Abstract
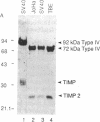
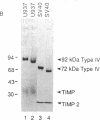
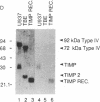
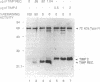
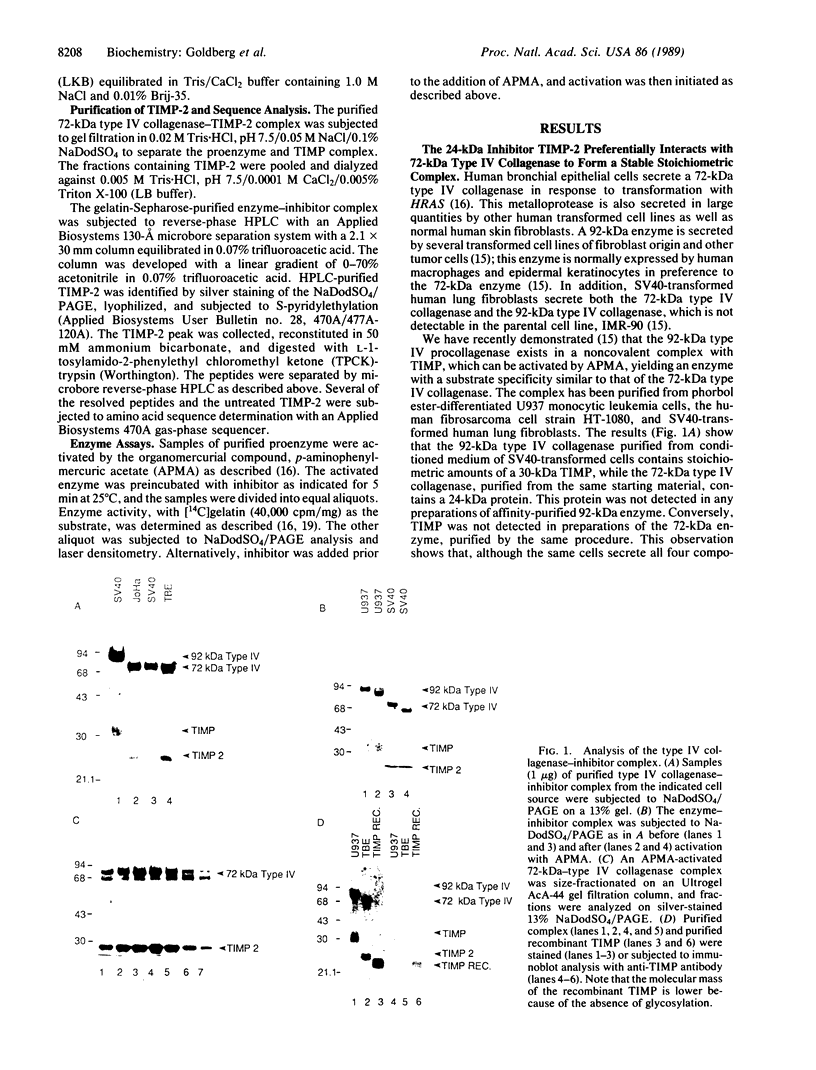
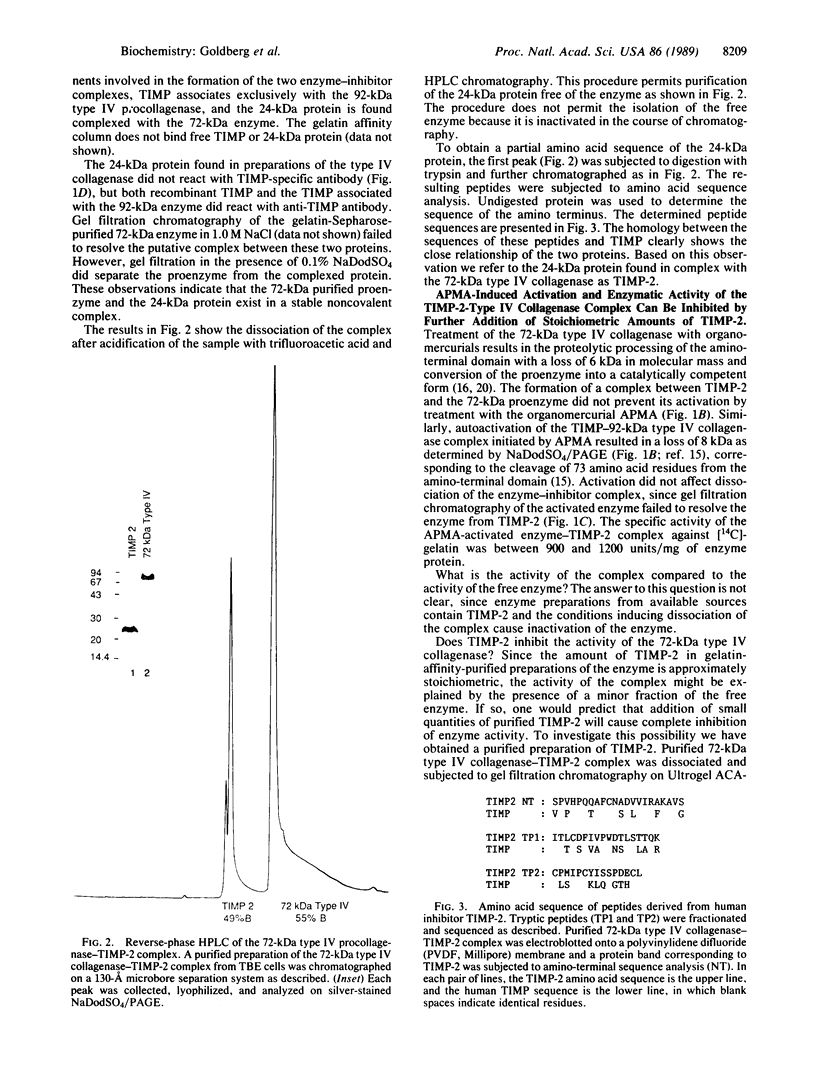
Simian virus 40 (SV40)-transformed human lung fibroblasts secrete both 72-kDa type IV collagenase and a closely related 92-kDa type IV collagenase that was not detected in the parental cell line. The 92-kDa type IV procollagenase purified from these cells exists in a noncovalent complex with the tissue inhibitor of metalloproteases, TIMP. Here we report that the 72-kDa type IV procollagenase purified from HRAS-transformed human bronchial epithelial cells, SV40-transformed lung fibroblasts, and normal skin fibroblasts exists in a stable but noncovalent stoichiometric complex with a 24-kDa inhibitor referred to here as "TIMP-2." TIMP-2 is closely related to TIMP, as demonstrated by comparison of the partial amino acid sequence of this protein to that of TIMP, although it does not cross-react with TIMP-specific antibody. The TIMP-2 inhibitor interacts with the 72-kDa type IV collagenase in preference to the 92-kDa type IV collagenase that forms a complex exclusively with TIMP. The 72-kDa type IV collagenase-TIMP-2 complex can be activated with organomercurials to yield a catalytically competent enzyme. Activation occurs concomitantly with autoproteolytic cleavage of the amino terminus of the protein and does not require dissociation of the complex. Both activity and activation of the complex can be completely inhibited by further addition of stoichiometric quantities of purified TIMP-2 or recombinant TIMP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmichael D. F., Sommer A., Thompson R. C., Anderson D. C., Smith C. G., Welgus H. G., Stricklin G. P. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2407–2411. doi: 10.1073/pnas.83.8.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Cooper T. W., Eisen A. Z., Stricklin G. P., Welgus H. G. Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc Natl Acad Sci U S A. 1985 May;82(9):2779–2783. doi: 10.1073/pnas.82.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe B., Skup D. In vitro synthesis of the active tissue inhibitor of metalloproteinases encoded by a complementary DNA from virus-infected murine fibroblasts. J Biol Chem. 1988 Jan 25;263(3):1439–1443. [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Golde D. W., Kaufman S. E., Westbrook C. A., Hewick R. M., Kaufman R. J., Wong G. G., Temple P. A., Leary A. C., Brown E. L. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. 1985 Jun 27-Jul 3Nature. 315(6022):768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. A primary genetic map of the pericentromeric region of the human X chromosome. Genomics. 1988 May;2(4):294–301. doi: 10.1016/0888-7543(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Grant S. G., Stephenson D. A., Chapman V. M. Multilocus molecular mapping of the mouse X chromosome. Genomics. 1988 Oct;3(3):187–194. doi: 10.1016/0888-7543(88)90078-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. B., Allison K., Sudhalter J., Langer R. Purification and partial amino acid sequence of a bovine cartilage-derived collagenase inhibitor. J Biol Chem. 1986 Mar 25;261(9):4154–4159. [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Tryggvason K. Purification and characterization of a murine basement membrane collagen-degrading enzyme secreted by metastatic tumor cells. J Biol Chem. 1983 Mar 10;258(5):3058–3063. [PubMed] [Google Scholar]
- Schultz R. M., Silberman S., Persky B., Bajkowski A. S., Carmichael D. F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988 Oct 1;48(19):5539–5545. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Stricklin G. P., Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry. 1978 Jun 13;17(12):2331–2337. doi: 10.1021/bi00605a012. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z., Roswit W. T., Stricklin G. P. Human skin fibroblast collagenase: interaction with substrate and inhibitor. Coll Relat Res. 1985 Mar;5(2):167–179. doi: 10.1016/s0174-173x(85)80038-8. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P., Eisen A. Z., Bauer E. A., Cooney R. V., Jeffrey J. J. A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem. 1979 Mar 25;254(6):1938–1943. [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]