh S. M., Mingoia J. T., France S. C.2004Mitochondrial DNA sequence variation in deep-sea bamboo coral (Keratoisidinae) species in the southwest and northwest Pacific Ocean. Mar. Biol. 144, 253–261 (doi:10.1007/s00227-003-1206-5) [Google Scholar]
- Snelgrove P. V. R., Smith C. R.2002aA riot of species in an environmental calm: the paradox of the species-rich deep-sea floor. Oceanogr. Mar. Biol. 40, 211–242 [Google Scholar]
- Snelgrove P. V. R., Smith C. R.2002bA riot of species in an environmental calm: the paradox of the species-rich deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 40, 311–342 [Google Scholar]
- Soberon J.2007Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 (doi:10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- Soberon J., Nakumura M.2009Niche and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19 644–19 650 (doi:10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen J., McDonald R., Hillebrand H.2007The distance decay of similarity in ecological communities. Ecography 30, 3–12 [Google Scholar]
- Somero G. N.1998Adaptation to cold and depth: contrasts between polar and deep-sea animals. In Cold ocean physiology (eds Portner H. O., Payle R. C.), pp. 33–57 Cambridge, UK: University Press [Google Scholar]
- Sotka E. E., Palumbi S. R.2006The use of genetic clines to estimate dispersal distances of marine larvae. Ecology 87, 1094–1103 (doi:10.1890/0012-9658(2006)87[1094:TUOGCT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Strugnell J. M., Rogers A. D., Prodöhl P. A., Collins M. A., Allcock A. L.2008The thermohaline expressway: the Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24, 853–860 (doi:10.1111/j.1096-0031.2008.00234.x) [DOI] [PubMed] [Google Scholar]
- Stuart C. T., Arbizu P. M., Smith C. R., Molodtsova T., Brandt A., Etter R. J., Escobar-Briones E., Fabri M.-C., Rex M. A.2009CeDAMar global database of abyssal biological sampling. Aquat. Biol. 4, 143–145 (doi:10.3354/ab00097) [Google Scholar]
- Taylor J. W., et al. 2000Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31, 21–32 (doi:10.1006/fgbi.2000.1228) [DOI] [PubMed] [Google Scholar]
- Thoma J. N., Pante E., Brugler M. R., France S. C.2009Deep-sea octocorals and antipatharians show no evidence of seamount-scale endemism in the NW Atlantic. Mar. Ecol. Prog. Ser. 397, 25–35 (doi:10.3354/meps08318) [Google Scholar]
- Thorson G.1950Reproductive and larval ecology marine bottom invertebrates. Biol. Rev. Camb. Philos. Soc. 25, 1–45 (doi:10.1111/j.1469-185X.1950.tb00585.x) [DOI] [PubMed] [Google Scholar]
- Thurston M. H.1990Abyssal necrophagous amphipods (Crustacea: Amphipoda) in the northeast and tropical Atlantic Ocean. Prog. Oceanogr. 24, 257–274 (doi:10.1016/0079-6611(90)90036-2) [Google Scholar]
- Tittensor D. P., et al. 2009Predicting habitat suitability for stony corals on seamounts. J. Biogeogr. 36, 1111–1128 (doi:10.1111/j.1365-2699.2008.02062.x) [Google Scholar]
- Tittensor D. P., et al. 2010Endemism at low sampling effort: real or artifact. 12th Int. Deep-Sea Biology Symp. Reykjavik, Iceland [Google Scholar]
- Tokuda G., Yamada A., Nakano K., Arita N., Yamasaki H.2006Occurence and recent long-distance dispersal of deep-sea hydrothermal vent shrimps. Biol. Lett. 2, 257–260 (doi:10.1098/rsbl.2005.0420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliffe V., Fowler C. M. R.1996Influence of sea-floor spreading on the global hydrothermal vent fauna. Nature 379, 531–533 (doi:10.1038/379531a0) [Google Scholar]
- Tyler P. A.1980Deep-sea ophiuroids. Oceanogr. Mar. Biol. Annu. Rev. 18, 125–153 [Google Scholar]
- Tyler P. A.1988Seasonality in the deep sea. Oceanogr. Mar. Biol. Annu. Rev. 26, 227–258 [Google Scholar]
- Tyler P. A., Young C. M.1999Reproduction and dispersal at vents and cold seeps. J. Mar. Biol. Assoc. UK 79, 193–208 (doi:10.1017/S0025315499000235) [Google Scholar]
- Tyler P. A., Young C. M.2003Dispersal at hydrothermal vents: a summary of recent progress. Hydrobiologia 503, 9–19 (doi:10.1023/B:HYDR.0000008492.53394.6b) [Google Scholar]
- Tyler P. A., Young C. M., Clarke A.2000Temperature and pressure tolerances of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri (Echinodermata: Echinoidea): potential for deep-sea invasion from high latitudes. Mar. Ecol. Prog. Ser. 192, 173–180 (doi:10.3354/meps192173) [Google Scholar]
- Van Dover C. L.1995Ecology of Mid-Atlantic Ridge hydrothermal vents. In Hydrothermal vents and processes (eds Parson L. M., Wlaker C. L., Dixon D. R.), pp. 257–294 London, UK: Geological Society of London Special Publication [Google Scholar]
- Van Dover C. L.2000The ecology of deep-sea hydrothermal vents. Princeton, NJ: Princeton University Press [Google Scholar]
- Van Dover C. L., Jenkins C. D., Turnipseed M.2001Corralling of larvae in the deep sea. J. Mar. Biol. Assoc. UK 81, 823–826 (doi:10.1017/S0025315401004659) [Google Scholar]
- Van Dover C. L., German C. R., Speer K. G., Parson L. M., Vrijenhoek R. C.2002Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295, 1253–1257 (doi:10.1126/science.1067361) [DOI] [PubMed] [Google Scholar]
- Vanreusel A., et al. 2010The contribution of deep-sea macrohabitat heterogeneity to global nematode diversity. Mar. Ecol. 31, 6–20 (doi:10.1111/j.1439-0485.2009.00352.x) [Google Scholar]
- Vetter E. W., Dayton P. K.1998Macrofaunal communities within and adjacent to a detritus-rich submarine canyon system. Deep-Sea Res. II 45, 25–54 (doi:10.1016/S0967-0645(97)00048-9) [Google Scholar]
- Villalobos F. B., Tyler P. A., Young C. M.2006Temperature and pressure tolerance of embryos and larvae of the Atlantic seastars Asterias rubens and Marthasterias glacialis (Echinodermata: Asteroidea): potential for deep-sea invasion. Mar. Ecol. Prog. Ser. 314, 109–117 (doi:10.3354/meps314109) [Google Scholar]
- Vinogradova N. G.1997Zoogeography of the abyssal and hadal zones. Adv. Mar. Biol. 32, 325–387 (doi:10.1016/S0065-2881(08)60019-X) [Google Scholar]
- Voight J. R.2009Deep-sea wood-boring bivalves of Xylophaga (Myoida: Pholadidae) on the continental shelf: a new species described. J. Mar. Biol. Assoc. UK 88, 1459–1464 [Google Scholar]
- Vrijenhoek R.1997Gene flow and genetic diversity in naturally fragmented metapopulations of deep-sea hydrothermal vent animals. J. Heredity 88, 285–293 [DOI] [PubMed] [Google Scholar]
- Vrijenhoek R.2008Cryptic species, phenotypic plasticity, and complex life histories: assessing deep-sea faunal diversity with molecular markers. Deep-Sea Res. II 56, 1713–1723 (doi:10.1016/j.dsr2.2009.05.016) [Google Scholar]
- Waelbroeck C., Labeyrie L., Michel E., Duplessy J. C., McManus J. F., Lambeck K., Balbon E., Labracherie M.2001Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quat. Sci. Rev. 21, 295–305 (doi:10.1016/S0277-3791(01)00101-9) [Google Scholar]
- Watanabe H., et al. 2004Larval development and intermould period of the hydrothermal vent barancle Neoverruca sp. J. Mar. Biol. Assoc. UK 94, 743–745 [Google Scholar]
- Wilson G. D. F.1999Some of the deep-sea fauna is ancient. Crustaceana 72, 1019–1030 (doi:10.1163/156854099503915) [Google Scholar]
- Wilson R. R., Kaufmann R. S.1987Seamount biota and biogeography. In Seamounts, islands, and atolls (ed. Keating B. H., et al.), pp. 227–237 Washington, DC: American Geophysical Union [Google Scholar]
- Won Y., Young C. R., Lutz R. A., Vrijenhoek R. C.2003Dispersal barriers and isolation among deep-sea mussel populations (Mytilidae: Bathymodiolus) from eastern Pacific hydrothermal vents. Mol. Ecol. 12, 169–184 (doi:10.1046/j.1365-294X.2003.01726.x) [DOI] [PubMed] [Google Scholar]
- Wright S.1943Isolation by distance. Genetics 28, 114–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. M.2004Reproduction, development and life-history traits. In Ecosystems of the deep oceans (ed. Tyler P. A.), pp. 381–426 Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- Young C. M., George S. B.2000Larval development of the tropical deep-sea echinoid Aspidodiadema jacobyi: phylogenetic implications. Biol. Bull. 198, 387–395 (doi:10.2307/1542694) [DOI] [PubMed] [Google Scholar]
- Young C. M., Sewell M. A., Tyler P. A., Metaxas A.1997Biogeographic and bathymetric ranges of Atlantic deep-sea echinoderms and ascidians: the role of larval dispersal. Biodivers. Conserv. 6, 1507–1522 (doi:10.1023/A:1018314403123) [Google Scholar]
- Young C. R., Fujio S., Vrijenhoek R. C.2008Directional dispersal between mid-ocean ridges: deep-ocean circulation and gene flow in Ridgeia piscesae. Mol. Ecol. 17, 1718–1731 (doi:10.1111/j.1365-294X.2008.03609.x) [DOI] [PubMed] [Google Scholar]
- Zardus J. D., Etter R. J., Chase M. R., Rex M. A., Boyle E. E.2006Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939). Mol. Ecol. 15, 639–651 (doi:10.1111/j.1365-294X.2005.02832.x) [DOI] [PubMed] [Google Scholar]
- Zulliger D. E., Tanner S., Ruch M., Ribi G.2009Genetic structure of the high dispersal Atlanto-Mediterreanean sea star Astropecten aranciacus revealed by mitochondrial DNA sequences and microsatellite loci. Mar. Biol. 156, 597–610 (doi:10.1007/s00227-008-1111-z) [Google Scholar]