Abstract
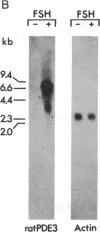
To elucidate the mechanisms by which hormones regulate cAMP phosphodiesterases (PDEs), a group of cDNA clones that had been isolated from a rat Sertoli cell library were characterized. These cDNAs are derived from a single gene (ratPDE3). The deduced amino acid sequence of the ratPDE3 cDNA corresponds to a 66,200-Da protein homologous to other testicular PDEs, to the Drosophila melanogaster dunce-encoded cAMP PDE, and to bovine and yeast PDEs. Expression of ratPDE3 in eukaryotic and prokaryotic cells leads to the appearance of a cAMP PDE with properties identical to the cAMP PDE purified from Sertoli cells. Although of different size, transcripts corresponding to ratPDE3 were present in all organs studied. In the immature Sertoli cell in culture, the level of mRNA transcripts of ratPDE3 was increased more than 100-fold by follicle-stimulating hormone or N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate treatment. Stimulation of ratPDE3 mRNA by N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate was also observed in a C6 glioma cell line. These data demonstrate that cAMP regulates the expression of one of its own degrading enzymes by an intracellular feedback mechanism that involves changes in mRNA levels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Tomkins G. M., Dion S. Regulation of phosphodiesterase synthesis: requirement for cyclic adenosine monophosphate-dependent protein kinase. Science. 1973 Sep 7;181(4103):952–954. doi: 10.1126/science.181.4103.952. [DOI] [PubMed] [Google Scholar]
- Bubis J., Neitzel J. J., Saraswat L. D., Taylor S. S. A point mutation abolishes binding of cAMP to site A in the regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 1988 Jul 15;263(20):9668–9673. [PubMed] [Google Scholar]
- Charbonneau H., Beier N., Walsh K. A., Beavo J. A. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Birchmeier C., Michaeli T., O'Neill K., Riggs M., Wigler M. Isolation and characterization of a mammalian gene encoding a high-affinity cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 May;86(10):3599–3603. doi: 10.1073/pnas.86.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M., Geremia R., Adamo S., Stefanini M. Regulation of Sertoli cell cyclic adenosine 3':5' monophosphate phosphodiesterase activity by follicle stimulating hormone and dibutyrl cyclic AMP. Biochem Biophys Res Commun. 1981 Feb 27;98(4):1044–1050. doi: 10.1016/0006-291x(81)91216-x. [DOI] [PubMed] [Google Scholar]
- Conti M., Kasson B. G., Hsueh A. J. Hormonal regulation of 3',5'-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology. 1984 Jun;114(6):2361–2368. doi: 10.1210/endo-114-6-2361. [DOI] [PubMed] [Google Scholar]
- Conti M., Monaco L., Geremia R., Stefanini M. Effect of phosphodiesterase inhibitors on Sertoli cell refractoriness: reversal of the impaired androgen aromatization. Endocrinology. 1986 Mar;118(3):901–908. doi: 10.1210/endo-118-3-901. [DOI] [PubMed] [Google Scholar]
- Conti M., Toscano M. V., Petrelli L., Geremia R., Stefanini M. Regulation of follicle-stimulating hormone and dibutyryl adenosine 3',5'-monophosphate of a phosphodiesterase isoenzyme of the Sertoli cell. Endocrinology. 1982 Apr;110(4):1189–1196. doi: 10.1210/endo-110-4-1189. [DOI] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geremia R., Rossi P., Pezzotti R., Conti M. Cyclic nucleotide phosphodiesterase in developing rat testis. Identification of somatic and germ-cell forms. Mol Cell Endocrinol. 1982 Sep;28(1):37–53. doi: 10.1016/0303-7207(82)90039-9. [DOI] [PubMed] [Google Scholar]
- Gettys T. W., Vine A. J., Simonds M. F., Corbin J. D. Activation of the particulate low Km phosphodiesterase of adipocytes by addition of cAMP-dependent protein kinase. J Biol Chem. 1988 Jul 25;263(21):10359–10363. [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Macphee C. H., Reifsnyder D. H., Moore T. A., Lerea K. M., Beavo J. A. Phosphorylation results in activation of a cAMP phosphodiesterase in human platelets. J Biol Chem. 1988 Jul 25;263(21):10353–10358. [PubMed] [Google Scholar]
- Maganiello V., Vaughan M. Prostaglandin E 1 effects on adenosine 3':5'-cyclic monophosphate concentration and phosphodiesterase activity in fibroblasts (mouse L cells-tissue culture-enzyme kinetics-prostaglandin homologues). Proc Natl Acad Sci U S A. 1972 Jan;69(1):269–273. doi: 10.1073/pnas.69.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H., Kono T. Characterization of insulin-sensitive phosphodiesterase in fat cells. II. Comparison of enzyme activities stimulated by insulin and by isoproterenol. J Biol Chem. 1980 Aug 25;255(16):7850–7854. [PubMed] [Google Scholar]
- Manganiello V. C., Yamamoto T., Elks M., Lin M. C., Vaughan M. Regulation of specific forms of cyclic nucleotide phosphodiesterases in cultured cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:291–301. [PubMed] [Google Scholar]
- Monaco L., Conti M. Localization of adenosine receptors in rat testicular cells. Biol Reprod. 1986 Sep;35(2):258–266. doi: 10.1095/biolreprod35.2.258. [DOI] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onali P., Schwartz J. P., Hanbauer I., Costa E. Regulation by a beta-adrenergic receptor of a Ca2+-independent adenosine 3',5'-(cyclic)monophosphate phosphodiesterase in C6 glioma cells. Biochim Biophys Acta. 1981 Jul;675(2):285–292. doi: 10.1016/0304-4165(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A. Cyclic nucleotides in bacteria. Adv Cyclic Nucleotide Res. 1976;7:1–48. [PubMed] [Google Scholar]
- Sass P., Field J., Nikawa J., Toda T., Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J., Meyer H., Epplen J. T. Cyclic AMP phosphodiesterases of the rat ovary. Oestrous cycle dependent activity change of high affinity form. Acta Endocrinol (Copenh) 1980 Nov;95(3):404–411. doi: 10.1530/acta.0.0950404. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Passonneau J. V. Cyclic AMP-mediated induction of the cyclic AMP phosphodiesterase of C-6 glioma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3844–3848. doi: 10.1073/pnas.71.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Zoller M. J., Uhler M. D., Helfman D. M., McKnight G. S., Krebs E. G. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]