Abstract
A protocol for the rapid determination of the absolute configuration and enantiomeric excess of α-chiral primary amines with potential applications in asymmetric reaction discovery has been developed. The protocol requires derivatization of α-chiral primary amines via condensation with pyridine carboxaldehyde to quantitatively yield the corresponding imine. The Cu(I) complex with 2,2'-bis (diphenylphosphino)-1,1'-dinaphthyl (BINAP -CuI) with the imine yields a metal-to-ligand-charge-transfer band (MLCT) in the visible region of the circular dichroism spectrum upon binding. Diastereomeric host-guest complexes give CD signals of the same signs, but different amplitudes, allowing for differentiation of enantiomers. Processing the primary optical data from the CD spectrum with linear discriminant analysis (LDA) allows for the determination of absolute configuration and identification of the amines, and processing with a supervised multi-layer perceptron artifical neural network (MLP-ANN) allows for the simultaneous determination of ee and concentration. The primary optical data necessary to determine the ee of unknown samples is obtained in 2 minutes per sample. To demonstrate the utility of the protocol in asymmetric reaction discovery, the ee's and concentrations for an asymmetric metal catalyzed reaction are determined. The potential of the protocol's application in high-throughput screening (HTS) of ee is discussed.
Keywords: Enantiomeric Excess, Amines, Circular Dichroism, High-Throughput, ANN
Introduction
Asymmetric catalysis is a commonly employed method for the synthesis of enantiomerically enriched compounds.1 Traditionally, asymmetric catalysts are developed from a combination of intuition, knowledge of the reaction mechanism, molecular modeling, and trial and error. This is a time-consuming process. Combining traditional techniques with those used in the screening of large libraries of potential asymmetric catalysts could potentially hasten the discovery process.2 However, this requires the rapid determination of ee, which is difficult with chromatographic techniques such as HPLC and GC.3 A variety of methods are currently being developed to overcome this problem.4
For instance, optical techniques for the determination of ee have emerged as a powerful approach to HTS because they are fast, simple, inexpensive, and easily adaptable to determine concentration.5 For example, our group and others have exploited the use of optical techniques based on enantioselective indicator-displacement assays (eIDAs).6
Recently, we reported a simple protocol based on the combination of circular dichroism (CD) spectroscopy7 and pattern recognition based-techniques.8 It is based on monitoring changes in the metal-to-ligand charge transfer (MLCT) bands of chiral and racemic metal complexes upon the addition of chiral diamine analytes. This technique allows for rapid screening of ee and the determination of concentration.
α-Chiral primary amines are important building blocks in chemical and pharmaceutical transformations.9 Several methods for the asymmetric synthesis of chiral amines are under development.10 Rapid screening of asymmetric reactions might hasten the discovery of an efficient route to these versatile building blocks. Thus, a method for the rapid screening of ee for α-chiral primary amines is desirable.11
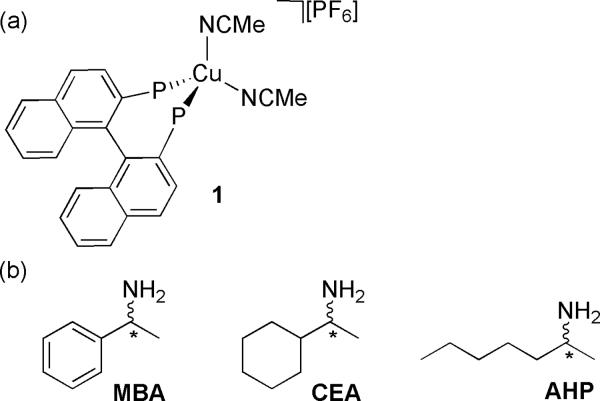
Herein, we describe a protocol for the rapid screening of ee of α-chiral primary amines. The protocol utilitizes the receptors (R)-and (S)-[Cu(I)(BINAP)(NCMe)2]PF6 (1), where BINAP is 2,2'-bis (diphenylphosphino)-1,1'-dinaphthyl (See Figure 1a). Both R- and S-1 complexes show MLCT bands around 340 nm in the CD spectra, and the changes in these MLCT bands after the addition of enantiomerically enriched chiral guests give a method for enantiodiscrimination and concentration determination of the chiral mono-amines (shown in Figure 1b). To test the practicality of the protocol, a sample from an asymmetric reaction was prepared. The ee of the sample was determined by the CD protocol and compared with values obtained from the well-accepted 1H NMR protocol developed by James,11a,11b showing that the CD protocol allows for rapid-screening with comparable accuracy to standard iterative protocols.
Figure 1.
(a) (R)- and (S)-1 complexes employed as receptors; (b) Chiral primary amines employed as analytes: a-methyl-benzylamine (MBA), 1-cyclohexyl-ethylamine (CEA), 2-aminoheptane (AHP).
Results and Discussion
1. Design
In order for the protocol to be of utility in rapid screening, a system in which the analyte (chiral amine) does not have to be derivatized is preferable. Derivatization is time consuming and frequently requires purification before and after derivatization. Additionally, we sought either a commercially available receptor, or one that required only a one to two step synthesis, because this would increase its utility to the general chemistry community. Unfortunately, there was no signal modulation via addition of an underivatized chiral amine to (R)-1. Thus, we developed a method for derivatization that is fast, and does not require purification. This minimizes the amount of time required for the derivatization step and still allows the protocol to be amenable to rapid ee determination.
2. Detection Scheme
As previously mentioned, signal modulation of the receptor is not possible upon the addition of underivatized chiral amines. In order to overcome this problem the chiral amine is condensed with 2-pyridine-carboxaldehyde to form the corresponding chiral Schiff base in situ (Scheme 1). The overall reaction was fully complete in under 2 hours, and resulted in quantitative formation of the imine. The imines were used directly without purification.12
Scheme 1.
Derivatization of the amines to form the corresponding Schiff bases.
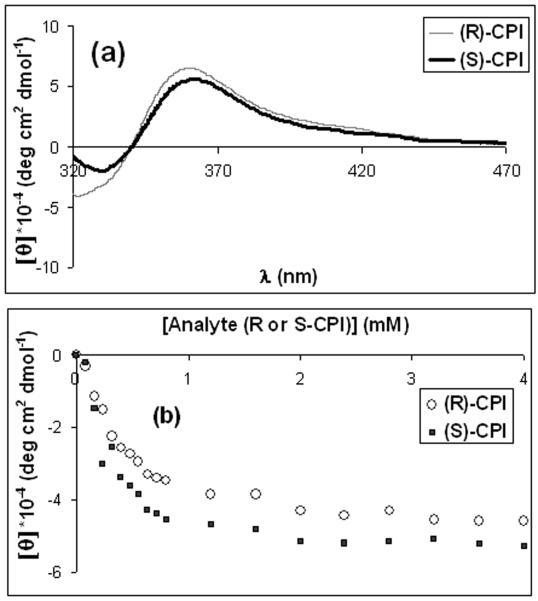
The addition of the chiral imines to a solution of receptor (R)-1 modulates the signal in the CD spectrum spanning from approximately 320nm to 470nm (Figure 2). No CD signals above 320 nm are observed for the chiral imines, for copper alone with the imines, or for free BINAP. Thus, the signal is the result of a MLCT in the coordination complex of the imines with (R)-1.
Figure 2.
(a) CD spectrum for (R)-1 [0.4 mM] and the enantiomers of CPI [0.8 mM]; (b) Titration of (R)-1 [0.4 mM] with (R)- and (S)-CPI in acetonitrile at 354 nm.
3. Identification of Amine and Enantiodiscrimination
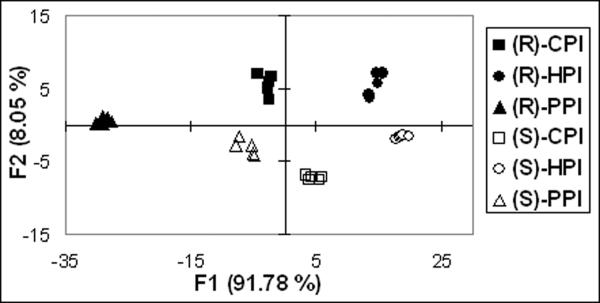
The absolute configuration and the identity of the amines can be determined by analyzing the primary optical data via LDA.13 Titration of (R)-1 with the imines shows that saturation is reached at 2 equivalents (see Figure 2b). For this system, saturation gives the maximum signal to noise ratio, and is thus best suited for data collection and analysis; hence, all data was collected at 2 equivalents. Each experiment was repeated 5 times to ensure reproducibility. The ellipticities were recorded at four different wavelengths (350, 355, 360 and 380 nm) and analyzed with LDA. The selected wavelengths were chosen around the positive maximum presented in the Cotton effect to try to get all the possible information of the spectra. The use of more wavelengths does not give any improvement to the resulting LDA plot. The analytes show good separation in the LDA plot (Figure 3). Positive scores along F2 are found for the (R)-amines and negative scores for the (S)-amines. As expected, the opposite behavior is observed for receptor (S)-1 (See Supporting Information). Jackknife analysis leads to 100% identification and enantioselective discrimination of the chiral primary amines.14 Thus, this method permits the identification and determination of handedness of chiral primary amines.
Figure 3.
LDA plot of receptor (R)-1 [0.4 mM] with all the analytes [0.8 mM].
4. Quantitative Determination of ee and Concentration
The next goal was the quantitative determination of ee and concentration for the chiral guest CEA utilizing CD data and an MLP-ANN.15 All optical data was collected via a CD spectrometer and a robotically controlled 96-well plate interface (JASCO ASU-605).
Because a supervised MLP-ANN was used, a training set was necessary. The training set consisted of the ellipticities from [θ]340 to [θ]400 at 1nm intervals for 0.4 mM of receptor (R)-1 in acetonitrile, charged with three different concentrations (0.2 mM, 0.8 mM, and 1.4 mM) of CPI (the imine derivative of CEA), along with eleven ee values for each concentration (1, 0.8, 0.6, 0.4, 0.2, 0, −0.2, −0.4, −0.6, −0.8, and −1), giving 33 solutions as the training set.
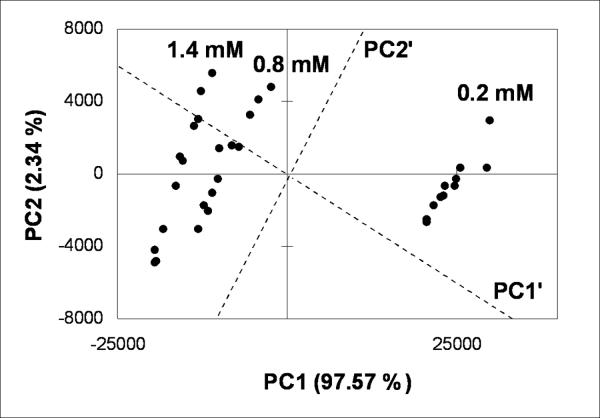
First, this training set was analyzed using Principle Component Analysis (PCA)13 to demonstrate that simultaneous ee and concentration determination is feasible (Figure 4). A slightly rotated axis shows concentration along PC1' and ee along PC2'. This PCA plot was performed to simply display the ANN training data in two dimensional space, and not to show reproducibility with multiple replicates. The three strips parallel to PC2' span ee from −1 to 1 in the eleven values listed above. The relative weights of the PC1 and PC2 axis clearly show that this system is much more responsive to concentration than ee values. Yet, as described below the errors found for ee values are still acceptable.
Figure 4.
Two-dimensional PCA plot of CPI with three ee trainings sets at three different [G]t values: 0.2 mM, 0.8 mM and 1.4 mM.
A MLP-ANN was trained with the ellipticities found for the 33 samples of varying concentrations and ee values. The number of hidden layers was varied until the MLP-ANN could accurately generate the outputs from the optical inputs. An MLP-ANN with 11 hidden layers gave the best results.
In order to assess the generality of the function developed by the MLP-ANN, the ellipticities from [θ]340 to [θ]400 for six unknown samples, independent of the training set, were collected. These ellipticities were used as inputs in the MLP-ANN, and its ability to generate the correct ee's and concentrations was tested. The MLP-ANN calculates the concentration ([G]t) with an average error of 14.7% and calculates the ee with an average error of 11.7%. The results are summarized in Table 1.
Table 1.
Determination of concentration and %ee of CEA test samples using ANN.
| Samples | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| [G]t (mM) | 0.80 | 1.40 | 1.00 | 0.50 | 0.30 | 1.20 |
| [G]t (mM) (ANN) | 0.77 | 1.47 | 1.13 | 0.24 | 0.07 | 1.36 |
| %ee | 80 | 60 | −40 | 0 | −20 | −60 |
| %ee (ANN) | 94 | 78 | −48 | 8 | 10 | −72 |
5. Analyzing an Asymmetric Reaction
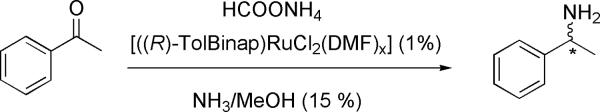
The applicability of this technique in the screening of asymmetric reactions was demonstrated. To show the generality of the method, a different amine than CEA was analyzed. Asymmetric synthesis of α-methylbenzylamine (MBA) was done by reacting acetophenone and ammonium formate in the presence of a chiral ruthenium catalyst according to a literature procedure (See Scheme 2).10b The crude sample was identified as α-methylbenzylamine by 1H NMR and 13C NMR spectroscopy. The enantiopurity of the sample was first determined by the James NMR protocol,11a,11b giving a value of 72% R enantiomer (44% ee) (Supporting Information).
Scheme 2.
Asymmetric reaction used to synthesize a sample of MBA of unknown ee.
Next, the ee was also determined using the protocol reported here. A training set was loaded onto a 96-well plate consisting of PPI (the imine derivative of MBA) at three different concentrations (0.2mM, 0.8mM, and 1.4mM), and eleven ee values at each concentration (1, 0.8, 0.6, 0.4, 0.2, 0, −0.2, −0.4, −0.6, −0.8, and −1). An MLP-ANN was trained using the ellipticities from [θ]340 to [θ]400 at 2nm intervals as inputs. A network containing 20 hidden layers gave the best results.
The imine (PPI) was synthesized in situ by direct addition of 2-pyridine-carboxaldehyde to the crude reaction mixture (Scheme 2). Without purification, the imine was loaded onto the same 96-well plate used for the training set. There were three sets at two different concentrations (0.7 and 1.1mM). The samples were run in triplicate at two different concentrations to demonstrate both that this protocol works at different concentrations, and that even with impurities from the reaction mixture the average errors would be comparable to those previously obtained using prepared solutions of pure imines.
The ee and concentration values calculated using the MLP-ANN are summarized in Table 2. The average %ee value obtained was 61. Assuming that the James protocol yields the real value for the ee, the average error for ee is 17%, which is higher than but comparable to that of 11.7% calculated using solutions of the pure imine CPI. With respect to concentration, the average error for the asymmetric reaction is 13.5%, which is also similar for the error of 14.7% calculated using solutions of a pure imine. The error found represents the accuracy of our method, which we note consistently gives a low ee value compared to the accepted method. In contrast, the precision, as represented by a standard deviation of the ee values is much better (5.2 %).
Table 2.
Determination of %ee and [G]t of MBA for unknown samples from the asymmetric reaction.
| Samples | 1_1 | 1_2 | 1_3 | 2_1 | 2_2 | 2_3 |
|---|---|---|---|---|---|---|
| %ee (ANN) | 60 | 62 | 62 | 52 | 62 | 68 |
| [G]t(mM) (ANN) | 0.84 | 0.86 | 0.86 | 1.23 | 1.21 | 1.21 |
| [G]t (mM) | 0.70 | 0.70 | 0.70 | 1.10 | 1.10 | 1.10 |
6. Applications in Rapid and High-Throughput Screening of ee
The term “rapid screening” is used throughout this paper instead of high-throughput screening (HTS). This is because true HTS requires the screening of a few thousand samples per day1, which is not possible with this protocol. However, the primary optical data in this protocol is collected in only 2 minutes per sample. More traditional techniques such as chiral HPLC takes 10–20 minutes per sample.1 Thus, this protocol outperforms traditional techniques and can reasonably be described as a “rapid screening” method. Additionally, HPLC and GC usually require purification of the sample prior to analysis. While the reported protocol requires a 2 hour derivatization, it can be done in situ and in parallel in 96 well plates. Thus, for the large sample sizes this protocol is designed for, the 2 hour time period is a relatively short amount of time when compared to the screening time.
The speed at which the optical data is collected could be improved allowing for HTS. Previously, our group has demonstrated that a 96-well plate reader can collect optical data for 84 samples in 2 minutes.6i However, in the case of the CD spectrometer, the commercially available automated plate interface uses a pump to inject samples from a 96-well plate into a cuvette. Injection of the samples is the most time-consuming part of the process, with the collection of the primary optical data occupying a relatively small portion of the time. Thus, with improvements to the automated plate interface, the protocol could approach sample sizes comparable to those previously reported for UV-Vis, which would allow for true high-throughput screening.
Conclusions
Our previous eIDA and CD methods analyzed chiral bidentate analytes: diols, amino acids, and diamines. As described above, the monodentate primary amines did not show an optical response, whereas conversion to bidentate imines gave enantioselective discrimination. Although here the scope was the determination of ee and concentration of chiral mono-amines, the approach of conversion to bidentate chelating ligands may prove to be a general strategy for analysis of simple functional groups, allowing the extension of this protocol to many functional groups using a single step in situ derivatization.
In summary, a new protocol that allows for the rapid and simultaneous determination of ee and concentration of chiral primary amines has been developed. Furthermore, the ee of a sample from an asymmetric reaction was determined using this CD technique and displayed close agreement with a literature protocol. The speed, accuracy, and simplicity of this method, as well as the possibility of simultaneous concentration determination, make this method clearly amenable to rapid screening. Additionally, improvements to the automated plate reader on the CD spectrometer may allow for this protocol to be applied in true HTS. We are currently employing this technique in the analysis of a wide variety of asymmetric reactions and other organic functional groups.
Experimental Section
Preparation of Imines
A stoichiometric amount of the corresponding chiral amines was added to a stirred solution of 2-pyridine-carboxaldehyde (9.5 ml, 0.1 mmol) in 2 mL of acetonitrile with 4 Å molecular sieves. After stirring for 2 h at room temperature the solvent was removed in vacuo yielding the corresponding crude imines as pale yellow oils. These imines were used directly in the analysis.
(R)- and (S)-N-(1-Phenylethyl)-2-pyridylmethanimine [(R)- and (S)-PPI]
Yield: 19.3 mg, 92%. 1H NMR (400 MHz, CDCl3): d 8.64–8.63 (m, 1H), 8.48 (s, 1H), 8.06–8.04 (m, 1H), 7.85–7.81 (m, 1H), 7.49–7.26 (m, 6H), 4.77 (q (6.7), 1H), 1.56 (d (6.6), 3H).
(R)- and (S)-N-(1-Cyclohexylethyl)-2-pyridylmethanimine [(R)- and (S)-CPI]
Yield: 19.7 mg, 91%. 1H NMR (400 MHz, CDCl3): d 8.62 (d (4.8), 1H), 8.3 (s, 1H), 7.98–7.96 (m, 1H), 7.84–7.79 (m, 1H), 7.40–7.37 (m, 1H), 3.18–3.15 (m, 1H), 1.84–1.68 (m, 4H), 1.48–1.46 (m, 2H), 1.32–1.24 (m, 2H), 1.21 (d (6.5), 3H), 1.19–0.96 (m, 2H).
(R)- and (S)-N-(2-hepthyl)-2-pyridylmethanimine [(R)- and (S)-HPI]
Yield: 19.2 mg, 94%. 1H NMR (400 MHz, CDCl3): d 8.63–8.62 (m, 1H), 8.34 (s, 1H), 7.97–7.95 (m, 1H), 7.83–7.82 (m, 1H), 7.39–7.37 (m, 1H), 3.42 (m, 1H), 1.58–1.57 (m, 2H), 1.29–1.22 (m, 9H), 1.11–1.10 (m, 3H).
James procedure
In a dry vial with 4 Å molecular sieves 2-formylphenylboronic acid (14.9 mg, 0.1 mmol) and (S)-1,1'-bi-2-naphthol (28.6 mg, 0.1 mmol) were dissolved in CDCl3 (1 mL). α-Methylbenzylamine (12.7 mL, 0.1 mmol) obtained from the asymmetric reaction was added onto the solution (Scheme S1). The mixture was stirring during 5 min. and the corresponding 1H NMR of the resulting solution is shown in Figure S2.
Linear Discriminant Analysis (LDA)
The XLSTAT program was used to carry out the LDA studies. LDA studies allow for differentiation and classification of the analytes. The generalization error of this classification method is measured using Jackknife analysis.
Principle Component Analysis (PCA)
XLSTAT was also used for PCA analysis. PCA allows multivariate data to be represented in lower-dimensional space. The first two principal components are invoked to visualize the objects in two-dimensional space. The first principal component (PC1) is directed along the maximum variance. The second principal component (PC2) is orthogonal to PC1 and carries the second maximum extent of variance.
Artificial Neural Network (ANN)
The ANN analyses were carried out employing the program Statistica Neural Networks 8.0.S6 The Statistica Neural Networks program has an embedded intelligent problem solver (IPS) function, which was requested to search for MLP consisting of networks three layers. During learning, output values from the ANN are compared to true values and the coupling weights are adjusted to give the best network. The hidden activation chosen was hyperbolic tangent and the output activation was identity. In the case of the training set data corresponding to MBA at 0.2 mM, the ellipticites values at 10, 30 and 50 %R were discarded.
Supplementary Material
Acknowledgements
This work was supported by The National Institutes of Health, GM77437 and the Welch Foundation.
Footnotes
Supporting information for this article is available on the WWW under http://www.eurjoc.org/ or from the author.
References
- [1].Reetz MT. Angew. Chem. 2001;113:292–320. Angew. Chem. Int. Ed. 2001, 40, 284–310. [Google Scholar]
- [2].(a) Wahler D, Reymond J-L. Curr. Opin. Biotechnol. 2001;12:535–544. doi: 10.1016/s0958-1669(01)00260-9. [DOI] [PubMed] [Google Scholar]; (b) Stambuli JP, Hartwig JF. Curr. Opin. Chem. Biol. 2003;7:420–426. doi: 10.1016/s1367-5931(03)00056-5. [DOI] [PubMed] [Google Scholar]; (c) Gennari C, Piarulli U. Chem. Rev. 2003;103:3071–3100. doi: 10.1021/cr020058r. [DOI] [PubMed] [Google Scholar]
- [3].(a) Welch CJ, Szczerba T, Perrin SR. J. Chromatogr. A. 1997;758:93–98. [Google Scholar]; (b) Welch CJ, Grau B, Moore J, Mathre DJ. J. Org. Chem. 2001;66:6836–6837. doi: 10.1021/jo015966n. [DOI] [PubMed] [Google Scholar]; (c) Welch CJ, Fleitz F, Antia F, Yehl P, Waters R, Ikemoto N, Armstrong IJD, Mathre D. J. Org. Process Res. Dev. 2004;8:186–191. [Google Scholar]; (d) Sigman MS, Jacobsen EN. J. Am. Chem. Soc. 1998;120:4901–4902. [Google Scholar]; (e) Wolf PAC, Hawes J. Org. Chem. 2002;67:2727–2729. doi: 10.1021/jo025534s. [DOI] [PubMed] [Google Scholar]; (f) Traverse JF, Snapper ML. Drug Discovery Today. 2002;7:1002–1012. doi: 10.1016/s1359-6446(02)02436-4. [DOI] [PubMed] [Google Scholar]; (g) Kuntz KW, Snapper ML, Hoveyda AH. Curr. Opin. Chem. Biol. 1999;3:313–319. doi: 10.1016/S1367-5931(99)80048-9. [DOI] [PubMed] [Google Scholar]
- [4].For recently representative examples see: Bailey D M, Hennig A, Uzunova V D, Nau W M. Chem. Eur. J. 2008;14:6069–6077. doi: 10.1002/chem.200800463.. Truppo M D, Escalettes F, Turner N J. Angew. Chem. 2008;120:2679–2681. doi: 10.1002/anie.200705046. Angew. Chem. Int. Ed. 2008, 47, 2639–2641.. Chen Z-H, He Y-B, Hu C-G, Huang X-H, Hu L. Aust. J.Chem. 2008;61:310–315.. Ingle J R, Busch K W, Kenneth W, Busch M A. Talanta. 2008;75:572–584. doi: 10.1016/j.talanta.2007.11.056.. Tanaka H, Matilde S. Chirality. 2008;20:307–312. doi: 10.1002/chir.20439.. Yashima E, Katsuhiro M. Macromolecules. 2008;41:3–12.. Young B L, Cooks R G. Int. J. Mass Spectrom. 2007;267:199–204.. Li Z-B, Lin J, Sabat M, Hyacinth M, Pu L. J. Org, Chem. 2007;72:4905–4916. doi: 10.1021/jo0704715.. Hoogenraad M, Klaus G M, Elders N, Hooijschuur S M, McKay B, Smith A A, Damen EW P. Tetrahedron:Asymmetry. 2004;15:519–523.. van der Linden J B, Ras E-J, Hooijschuur S M, Klaus G M, Luchters N T, Dani P, Verspui G, Smith A A, Damen EW P, McKay B, Hoogenraad M. QSAR Comb. Sci. 2005;24:94–98.. Mazurek S, Ward T R, Novic M. Mol. Divers. 2007;11:141–152. doi: 10.1007/s11030-008-9068-x.
- [5].See for example: Lin J, Zhang H C, Pu L. Org. Lett. 2002;4:3297–3300. doi: 10.1021/ol026565c.. Lee S J, Lin W. J. Am. Chem. Soc. 2002;124:4554–4555. doi: 10.1021/ja0256257.. Ahn K H, Ku H-Y, Kim Y, Kim S-G, Kim Y K, Son H S, Ku J K. Org. Lett. 2003;5:1419–1422. doi: 10.1021/ol034041m.. Pu L. Chem. Rev. 2004;104:1687–1716. doi: 10.1021/cr030052h.. Corradini R, Paganuzzi C, Marchelli R, Pagliari S, Sforza S, Dossena A, Galaverna G, Duchateau A. J. Mater. Chem. 2005;15:2741–2746. doi: 10.1002/chir.10272.. Li Z-B, Lin J, Pu L. Angew. Chem. 2005;117:1718–1721. Angew. Chem. Int. Ed. 2005, 44, 1690–1693.. Zhao J, James T D. J. Mater. Chem. 2005;15:2896–2901.. Mei X, Wolf C. Tetrahedron Lett. 2006;47:7901–7904.. Wolf C, Liu S, Reinhardt B C. Chem. Commun. 2006;47:4242–4244. doi: 10.1039/b609880k.. Liu S, Pestano J P C, Wolf C. J. Org. Chem. 2008;73:4267–4270. doi: 10.1021/jo800506a.
- [6].(a) Zhu L, Anslyn EV. J. Am. Chem. Soc. 2004;126:3676–3677. doi: 10.1021/ja031839s. [DOI] [PubMed] [Google Scholar]; (b) Folmer-Andersen JF, Lynch VM, Anslyn EV. J. Am. Chem. Soc. 2005;127:7986–7987. doi: 10.1021/ja052029e. [DOI] [PubMed] [Google Scholar]; (c) Zhu L, Zhong Z, Anslyn EV. J. Am. Chem. Soc. 2005;127:4260–4269. doi: 10.1021/ja0435945. [DOI] [PubMed] [Google Scholar]; (d) Folmer-Andersen JF, Kitamura M, Anslyn EV. J. Am. Chem. Soc. 2006;128:5652–5653. doi: 10.1021/ja061313i. [DOI] [PubMed] [Google Scholar]; (e) Mei X, Wolf C. J. Am. Chem. Soc. 2006;128:13326–13327. doi: 10.1021/ja0636486. [DOI] [PubMed] [Google Scholar]; (f) Kacprzak K, Grajewski J, Gawronski J. Tetrahedron: Asymmetry. 2006;17:1332–1336. [Google Scholar]; (g) Zhu L, Shabbir SH, Anslyn EV. Chem. Eur. J. 2007;13:99–104. doi: 10.1002/chem.200600402. [DOI] [PubMed] [Google Scholar]; (h) Leung D, Folmer-Andersen JF, Lynch VM, Anslyn EV. J. Am. Chem. Soc. 2008;130:12318–12327. doi: 10.1021/ja803806c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Leung D, Anslyn EV. J. Am. Chem. Soc. 2008;130:12328–12333. doi: 10.1021/ja8038079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakanishi K, Berova N, Woody RW. Circular Dichroism. Principles and Applications. VCH Publishers, Inc.; 1994. [Google Scholar]
- [8].(a) Nieto S, Lynch VM, Anslyn EV, Kim H, Chin J. J. Am. Chem. Soc. 2008;130:9232–9233. doi: 10.1021/ja803443j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nieto S, Lynch VM, Anslyn EV, Kim H, Chin J. Org. Lett. 2008;10:5167–5169. doi: 10.1021/ol802085j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gadamasetti KG, Braish T. Process Chemistry in the Pharmaceutical Industry. Volume 2. CRC Press, Inc.; 1973. [Google Scholar]
- [10].See for example: Braun H, Felber H, Knesse G, Ritter A, Schmidtchen F P, Schneider A. Tetrahedron. 2001;47:3313–3328.. Kadyrov R, Riermeier T H. Angew. Chem. 2003;115:5630–5632. doi: 10.1002/anie.200352503. Angew. Chem. Int. Ed. 2003, 42, 5472–5474.. Huang X, Ortiz-Marciales M, Huang K, Stepanenko V, Merced F G, Ayala A M, Correa W, De Jesus M. Org. Lett. 2007;9:1793–1795. doi: 10.1021/ol0704791.. Lin G-Q, Xu M-H, Zhong Y-W, Sun X-W. Acc. Chem. Res. 2008;41:831–840. doi: 10.1021/ar7002623.. Hili R, Baktharaman S, Yudin A K. Eur. J. Org. Chem. 2008:5201–5213.. Hou G, Gosselin F, Li W, McWilliams C, Sun Y, Weisel M, O'Shea PD, Chen C, Davies IW, Zhang X. J. Am. Chem. Soc. 2009;131:9882–9883. doi: 10.1021/ja903319r.
- [11].See for example: Perez-Fuertes Y, Kelly A M, Fossey J S, Powell M E, Bull S D, James T D. Nat. Protoc. 2008;3:210–214. doi: 10.1038/nprot.2007.524.. Perez-Fuertes Y, Kelly A M, Johnson A L, Arimori S, Bull S D, James T D. Org. Lett. 2006;8:609–612. doi: 10.1021/ol052776g.. Cheng J, Kang J. Electrophoresis. 2006;27:865–871. doi: 10.1002/elps.200500589.
- [12].See for example: Haddlenton D M, Duncalf D J, Kukulj D, Heming A M, Shooter A J, Clark A J. J. Mater. Chem. 1998;8:1525–1532.. Ziessel R, Nguyen P. Synthesis. 2005:223–232.
- [13].(a) From xlstat.com.; (b) Jurs PC, Baken GA, McClelland HE. Chem. Rev. 2000;100:2649–2678. doi: 10.1021/cr9800964. [DOI] [PubMed] [Google Scholar]
- [14].Gong G. J. Am. Stat. Assoc. 1986;81:108–113. [Google Scholar]
- [15].Burns JA, Whitesides GM. Chem. Rev. 1993;93:2583–2601. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.