Abstract
Chamomile has long been used in traditional medicine for the treatment of inflammation-related disorders. In this study we aimed to investigate the inhibitory effects of chamomile on nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression, and to explore its potential anti-inflammatory mechanisms using RAW 264.7 macrophages. Chamomile treatment inhibited LPS-induced NO production and significantly blocked IL-1β , IL-6 and TNFα-induced NO levels in RAW 264.7 macrophages. Chamomile caused reduction in LPS-induced iNOS mRNA and protein expression. In RAW 264.7 macrophages, LPS-induced DNA binding activity of RelA/p65 was significantly inhibited by chamomile, an effect that was mediated through the inhibition of IKKβ , the upstream kinase regulating NF-κ B/Rel activity, and degradation of inhibitory factor-κ B. These results demonstrate that chamomile inhibits NO production and iNOS gene expression by inhibiting RelA/p65 activation and supports the utilization of chamomile as an effective anti-inflammatory agent.
Keywords: inflammation, nitric oxide, chamomile, nuclear factor-κ B, macrophages, pro-inflammatory cytokines
INTRODUCTION
Chronic inflammation of longstanding duration plays a critical role in the initiation and development of various human diseases including cancer [1]. Macrophages play a central role in the inflammatory response and produce excess amounts of pro-inflammatory cytokines, pro-inflammatory enzymes and inflammatory mediators, the accumulation of which leads to disease development [2, 3]. Activated macrophages transcriptionally express inducible nitric oxide synthase (iNOS), which catalyzes the oxidative deamination of L-arginine to produce nitric oxide (NO), and is responsible for prolonged and profound production of NO [4]. High output of NO by iNOS induces deleterious effects such as inflammation and cancer [5, 6].
Molecular cloning and sequencing analysis studies have demonstrated the existence of at least 3 main types of NOS isoforms, endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) [7]. Expression of the iNOS gene is regulated at the transcriptional level by the NF-κ B/Rel family of transcription factors, which are involved in regulation of immune and inflammatory responses. The murine iNOS promoter contains two NF-κ B/Rel binding sites located at 55 and 971 base pairs upstream of the TATA box. Moreover, it has been reported that the protein binding to both of these–κ B sites is necessary for the full induction of the iNOS gene by pro-inflammatory mediators such as lipopolysaccharide [8, 9]. In un-stimulated cells, NF-κ B/Rel exists in an inactive state in the cytoplasm, complexed with an inhibitory protein called Iκ B. Upon activation, Iκ B undergoes phosphorylation and degradation and the NF-κ B/Rel heterodimer is translocated into the nucleus where it binds to DNA and activates transcription [10]. A number of inflammatory stimuli and pro-inflammatory cytokines activate immune cells to upregulate inflammation, and consequently are potential targets for exploration of the molecular mechanisms underlying the activation processes, with the aim of developing effective anti-inflammatory drugs to ameliorate their untoward effects.
Chamomile is an herbal plant that has been used for centuries in many human cultures to treat various inflammatory conditions such as eczema, ulcers, gout, neuralgia and rheumatic pains [11, 12]. Dried flowers of Matricaria chamomilla L. are used in the preparation of tea, which is consumed at a rate of more than a million cups per day [13]. The beneficial effects of chamomile are related to the presence of several flavonoid constituents and the core structure consists of either flavone (apigenin, luteolin) or flavonol-derivatives (quercetin, patuletin). These occur in various forms such as aglyco- mono- and di-glycosides and/or acyl-derivatives. Other principal components are essential oils such as terpenoids, α-bisabolol and its oxides, azulenes including chalmuzene and acetylene derivatives [14].
Chamomile has been approved by the German Commission E for oral consumption in the management of various inflammatory diseases of the gastrointestinal tract, and for topical application in the treatment of various skin disorders and inflammatory disorders of certain mucosal surfaces, such as the oral cavity and ano-genital areas [15]. Recent studies have demonstrated its antioxidant, hypocholesterolemic, anti-parasitic, anti-aging, and anticancer properties, supporting its longstanding traditional use for treating various human ailments [16–18]. In previous investigations, we have demonstrated that chamomile is a selective COX-2 inhibitor with anti-inflammatory activities [19]. In the current study, we investigated the effects of chamomile on NO synthesis in LPS activated macrophages and analyzed the underlying mechanisms of action using RAW 264.7 murine macrophages.
MATERIALS AND METHODS
Materials
Dry chamomile flower of Egyptian origin was purchased from Bec's Tea Nirvana, Cleveland, Ohio. Cell culture medium, DMEM, fetal bovine serum, penicillin–streptomycin cocktail and phosphate buffered saline were purchased from Cellgro Mediatech, Inc. (Herndon, VA). Lipopolysaccharide (LPS, E coli), and apigenin 7-O-glucoside (>95% pure) were purchased from Sigma (St. Louis, MO). Mouse rTNF- α , mouse rIL-6, and mouse rIL-1β were purchased from R&D Systems (Minneapolis, MN). L-NMMA NG-monomethyl-L-arginine, monoacetate salt was purchased from Calbiochem (Brookfield, WI). All reagents used in the experiments were of analytical reagent grade or HPLC grade where applicable.
Preparation of extracts
Dry chamomile flowers were weighed and crushed to powder with a marble pestle and mortar and a 5% w/v suspension was prepared in a flask by adding hot boiled water. The flask was then placed on a shaker (200 rpm) for 4 h and the temperature was maintained at 37°C. After shaking, the flask was brought to room temperature and the suspension was filtered through a series of Whatman filters and finally passed through 0.22 micron filter (Millipore, Billerica, MA). The filtered aqueous extract was freeze-dried and stored at −20°C until use. For cell culture studies, the dried material from aqueous extract was weighed and dissolved in culture medium to achieve desired concentration.
Cell culture
Murine RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco's modified essential medium in appropriate culture conditions. Cell stimulation was performed with 1 μg/mL of LPS, mouse rTNF-α , mouse rIL-6, and mouse rIL-1β .
Cell viability assay
Cell respiration, an indicator of cell viability, was determined by the mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan. After the supernatants were removed for nitrite assay, cells were incubated at 37° with MTT (0.5 mg/mL) for 45 min. The medium was aspirated and cells were solubilized in dimethyl sulfoxide (250 μL) for at least 2 h in the dark. The extent of reduction of MTT was quantified by optical density measurement at 550 nm.
Nitrite estimation
RAW 264.7 macrophages were cultured in 6 well plates. After incubation with LPS and other cytokines and various doses of chamomile for 12–24 h, nitric oxide synthesis was determined by assaying the culture medium for nitrite, which is the stable reaction product of nitric oxide with molecular oxygen, using Griess reagent kit obtained from Biotium (Hayward, CA).
Western blot analysis
Macrophages, grown in 6-well plates to confluence, were incubated with or without LPS in the absence or presence of the test agents for 16 h. Cells were washed with ice-cold PBS and stored at 70° until further analysis. Frozen plates were put on ice and cells were lysed in 1% Triton X-100, 0.15 M NaCl, and 10 mM Tris-HCl pH 7.4 for 30 min. Lysates were homogenized through a 22 G needle and centrifuged at 10,000 g for 10 min at 4°. The supernatants were collected and protein was measured by the method according to Bradford, 1976 [20]. Cell lysates, containing equal amounts of protein, were boiled in SDS sample buffer for 5 min before running on a 10% SDS–polyacrylamide gel. Proteins were transferred to polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA). Membranes were blocked with 5% fat-free dry milk in TBS-T pH 8.0 (Tris-buffered saline [50 mM Tris, pH 8.0, 150 mM NaCl] with 0.1% Tween 20) and then incubated with antibodies viz. anti-iNOS (SC-7271), anti-Iκ Bα (SC- 1643), anti-NF-κ B/RelA (SC-8008) anti-β-actin (SC-47778) obtained from SantaCruz (SantaCruz, CA), anti-p-IKKα /IKKβ (Ser180/181; Cat#2681), anti-p-Iκ Bα (Ser32/36; Cat#9246) obtained from Cell Signaling Technology (Beverly, MA) with appropriate dilutions and incubated overnight at 4°C. After washing 3 times with TBS-T, and the bands were visualized by using appropriate IgG:horseradish peroxidase conjugate and the enhanced chemiluminescence system (ECL , Amersham Pharmacia Biotech). Signal intensities were evaluated by densitometric analysis (Kodak Digital Science Image Station 2000R Life Science Products).
Reverse transcriptase (RT)-PCR analysis
RAW 264.7 cells (5 × 106 cells-100 mm dish) were incubated for 8 h with or without various concentrations of chamomile and LPS (1 μg/ml). After washing with PBS twice, total RNA was isolated from the cell pellet using RNA isolation kit (Invitrogen, CA). The total amount of RNA was determined by absorbance at 260 nm. One microgram (μg) of RNA was reverse transcribed into cDNA using avian myeloblastosis virus (AMV) reverse transcriptase and oligo(dT)15 primer (Promega Co., Madison, WI, USA). The PCR samples contained 50 μl of the reaction mixture, comprised of 50 mM KCl, 5 mM MgCl2, 0.16 mM dNTP, 5.0 units of Taq DNA polymerase (Qiagen, Valencia, CA, USA), and 20 pmol of sense and antisense primers in 10 mM Tris-HCl (pH 8.3). The primer for iNOS were 5’-CAGTTCTGCGCCTTTGCTCAT-3’ (sense) and 5’-GGTGGTGCGGCTGGACTTT-3’ (antisense), and those for GAPDH 5’-AGGCCGGTGCTGAGTATGTC-3’ (sense) and 5’-TGCCTGCTTCACCACCTTCT-3’ (antisense). The PCR amplification was performed under the following conditions: 30 cycles of denaturation at 98°C for 30 sec, annealing at 60°C for 30 sec and extension at 74 °C for 1 min, using a thermal cycler (Px2, Thermo Electron Corporation). The amplified PCR products were run on a 2% agarose gel and visualized by SYBR Gold staining.
Electrophoretic mobility shift assay (EMSA)
EMSA for NF-κ B was performed in the nuclear fraction of RAW 264.7 macrophages incubated for 16 h with or without various concentrations of chamomile and LPS (1 μg/ml) using LightshiftTM Chemiluminiscent EMSA kit (Pierce Biotechnology, Rockford, IL) following manufacturer’s protocol as previously described [21].
Statistical analysis
Nitrite estimation and cell viability were performed in triplicate. All experiments were repeated at least twice. Results are expressed as mean values ± SEM. Statistical comparisons were made by ANOVA followed by a Dunnett's multiple comparison test. P values < 0.05 were considered significant.
RESULTS
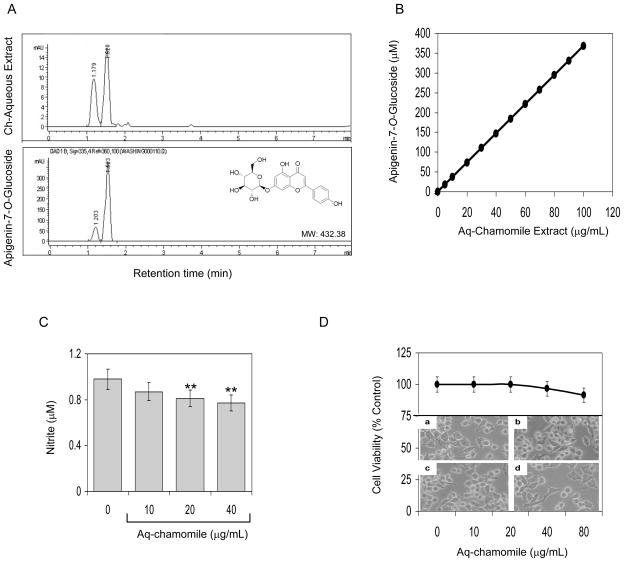
First, we performed HPLC analysis on aqueous chamomile extract with reference to apigenin 7-O-glucoside, the major constituent of chamomile. As shown in figure 1A, HPLC analysis demonstrated two major peaks with retention times of 1.179 min (27.7%) and 1.520 min (63.3%) and 5 other minor peaks which together constitute 90% of the total flavonoids. The two major peaks in the aqueous chamomile extract correspond to apigenin 7-O-glucoside (63.3%) and apigenin 7-O-neohesperidoside (27.7%), which was further confirmed by LC-MS analysis as previously demonstrated [12].
Figure 1.
(A) HPLC chromatogram of aqueous chamomile extract demonstrates apigenin 7-O-glucoside as major constituent (B) standardization of chamomile extract with apigenin 7-O-glucoside concentration (C) effect of chamomile on endogenous nitrite levels in culture medium of RAW 264.7 macrophages. Bars represent mean ± SEM of at least 3 independent experiments each performed in triplicate **P<0.05 (ANOVA) and (D) effect of chamomile on cell viability as determined by MTT assay and lower panel shows photograph of macrophages (a–d) after treatment with 0, 10, 20 and 40 μg/mL chamomile extract. Details are described in ‘Materials and methods’ section.
Next we standardized aqueous chamomile extract with doses equivalent to molar concentration of apigenin 7-O-glucoside. For this study, different concentration of apigenin 7-O-glucoside were prepared in methanol and subjected to HPLC (Figure 1A). The peak area (retention time 1.5 to 1.7 min) were calculated and plotted to obtain a standard curve, which corresponded to the concentration of apigenin 7-O-glucoside in the aqueous extract on the basis of peak area (Figure 1B).
Following this, we determined the effect of aqueous chamomile extract on inhibition of constitutive NOS expression in RAW 264.7 cells by measuring the levels of total nitrite. The amount of nitrite, a stable metabolite of NO in the cell culture medium was estimated using Griess reagent as an index for nitric oxide. RAW 264.7 macrophages in the un-stimulated state produced 0.98 + 0.01 μM nitrite in the medium. Treatment of macrophages with chamomile cause a modest decrease in the endogenous NO levels in RAW 264.7 cells which was more pronounced at 20- and 40-μg/mL doses of chamomile (Figure 1C). Chamomile exposure did not affect cell viability at the test concentration up to 40-μg/mL. At 80-μg/mL chamomile, a modest decrease in cell viability was observed.
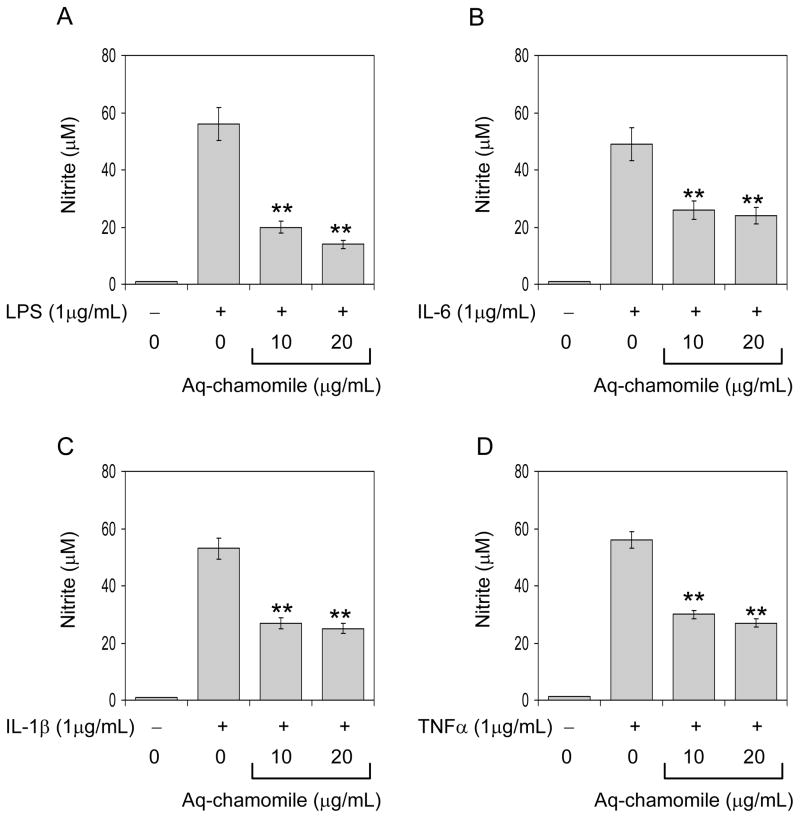
Since IL-1β , IL-6 and TNFα are known pro-inflammatory cytokines and LPS causes NO production, we examined whether chamomile is capable of inhibiting NO levels after challenge with these pro-inflammatory mediators. As shown in figure 2A-D, chamomile at 10- and 20-μg/mL doses significantly blocked NO production after challenge of RAW 264.7 cells with these molecules up to 40–60%. Since the NO production was highest with LPS challenge, therefore for further experiments we used LPS (1μg/mL).
Figure 2.
Effect of chamomile on NO production in culture medium of RAW 264.7 cells (A) RAW 264.7 cells activated with 1μg/mL LPS (B) 1μg/mL IL-6, (C) 1μg/mL IL-1β , and (D) 1μg/mL TNFα in the absence and presence of chamomile (10 and 20 μg/mL) for 16 h. Bars represent mean ± SEM of at least 3 independent experiment each performed in triplicate, **P<0.001 (ANOVA), compared to LPS-challenge group. Details are described in ‘Materials and methods’ section.
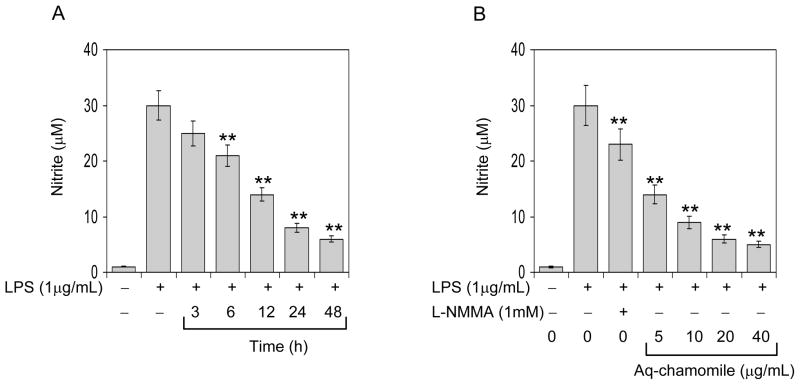
Next we performed time course experiments to determine the decrease in levels of NO by chamomile. As shown in figure 3A, treatment of RAW 264.7 cells with 10-μg/mL for 3 h to 48 h caused a significant decrease in NO production in the culture medium by 16.6% starting as early as 3 h and 79.8% at 48 h, respectively. In dose-dependent assay, treatment of RAW 264.7 macrophages with LPS for 24 h, the nitrite concentration in the culture medium increased to about 30-fold. Treatment with chamomile caused a significant decrease in NO production with 53.3% at 5-μg/mL to 83.3% at the highest dose of 40-μg/mL, respectively. However, a decrease of 23.3% in NO production was achieved after treatment of RAW 264.7 macrophages with L-NMMA, a non-selective inhibitor of all NOS isoforms (Figure 4B).
Figure 3.
Effect of chamomile on NO production in the culture medium of RAW 264.7 macrophages (A) time-dependent effect using 1 μg/mL LPS in the absence and presence of 10 μg/mL chamomile for indicated times. (B) dose-dependent effect using 1μg/mL LPS for 16 h in the presence and absence of chamomile at indicated doses. L-NMMA (1 mM), a non-selective inhibitor of all NOS isoforms was used as positive control. Bars represent mean ± SEM of at least 3 independent experiments each performed in triplicate, **P<0.001 (ANOVA) compared to LPS challenge group. Details are described in ‘Materials and methods’ section.
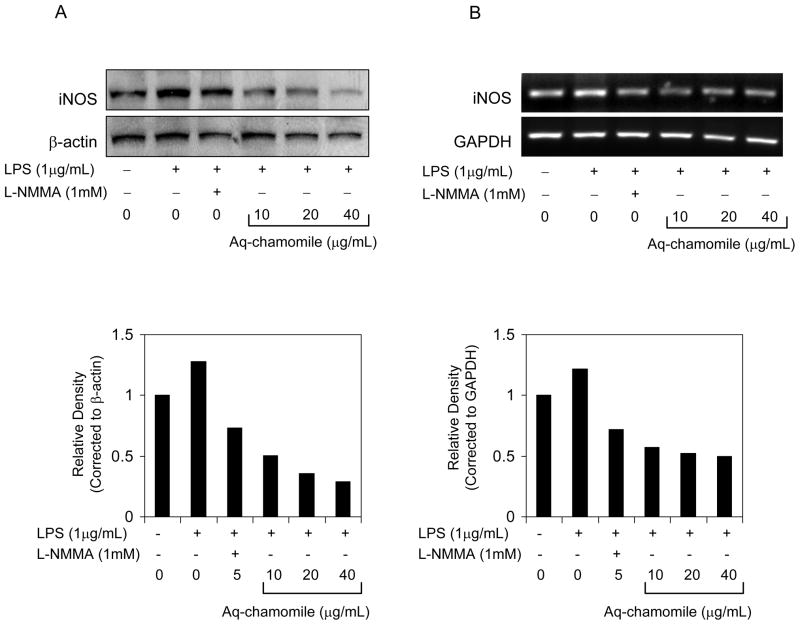
Figure 4.
Effect of chamomile on iNOS expression in RAW 264.7 macrophages. (A) Western blot for iNOS protein expression, and (B) mRNA expression of iNOS in RAW 264.7 macrophages stimulated with 1 μg/mL LPS and LPS and chamomile as indicated. Graph represents protein and mRNA levels of iNOS corrected to the corresponding controls. L-NMMA, a non-selective inhibitor of all NOS isoforms was used as positive control. Details are described in ‘Materials and methods’ section.
The marked reduction in nitrite production with chamomile suggests that it might also affect the iNOS protein expression. Therefore, we examined iNOS protein expression by Western Blot analysis. As shown in Figure 4A, RAW 264.7 macrophages incubated with LPS (1μg/mL) in the absence and presence of chamomile at 10–40 μg/mL concentration for 16 h and the cell extract were examined for 133 KDa iNOS protein. As shown in figure 4A, reduced iNOS protein expression were observed after chamomile treatment compared to LPS alone challenge, which was dose-dependent. At the message level, chamomile treatment to LPS challenged RAW 264.7 macrophages resulted in a modest decrease in mRNA expression (Figure 4B).
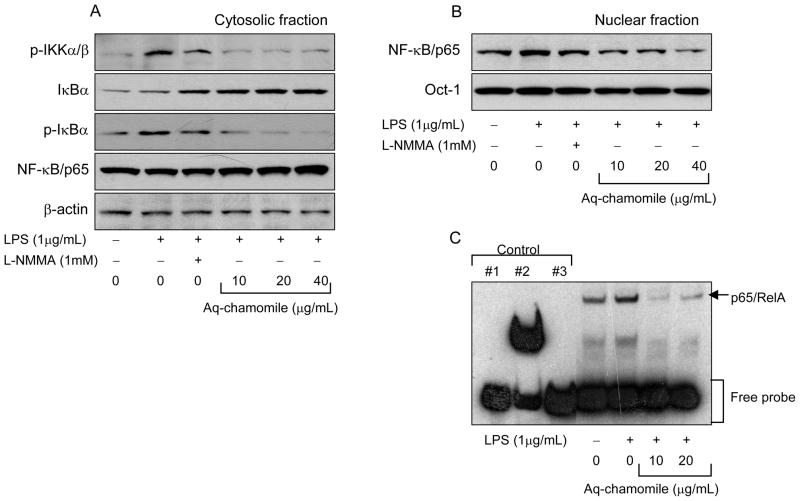
Since LPS-mediated activation of NF-κ B is associated with prolonged activation of IKK, the upstream kinase, we first sought to determine whether chamomile can alter the level of this kinase. As shown in figure 5A, LPS challenge to RAW 264.7 macrophages resulted in a significant increase in phosphorylation of IKKα /β which was markedly reduced with chamomile treatment in dose-dependent manner. Activation of IKK leads to the hyper-phosphorylation of Iκ Bα as well as its subsequent degradation therefore next we measured the cytosolic levels of Iκ Bα and its phosphorylated forms. Treatment with chamomile resulted in an increase in the total levels of Iκ Bα in the cytosol whereas a significant decrease in the p-Iκ Bα at Serine 32/36 was observed after chamomile treatment which was dose-dependent. Furthermore, treatment of RAW 264.7 cells with chamomile caused a decrease in the nuclear levels of NF-κ B/p65 which correlated with a simultaneous increase in the cytosol in dose-dependent manner (Figure 5B).
Figure 5.
Effect of chamomile on NF-κ B activity. (A) Western blot analysis for protein expression of p- IKKα /β , Iκ Bα and its phosphorylation, and RelA/p65 in the cytosolic fraction of RAW 264.7 macrophages stimulated with 1μg/mL LPS and LPS and chamomile for indicated doses, (B) RelA/p65 expression in the nuclear fraction, (C) EMSA assay. EMSA was performed to determine the effect of chamomile on the nuclear translocation of NF-κ B dimers and their binding to DNA. Controls: #1 Biotin-EBNA control DNA, #2 Biotin-EBNA control DNA+EBNA extract, #3 Biotin-EBNA control DNA + EBNA extract + 20-fold molar excess of unlabeled EBNA DNA. L-NMMA, a non-selective inhibitor of all NOS isoforms was used as positive control. The details are described in ‘Materials and Methods’ section.
To further confirm NF-κ B-mediated iNOS gene regulation, an EMSA was performed using an oligonucleotide containing a consensus RelA/p65 binding sequence in the nuclear fractions prepared after LPS challenge and chamomile treatment. A strong NF-κ B band was observed in response to LPS- induced activation which was reduced with chamomile treatment (Figure 5C). These results demonstrate that chamomile blocked NF-κ B activation, which might account for the inhibition of iNOS induction in RAW 264.7 macrophages.
DISCUSSION
Chamomile is known to possess anti-inflammatory and antioxidant effects. In the present study, we demonstrated that chamomile inhibits NO production and iNOS expression in macrophages, and showed that these effects are mediated through the inhibition of NF-κ B/Rel transcription factor. As stated earlier NO plays an important role in the pathogenesis of various inflammatory diseases, including cancer. Therefore, the inhibitory effect of chamomile on iNOS gene expression suggests that this is one of the mechanisms responsible for its anti-inflammatory properties.
Macrophages play a central role in a host’s defense against various infections by nature of their phagocytic, cytotoxic, and intracellular killing capacities [22]. Stimulation of murine macrophages by LPS, the major component of Gram-negative bacterial cell walls, results in expression of iNOS [23]. Increased production of NO plays a critical role in the process of macrophage activation and is associated with acute and chronic inflammations [24]. Therefore, the suppression of NO production by inhibition of iNOS expression and/or enzyme activity is potentially a very important therapeutic strategy in the development of therapeutic anti-inflammatory agents. In the current study, we have demonstrated that chamomile inhibits LPS-induced NO production in RAW 264.7 macrophages. The inhibitory effect of chamomile was mediated via a reduction in iNOS both at the protein and message levels.
Macrophages are capable of secreting various mediators such as IL-1, IL-6, granulocyte- macrophage colony-stimulating factor (GM-CSF) and TNFα , which lead to secondary immune responses such as proliferation of T and B cells, activation of macrophages for phagocytosis, and killing of microorganisms. Among these mediators, pro-inflammatory cytokines such as IL-1β , IL-6 and TNFα can be generated in response to immunological reaction, inflammation and microbial invasion [25]. Therefore, we sought to examine whether chamomile could alter NO production after incubation of RAW 264.7 macrophages with these cytokines along with chamomile. Our results demonstrate that chamomile may, in part, exert inhibitory effects on pro-inflammatory cytokines through inhibition of NO production in RAW 264.7 macrophages.
NO production by iNOS is regulated mainly at the transcriptional level, and the expression of the iNOS gene in macrophages is under the control of several transcription factors, which include NF- κ B/RelA [26]. The cis-acting NF-κ B element's presence has been demonstrated in the 5′ flanking regions of both the COX-2 and iNOS genes [27, 28]. Recently we have demonstrated that chamomile differentially inhibits LPS-induced COX-2 activity and expression in RAW 264.7 macrophages [19]. NF-κ B is functional as a hetero- or homo-dimeric form of the Rel family proteins, including RelA (p65), RelB, cRel, p50 and p52 [29. In resting cells, cytoplasmic Iκ B proteins (Iκ Bα , Iκ Bβ , and Iκ Bε) are associated with NF-κ B dimmers, and some stimuli, such as IL-1β and LPS, lead to prolonged activation of IKK, the upstream kinase regulating NF-κ B [30]. Our studies demonstrate that chamomile in a dose-dependent manner suppresses LPS-mediated IKK expression, NF-κ B activation and its translocation to the nucleus. These results suggest that chamomile inhibits the expression of iNOS and thus NO production, a process that is mediated through inactivation of NF-κ B by reducing Iκ Bα degradation and phosphorylation.
It is well known that RelA/p65 is a critical transactivation subunit for NF-κ B [31, 32]. Our studies performed through EMSA demonstrate that chamomile inhibits RelA/p65 activation. Moreover, recent studies have demonstrated that the transcriptional activity of RelA/p65 subunit is regulated by posttranslational modifications such as phosphorylation and acetylation [33]. It has been shown that the phosphorylation status of RelA determines whether it associates with CREB-binding protein/p300, which is a critical regulator of NF-κ B [34]. In this regard, it is possible that chamomile suppresses transcriptional activity of RelA/p65 by modifying the phosphorylation or acetylation status of the RelA/p65 subunit. Further studies are required to understand as how chamomile regulates the transcriptional activity of the RelA/p65 subunit.
In summary, this study demonstrates that chamomile inhibits LPS-induced NO production and iNOS gene expression in macrophages and that these effects are mediated, at least in part, through blockage of NF-κ B/Rel transcriptional activation. The fact that NF-κ B/Rel is negatively regulated by chamomile is important, because this transcription factor plays a critical role in the regulation of a variety of genes that are involved in inflammatory responses. Since chamomile is a nontoxic and pharmacologically active compound that has demonstrable inhibitory effects on iNOS gene expression, and since NO plays an important role in mediating inflammatory responses, our study supports the utilization of chamomile as a potentially effective therapeutic anti-inflammatory agent.
Acknowledgments
Financial Support: This work was supported by grants from United States Public Health Services RO1 AT002709 and RO1 CA108512
Abbreviations
- EMSA
electrophoretic mobility shift assay
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HPLC
high performance liquid chromatography
- NO
nitric oxide
- NOS
nitric oxide synthase
- IL-1β
interleukin 1beta
- TNFα
tumor necrosis factor-alpha
- LPS
lipopolysaccharide
- NF-κ B
nuclear factor-κ appaB
- IKK
Iκ appaB kinase
References
- 1.Fujihara M, Muroi M, Tanamoto K, et al. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Ahn KS, Noh EJ, Zhao HL, et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by platycodon grandiflorum saponins via suppression of nuclear factor-kappa B activation in RAW 264.7 cells. Life Sci. 2005;76:2315–2328. doi: 10.1016/j.lfs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Makarov SS. NF-kappa B as a therapeutic target in chronic inflammation: recent advances. Molecular Medicine Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 4.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 5.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 6.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- 7.Noh EJ, Ahn KS, Shin EM, Jung SH, Kim YS. Inhibition of lipopolysaccharide-induced iNOS and COX-2 expression by dehydroevodiamine through suppression of NF-κB activation in RAW 264.7 macrophages. Life Sci. 2006;79:695–701. doi: 10.1016/j.lfs.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 9.Lowenstein CJ, Alley EW, Raval P, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice NR, Ernst MK. In vivo control of NF-κB activation by IκBα. EMBO (Eur Mol Biol Organ) J. 1993;12:4685–4695. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L) Phytother Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava JK, Gupta S. Antiproliferative and Apoptotic Effects of Chamomile Extract in Various Human Cancer Cells. J Agric Food Chem. 2007;55:9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 13.Speisky H, Rocco C, Carrasco C, Lissi EA, López-Alarcón C. Antioxidant screening of medicinal herbal teas. Phytother Res. 2006;20:462–467. doi: 10.1002/ptr.1878. [DOI] [PubMed] [Google Scholar]
- 14.Ganzera M, Schneider P, Stuppner H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006;78:856–861. doi: 10.1016/j.lfs.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 15.Ross SM. Chamomile: a spoonful of medicine. Holistic Nursing Practice. 2008;22:56–57. doi: 10.1097/01.HNP.0000306329.41708.c6. [DOI] [PubMed] [Google Scholar]
- 16.Babenko NA, Shakhova EG. Effects of Chamomilla recutita flavonoids on age-related liver sphingolipid turnover in rats. Exp Gerontol. 2006;41:32–39. doi: 10.1016/j.exger.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Lee KG, Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J Agric Food Chem. 2002;50:4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava JK, Gupta S. Health promoting benefits of chamomile in the elderly population. In: Watson Ronald R., editor. Complementary and Alternative Therapies in the Aging Population. Elsevier Inc., Academic Press; 2009. [Google Scholar]
- 19.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Shukla S, MacLennan GT, Fu P, et al. Nuclear Factor-κB/p65 (Rel A) Is Constitutively Activated in Human Prostate Adenocarcinoma and Correlates with Disease Progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 23.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 24.Choi MS, Lee SH, Cho HS, et al. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol. 2007;556:181–189. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Lee JH, Ha TY. Effects of capsaicin pretreatment on the function of mouse peritoneal macrophages. Kor J Immunol. 2000;22:39–49. [Google Scholar]
- 26.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 27.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 28.Lowenstein CJ, Alley EW, Raval P, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhou HY, Shin EM, Guo LY, et al. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Ballard DW, Dixon EP, Peffer NJ, et al. The 65-kDa subunit of human NF-kB functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci USA. 1992;89:1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz ML, Baeuerle P. The p65 subunit is responsible for the strong transcription activating potential of NF-kB. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeulen L, DeWilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]