Abstract
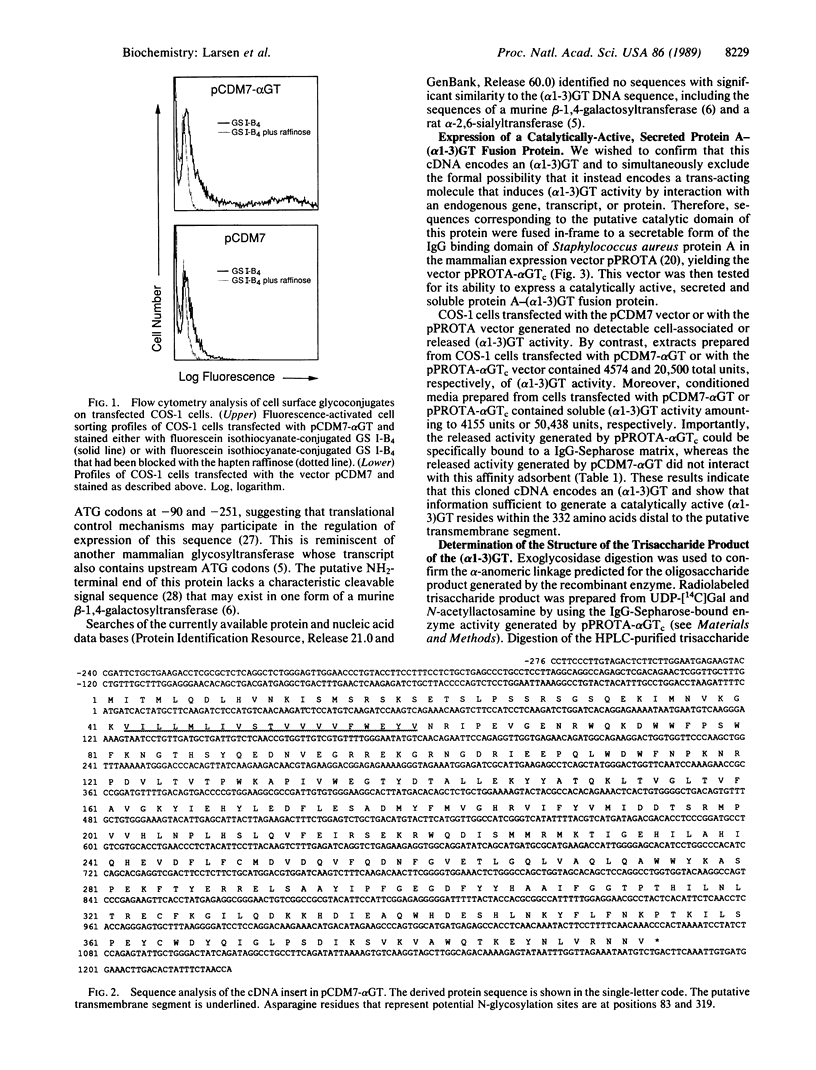
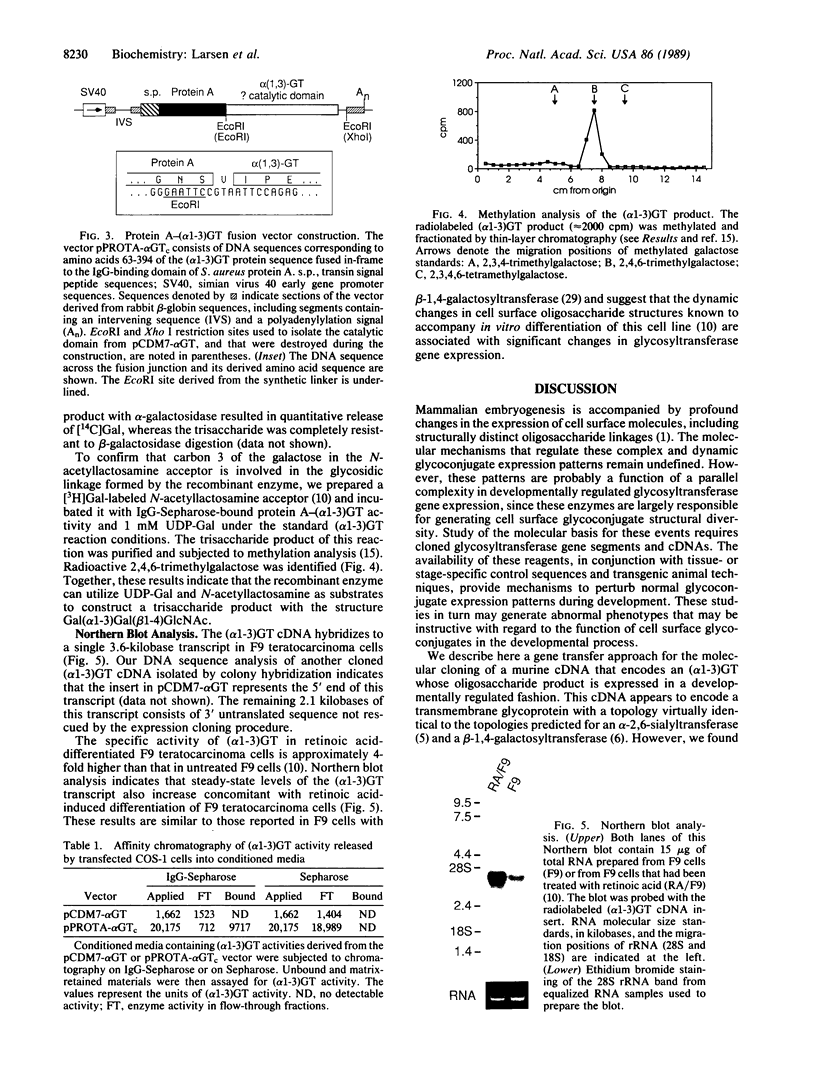
We have developed a genetic approach to isolate cloned cDNA sequences that determine expression of cell surface oligosaccharide structures and their cognate glycosyltransferases. A cDNA library was constructed in a mammalian expression vector by using mRNA from a murine cell line known to express a UDPgalactose:beta-D-galactosyl-1,4-N-acetyl-D-glucosaminide alpha-1,3-galactosyltransferase [(alpha 1-3)GT; EC 2.4.1.151]. This library was transfected into COS-1 cells, which lack expression of (alpha 1-3)GT. Transfected cells containing functional (alpha 1-3)GT cDNAs were detected and isolated with a lectin that recognizes the surface-expressed glycoconjugate product of the (alpha 1-3)GT enzyme. One cloned (alpha 1-3)GT cDNA was rescued from lectin-positive transfected cells. This cDNA contains a single long open reading frame that predicts a 394-amino-acid protein. No significant primary structure similarities were identified between this protein and other known sequences. However, the protein predicts a type II transmembrane topology similar to two other mammalian glycosyltransferases. This topology places the large COOH-terminal domain within the Golgi lumen; this domain was shown to be catalytically active when expressed in COS-1 cells as a portion of a secreted protein A fusion peptide. Biochemical analysis confirmed that this enzyme catalyzes a transglycosylation reaction between UDP-Gal and Gal(beta 1-4)GlcNAc to form Gal(alpha 1-3)Gal(beta 1-4)GlcNAc. This cloning approach may be generally applicable to the isolation of cDNAs encoding other mammalian glycosyltransferases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert H. E., Rutherford T. J., Tarr G. E., Wiest J. S., Thomford N. R., McCorquodale D. J. Isolation of a cDNA coding for human galactosyltransferase. Biochem Biophys Res Commun. 1986 Aug 29;139(1):163–168. doi: 10.1016/s0006-291x(86)80094-8. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken W. M., Van den Eijnden D. H. Biosynthesis of terminal Gal alpha 1----3Gal beta 1----4GlcNAc-R oligosaccharide sequences on glycoconjugates. Purification and acceptor specificity of a UDP-Gal:N-acetyllactosaminide alpha 1----3-galactosyltransferase from calf thymus. J Biol Chem. 1985 Oct 25;260(24):12927–12934. [PubMed] [Google Scholar]
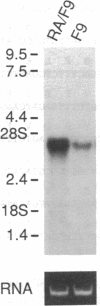
- Cummings R. D., Mattox S. A. Retinoic acid-induced differentiation of the mouse teratocarcinoma cell line F9 is accompanied by an increase in the activity of UDP-galactose: beta-D-galactosyl-alpha 1,3-galactosyltransferase. J Biol Chem. 1988 Jan 5;263(1):511–519. [PubMed] [Google Scholar]
- Delmas V., Bastien C., Scherneck S., Feunteun J. A new member of the polyomavirus family: the hamster papovavirus. Complete nucleotide sequence and transformation properties. EMBO J. 1985 May;4(5):1279–1286. doi: 10.1002/j.1460-2075.1985.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Blake D. A., Goldstein I. J. Purification and characterization of a UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase from Ehrlich ascites tumor cells. J Biol Chem. 1986 May 5;261(13):6064–6072. [PubMed] [Google Scholar]
- Ernst L. K., Rajan V. P., Larsen R. D., Ruff M. M., Lowe J. B. Stable expression of blood group H determinants and GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase in mouse cells after transfection with human DNA. J Biol Chem. 1989 Feb 25;264(6):3436–3447. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Guan J. L., Rose J. K. A membrane-anchored form but not the secretory form of human chorionic gonadotropin-alpha chain acquires polylactosaminoglycan. J Biol Chem. 1988 Apr 15;263(11):5314–5318. [PubMed] [Google Scholar]
- Galili U., Shohet S. B., Kobrin E., Stults C. L., Macher B. A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov 25;263(33):17755–17762. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Maillet C. M., Oleszkowicz K., Shur B. D. Cell surface and Golgi pools of beta-1,4-galactosyltransferase are differentially regulated during embryonal carcinoma cell differentiation. Mol Cell Biol. 1989 Jun;9(6):2370–2377. doi: 10.1128/mcb.9.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee R. F., Kavathas P., Berg P. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol Cell Biol. 1988 Jul;8(7):2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. Cloning and sequencing of cDNA of bovine N-acetylglucosamine (beta 1-4)galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4720–4724. doi: 10.1073/pnas.83.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Rastan S., Roelcke D., Feizi T. Saccharide structures of the mouse embryo during the first eight days of development. Inferences from immunocytochemical studies using monoclonal antibodies in conjunction with glycosidases. J Embryol Exp Morphol. 1985 Dec;90:335–361. [PubMed] [Google Scholar]
- Rajan V. P., Larsen R. D., Ajmera S., Ernst L. K., Lowe J. B. A cloned human DNA restriction fragment determines expression of a GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase in transfected cells. Evidence for isolation and transfer of the human H blood group locus. J Biol Chem. 1989 Jul 5;264(19):11158–11167. [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]