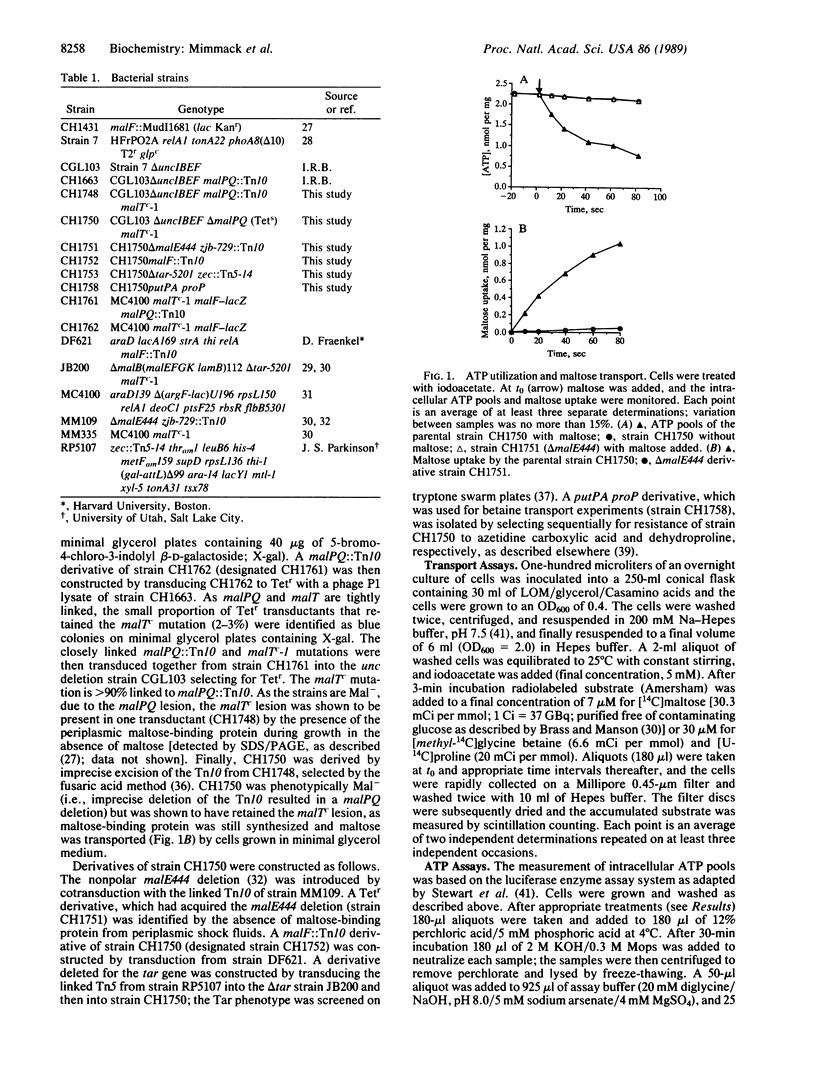
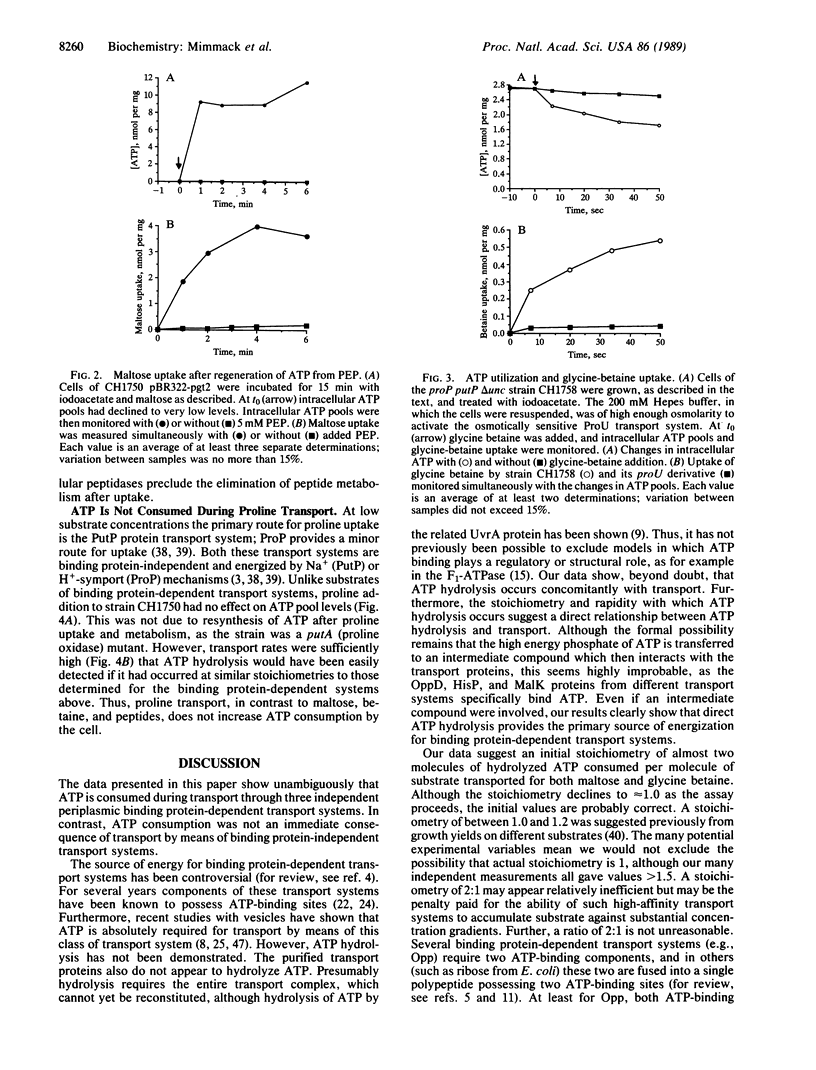
Abstract
Periplasmic binding protein-dependent transport systems mediate the accumulation of many diverse substrates in prokaryotic cells. Similar transport systems, including the P-glycoprotein responsible for multidrug resistance in human tumors, are also found in eukaryotes. The mechanism by which energy is coupled to the accumulation of substrate by these transport systems has been controversial. In this paper we demonstrate that ATP hydrolysis occurs in vivo concomitantly with transport. These data strongly suggest that ATP hydrolysis directly energizes substrate accumulation by these transport systems. The apparent stoichiometry is one to two molecules of ATP hydrolyzed per molecule of substrate transported.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K., Groarke J., Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J Biol Chem. 1989 Mar 5;264(7):3998–4002. [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass J. M., Manson M. D. Reconstitution of maltose chemotaxis in Escherichia coli by addition of maltose-binding protein to calcium-treated cells of maltose regulon mutants. J Bacteriol. 1984 Mar;157(3):881–890. doi: 10.1128/jb.157.3.881-890.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985 Dec;164(3):1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985 Dec;164(3):1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Higgins C. F., Booth I. R. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J Bacteriol. 1984 Oct;160(1):22–27. doi: 10.1128/jb.160.1.22-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Cross R. L., Nalin C. M. Adenine nucleotide binding sites on beef heart F1-ATPase. Evidence for three exchangeable sites that are distinct from three noncatalytic sites. J Biol Chem. 1982 Mar 25;257(6):2874–2881. [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A., Fikes J. D., Gehring K., Bassford P. J., Jr, Nikaido H. Active transport of maltose in membrane vesicles obtained from Escherichia coli cells producing tethered maltose-binding protein. J Bacteriol. 1989 Jan;171(1):503–510. doi: 10.1128/jb.171.1.503-510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G. The basis of multidrug resistance in mammalian cells: homology with bacterial transport. Cell. 1986 Nov 7;47(3):323–324. doi: 10.1016/0092-8674(86)90585-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M. P., Pearce S. R., Higgins C. F. Identification and localization of the membrane-associated, ATP-binding subunit of the oligopeptide permease of Salmonella typhimurium. Eur J Biochem. 1989 Mar 1;180(1):133–141. doi: 10.1111/j.1432-1033.1989.tb14623.x. [DOI] [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hazelbauer G. L. Maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Apr;122(1):206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gallagher M. P., Mimmack M. L., Pearce S. R. A family of closely related ATP-binding subunits from prokaryotic and eukaryotic cells. Bioessays. 1988 Apr;8(4):111–116. doi: 10.1002/bies.950080406. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Whalley K., Jamieson D. J. Nucleotide binding by membrane components of bacterial periplasmic binding protein-dependent transport systems. EMBO J. 1985 Apr;4(4):1033–1039. doi: 10.1002/j.1460-2075.1985.tb03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. Transport proteins. Export-import family expands. Nature. 1989 Aug 3;340(6232):342–342. doi: 10.1038/340342a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Hobson A. C., Weatherwax R., Ames G. F. ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7333–7337. doi: 10.1073/pnas.81.23.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Hong J. S., Kaback H. R. ATP-driven active transport in right-side-out bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3446–3449. doi: 10.1073/pnas.78.6.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. G., Hong J. Properties and characterization of binding protein dependent active transport of glutamine in isolated membrane vesicles of Escherichia coli. Biochemistry. 1983 Feb 15;22(4):844–850. doi: 10.1021/bi00273a021. [DOI] [PubMed] [Google Scholar]
- Joshi A. K., Ahmed S., Ferro-Luzzi Ames G. Energy coupling in bacterial periplasmic transport systems. Studies in intact Escherichia coli cells. J Biol Chem. 1989 Feb 5;264(4):2126–2133. [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. Energization of osmotic shock-sensitive transport systems in Escherichia coli requires more than ATP. Arch Biochem Biophys. 1976 Jan;172(1):312–315. doi: 10.1016/0003-9861(76)90080-1. [DOI] [PubMed] [Google Scholar]
- May G., Faatz E., Villarejo M., Bremer E. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):225–233. doi: 10.1007/BF00430432. [DOI] [PubMed] [Google Scholar]
- Milner J. L., Grothe S., Wood J. M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14900–14905. [PubMed] [Google Scholar]
- Muir M., Williams L., Ferenci T. Influence of transport energization on the growth yield of Escherichia coli. J Bacteriol. 1985 Sep;163(3):1237–1242. doi: 10.1128/jb.163.3.1237-1242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury S. F., Smith N. H., Higgins C. F. Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell. 1987 Dec 24;51(6):1131–1143. doi: 10.1016/0092-8674(87)90599-x. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Novel mutations affecting a signaling component for chemotaxis of Escherichia coli. J Bacteriol. 1980 Jun;142(3):953–961. doi: 10.1128/jb.142.3.953-961.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz E., Gee A., Ames G. F. Reconstitution of the histidine periplasmic transport system in membrane vesicles. Energy coupling and interaction between the binding protein and the membrane complex. J Biol Chem. 1989 Mar 25;264(9):5006–5014. [PubMed] [Google Scholar]
- Richarme G. Possible involvement of lipoic acid in binding protein-dependent transport systems in Escherichia coli. J Bacteriol. 1985 Apr;162(1):286–293. doi: 10.1128/jb.162.1.286-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Shuman H. A. The genetics of active transport in bacteria. Annu Rev Genet. 1987;21:155–177. doi: 10.1146/annurev.ge.21.120187.001103. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. The action of tributyltin chloride on the uptake of proline and glutamine by intact cells of Escherichia coli. Can J Biochem. 1979 Dec;57(12):1376–1383. doi: 10.1139/o79-183. [DOI] [PubMed] [Google Scholar]
- Slocum M. K., Parkinson J. S. Genetics of methyl-accepting chemotaxis proteins in Escherichia coli: organization of the tar region. J Bacteriol. 1983 Aug;155(2):565–577. doi: 10.1128/jb.155.2.565-577.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L. M., Bakker E. P., Booth I. R. Energy coupling to K+ uptake via the Trk system in Escherichia coli: the role of ATP. J Gen Microbiol. 1985 Jan;131(1):77–85. doi: 10.1099/00221287-131-1-77. [DOI] [PubMed] [Google Scholar]
- Sutherland L., Cairney J., Elmore M. J., Booth I. R., Higgins C. F. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986 Nov;168(2):805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



