Abstract
Objective
This functional magnetic resonance imaging (fMRI) study investigated the effects of pharmacotherapy on brain function underlying affect dysregulation and cognitive function in pediatric bipolar disorder (PBD).
Method
Healthy controls (HC) (n = 14; mean age = 14.1 ± 2.4 years) and unmedicated PBD patients with manic or hypomanic episodes (n = 17; mean age = 14.3 ± 1.1 years) were matched on intelligence quotient (IQ) and demographic factors. The fMRI studies were performed at baseline and after 14 weeks, during which PBD patients were treated initially with second-generation antipsychotics (SGAs) followed by lamotrigine monotherapy. The pediatric affective color-matching task was used where subjects matched the color of a positive, negative, or neutral word with one of the two colored circles below in each of the trials. There were five blocks of each emotional word type, with 10 trials per block.
Results
Behavioral data showed that the PBD group was modestly slower and less accurate than the HC, regardless of condition or treatment status. The blood oxygen level-dependent (BOLD) signal activity was reduced with treatment in the PBD group relative to the HC group during the negative versus neutral condition in bilateral dorsolateral prefrontal cortex (DLPFC), right posterior cingulate gyrus, parahippocampal gyrus, and inferior parietal lobule, but increased in left ventromedial prefrontal cortex (VMPFC). Similarly, during the positive versus neutral condition, the PBD group, relative to HC, showed reduced activity in right DLPFC, precuneus, and inferior parietal lobule and increased activity in the right VMPFC. However, within the PBD group, there was treatment related decrease in VMPFC and DLPFC. Improvement on Young Mania Rating Scale (YMRS) score significantly correlated with the decreased activity in VMPFC within the patient group.
Conclusions
Pharmacotherapy in PBD patients led to differential effort with persistently increased activity in the affective regions and decreased activity in the cognitive regions relative to HC, demonstrating altered mechanisms of affective and cognitive systems of brain function, regardless of symptom response.
Introduction
Pediatric bipolar disorder (PBD) is associated with significant affect dysregulation and cognitive dysfunction (Dickstein et al. 2004; McClure et al. 2005; Pavuluri et al. 2006; Dickstein et al. 2008; Pavuluri et al. 2008). These disturbances have typically been investigated in isolation, even though the interaction of cognitive and affective systems is a key to adaptive behavior. Emotions motivate and organize cognition and behavior, whereas cognition can regulate the degree and duration of emotional responses. The rich interaction of emotional and cognitive systems organizes behavior in day-to-day life. Therefore, understanding disturbances in such interactions and how treatment impacts them in PBD is crucial to advancing models of pathophysiology and treatment.
Using different paradigms that involved both emotion evaluation and emotion regulation, findings in PBD, relative to healthy controls (HC) showed either decreased (Pavuluri et al. 2007) or increased (Rich et al. 2006) right ventrolateral prefrontal cortex (VLPFC; Brodmann area (BA) 45, 47; inferior frontal gyrus) and ventromedial prefrontal cortex (VMPFC; medial frontal gyrus; BA 10) activity. Furthermore, another study that examined the influence of emotional challenge on cognitive function, patients with PBD demonstrated decreased activity in right VMPFC, VLPFC, and dorsolateral prefrontal cortex (DLPFC; BA 9; middle frontal gyrus) and increased activity in limbic regions when compared to HC (Pavuluri et al. 2008). In this latter study, examining the cognitive and affective circuitry interface, euthymic PBD patients and HC performed the cognitive task of matching the color of emotional words with one of the two colored circles below. The cognitive element of the task involved executive function consisting of attention and problem solving in matching the colors of the words with color of the circles below. The words served as emotional or neutral stimuli while performing the cognitive task. The PBD subjects, relative to HC, demonstrated a pattern of functional alteration with reduced activity in areas that provide top-down control concurrent with increased activity in subcortical regions that was thought to suggest a reduced capacity for emotion regulation during cognitive processing (Pavuluri et al. 2008). Using the same task and in a different sample, acutely ill PBD patients showed increased VLPFC activity when compared to children with attention-deficit/hyperactivity disorder (ADHD) or HC (Passarotti et al. 2010). Therefore, the emotional control region of VLPFC was shown to be either ineffective (Pavuluri et al. 2007; Pavuluri et al. 2008) or overactive and presumed to be deployed in modulating the subcortical regions (Rich et al. 2006; Passarotti et al. 2010). However, none of these earlier studies involved prospective longitudinal study design that would illustrate the mechanism of brain function underlying how treatment would alter the activity in the cognitive and affective neural systems.
To date, there are two pioneering functional magnetic resonance imaging (fMRI) treatment studies, one in adult and one in adolescent patients with bipolar disorder (BD), that suggested that lamotrigine may enhance cognitive (Haldane et al. 2008) and affective circuitry (Chang et al. 2008) function in BD. Chang et al. (Chang et al. 2008) studied the PBD subjects using blocks of negative, positive, and neutral pictures that were selected from the International Affective Picture Systems. Patients were asked to rate how these pictures made them feel. They demonstrated that addition of lamotrigine led to clinical improvement on Children's Depression Rating Scale (CDRS) scores that in turn significantly correlated with decrease in right amygdala activity. In adult bipolar euthymic patients, Haldane et al. (Haldane et al. 2008) showed that addition of lamotrigine led to increased prefrontal activity while performing a working memory task. There are no published treatment studies that simultaneously probed both the affective and cognitive circuitry function in patients, relative to HC.
Building on these earlier studies, the current study aimed to examine the effects of pharmacotherapy on both the affective and cognitive neural systems involved in emotion processing during the performance of a cognitive task in patients with PBD, relative to HC. Furthermore, our study differed from that of Chang et al. (Chang et al. 2008) in examining the treatment effects in manic and hypomanic patients. Also, treatment consisted of second-generation antipsychotics (SGAs) for acute mania followed by lamotrigine for maintaining stable mood (design shown in Fig. 1), as opposed to add-on treatment with lamotrigine for depression in PBD patients by Chang et al. (Chang et al. 2008). We opted to not use SGAs for maintenance treatment because they pose high risk for metabolic side effects if prescribed for long-term treatment (Correll and Carlson 2006), in addition to examining the effectiveness of lamotrigine on affective and cognitive neural systems. On the basis of our earlier fMRI studies with PBD patients (Pavuluri et al. 2007), and preliminary studies in pediatric (Chang et al. 2008) and adult (Haldane et al. 2008) bipolar patients, we hypothesized that: (1) At baseline, untreated PBD subjects will show an abnormal functional pattern in higher cortex in both the affective regions, such as VLPFC and VMPFC, and cognitive regions, such as DLPFC, when compared to HC. In addition, there may be abnormal activity in the limbic system. (2) Pharmacotherapy (with SGAs during acute phase followed by lamotrigine monotherapy) would control clinical symptoms of mania and hypomania and concurrently normalize the affective and cognitive circuitry function. To test these hypotheses, we administered an updated version of the color-matching task that we previously piloted in euthymic PBD patients to probe the impact of emotion processing on cognitive functioning (see the task description). The data presented in this paper for our PBD and HC sample at baseline and follow-up of lamotrigine treatment are novel and have never been published before.
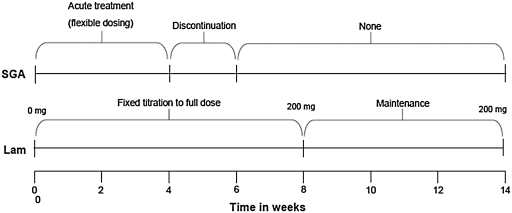
FIG. 1.
Trial design of pharmacotherapy. The treatment timeline over the 14 weeks of the study is shown. SGA = Second-generation antipsychotic; Lam = lamotrigine.
Methods
This is a prospective outpatient treatment trial for manic, mixed, and hypomanic episodes in BD types I and II, with fMRI blood oxygen level-dependent (BOLD) signal activation on a Pediatric Affective Color Matching Task as the primary outcome. The total duration of the trial was 14 weeks, with an initial 4 weeks of treatment with SGAs, followed by discontinuation in weeks 4–6, just as they were titrated up to the full dose of lamotrigine. During the first 8 weeks of the trial, lamotrigine is titrated up gradually, followed by the last 6 weeks of lamotrigine monotherapy at 200 mg/day. PBD patients (n = 17; mean age = 14.3 ± 1.1 years) and intelligence quotient (IQ) and demographically matched HC (n = 14; mean age = 14.1 ± 2.4 years) had fMRI studies performed at baseline and again at 14 weeks. HC subjects did not receive treatment, but they were retested to control for potential changes at retest due to familiarity with the behavioral paradigm or MRI scanning procedure in the patient group. This study was approved by the University of Illinois at Chicago (UIC) Institutional Review Board. Parents gave written consent and children gave assent or consent to participate in this trial.
Inclusion criteria for patients were: a Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) diagnosis of BD type I, a mixed (n = 5) or manic (n = 5) episode or type II hypomanic episode (n = 7), 10–18 years old, and a baseline score of >12 on the Young Mania Rating Scale (YMRS) (Young et al. 1978). Patients were already medication free, not requiring a wash out at study entry, or sufficiently unstable on medications to justify discontinuation of an ineffective treatment regime prior to beginning treatment with lamotrigine with the consent of parents and the assent or consent of patients. The wash-out period consisted of tapering previous medications over 1 week, allowing a medication-free period of at least 4–7 days prior to scanning. None of the patients was on fluoxetine that would have required a longer wash-out period. Inclusion criteria for HC were: Not meeting DSM-IV criteria for an axis I disorder, not having a family history of affective illness, 10–18 years old, and a baseline score of <12 on the YMRS. Exclusion criteria for PBD patients and HC included: Active substance abuse through urine toxicology screen; those with ADHD diagnosis before age 7 and prior to the onset of PBD; serious medical problems; a history of allergy to lamotrigine (in case of patients); IQ < 80; and contraindications to MRI studies, including metallic implants, retractors or braces, and claustrophobia. IQ was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI) (Psychological Corporation 1999).
Assessment procedures
Board-certified child psychiatrists completed the Washington University Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (Geller et al. 1998). Information from this interview and all other available clinical information were reviewed to make a consensus clinical diagnosis. Live diagnostic interviews of 10 cases were independently coded by two researchers to establish interrater diagnostic reliability, which was 0.94 by the Cohen kappa.
Efficacy and safety measures
The primary clinical treatment efficacy measure was the YMRS. Secondary measures included the Clinical Global Impression Scale for Bipolar Disorder (CGI-BP) (Spearing et al. 1997), and the Child Depression Rating Scale-Revised (CDRS-R) (Poznanski et al. 1984). For the purpose of this study, response for manic symptoms was defined as <12 on the YMRS, and response for depressive symptoms was defined as <40 on the CDRS-R.
SGAs during the first 4 weeks
Manic and hypomanic symptoms were addressed during the 8-week lamotrigine titration phase by using SGAs. This was an essential step in the study design because SGAs serve as rescue medications for acutely ill unmedicated patients until the subjects receive the full dose of lamotrigine. The order of preference for SGAs given for the first 4 weeks of acute illness was risperidone, aripiprazole, quetiapine, and ziprasidone. The order was modified according to reported previous ill effects of any SGA. For example, if a patient did not respond to risperidone and was agitated on aripiprazole, they received quetiapine. The SGA was slowly withdrawn over 2–4 weeks as tolerated (i.e., between the 4 th- to 8 th-week period). An overall guideline for withdrawal of SGAs was followed with reduction at 0.25 mg of risperidone, 2.5–5 mg of aripiprazole, 25–50 mg of seroquel, or 20–40 mg of ziprasidone every other day until they were off of the SGA. All patients were on a full fixed dose of 200 mg of lamotrigine monotherapy at the end of the 8-week dosing period. Benztropine was allowed on as-needed basis for extrapyramidal symptoms if on SGAs, but only during the first 4-week period.
Lamotrigine dosing over 14 weeks
The lamotrigine starting dose was 12.5 mg during the first week. It was increased at 12.5 mg/week for the first 4 weeks, 25 mg/week for the next 2 weeks, and titrated to 200 mg by 8 weeks. All patients were treated with a fixed dose of 200 mg for the last 6 weeks of treatment prior to being scanned at week 14.
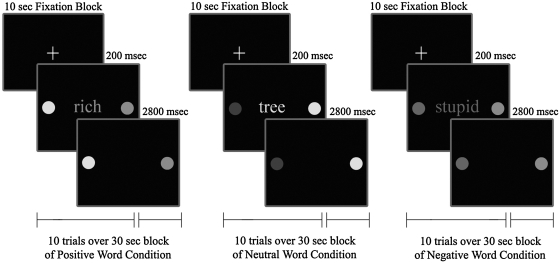
Pediatric Affective Color Matching Task
The task assessed the ability to match the color of a word to one of two colored dots presented on the left and right side of the word on a display screen (unlike the words appearing below the dots in our older version) (Pavuluri et al. 2008). Subjects were presented with separate blocks (30 seconds each) of positive, negative, and neutral word conditions (5 blocks of each condition) in a pseudo-random order, with each block consisting of 10 trials. Each block was separated by a 10-second fixation period (total of 15 fixation blocks) to provide rest periods during testing, and to allow for hemodynamic responses to return toward resting level before the next block of trials. In each 3-second trial, a word was presented for 200 msec, followed by a response period of 2,800 msec, during which subjects pressed a button (left or right) to match the color of the word to the correct color dot, as illustrated in Fig. 2. We moved the color dots from appearing below the words, as was the case in our previous paradigm (Pavuluri et al. 2008), to appear at the same level on either side of the word in the new design. This would avoid the confound of the frontal eye field activation generated by the eye movement while looking away from where the words are presented.
FIG. 2.
Pediatric Affective Color Matching Paradigm.
Subjects were told to “respond as quickly as possible.” Trials were balanced for correct responses (left or right dots) and colors used. The total duration of the task was exactly 10 minutes.
Affective words targeted affective domains relevant to PBD, such as feelings of depression, disappointment, and rejection. Positive words reflected feelings of happiness, energy, accomplishment, and success. Words were at an 8-year-old reading level and were equivalent across affect conditions in frequency of usage (Klein 1964; Kucera et al. 1967; Gilhooly and Logie 1980; Bradley 1999). No word was repeated during the task and, therefore, required no randomization. This task had three noteworthy characteristics. First, it directed voluntary attention to a simple perceptual characteristic of the words (color) rather than to their semantic meaning. Second, words were presented only briefly (200 msec) to minimize effects of variable scanning of stimulus words. Third, it required a simple cognitive operation and response choice (color matching) that could be easily performed by all subjects. In our updated version, we also ensured that all subjects were reading the words on each trial by asking subjects whether they recalled the words they had previously read (instead of just color matching without attention to the word valence). We found no group differences in the degree of recall. Together, these features maximize the impact of automatic affective responses on cognition, rather than deep semantic processing of the affective valence and meaning of words.
A color high-resolution liquid crystal display (LCD) projector projected visual stimuli onto a rear-projection screen that was viewed via an angled double-mirror system mounted on a standard GE head coil. During the scan, a camera monitored subject's right eye to ensure attention to visual stimuli. The accuracy and response time (RT) of button-press responses were averaged within each condition for each participant. Prior to imaging studies, participants spent approximately 20 minutes in a mock scanner to acclimate them to the scanner environment.
MRI protocol
MRI studies were performed using a 3.0 Tesla whole-body scanner (Signa, General Electric Medical System, Milwaukee, WI). Functional images were acquired using echo-planar imaging which is sensitive to regional alterations in blood flow via BOLD contrast effects. Twenty-five axial slices were acquired. Parameters for functional scans were: Echo time (TE) = 25 msec; flip angle = 90°; field of view = 20 × 20 cm2; acquisition matrix = 64 × 64; repetition time (TR) = 2.5 seconds; 5-mm slice thickness with 1-mm gap. Anatomical images were acquired in the axial plane (three-dimensional spoiled gradient recalled [SPGR], 1.5-mm-thick contiguous axial slices) to coregister and normalize the functional data.
Image processing and data analysis
For functional imaging data, FIASCO software (Functional Imaging Analysis Software-Computational Olio; Eddy et al. 1996) was used to implement three-dimensional motion estimation and correction and to remove slow signal drift. Individual volumes from the time series were excluded from the analysis if head displacement from the median head position in the time series was greater than 1.5 mm, or if head rotation from the median head position was greater than 0.5 degrees. The number of volumes retained after discarding those with motion artifact did not significantly differ across groups.
To evaluate subject-wise activation effects for statistical analyses, voxel-wise effect size (r) maps were calculated for each subject by contrasting activation for the negative and positive conditions versus the neutral condition. A Fisher z transform was applied to the r values to more closely approximate a normal distribution (zr) (Rosenthal 1991). Subjects' zr maps (effect size) and SPGR anatomical images were warped into Talairach space using the Analysis of Functional Neuroimages (AFNI) automated procedure (Talairach and Tournoux 1988). Functional maps were resampled to an isotropic 3 × 3 × 3-mm grid to provide a voxel dimension similar to that of the in-plane resolution of the acquired data.
The primary analysis for the study was a repeated-measures whole-brain analysis of variance (ANOVA) with the between-subjects factor of group (PBD, HC) and the within-subjects factors of word-matching conditions (negative vs. neutral, positive vs. neutral), and testing time (baseline, follow-up) that was carried out voxelwise on the zr maps. To correct for multiple comparisons, we applied a Bonferroni correction resulting in an adjusted probability for significance of p < 0.01. Then we used AFNI AlphaSim Monte Carlo simulations (Ward et al. 2000) to correct for voxel-wise multiple comparisons in the fMRI analyses. To do so, we identified significant voxel clusters by adopting a contiguity threshold (minimum volume threshold = 270 mm3; minimum clustering radius: 3.1 mm) that ensured an experiment-wise type 1 error rate of p < 0.025 (corrected p). On the basis of a significant three-way interaction in the whole-brain ANOVA, we carried out t-tests on clusters that were significant in the three-way interaction to further explore the directionality of within-group and between-group differences due to testing time and condition. Finally, we also performed Spearman correlation analyses to determine the relationship between symptom response as assessed by clinical measures (YMRS, CDRS-R) and fMRI activation in brain regions that resulted in significance in the ANOVA for the PBD group. Anatomical regions of interest (ROIs) for these regions were defined in standard Talairach space using AFNI tools. These regions in AFNI format, as well as anatomical ROI definitions, are available at: http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm; http://ccm.psych.uic.edu/Research/ResearchProgram/NormalBrain/ROIaffect_rules.aspx.
Results
Clinical and demographic data are summarized in Table 1. ANOVAs revealed no significant group differences for age, socioeconomic status (SES), and IQ. Fisher p tests revealed no significant group differences on handedness, gender, and racial composition (Table 1). There were 3 subjects (17.6%) with co-morbid diagnosis of generalized anxiety disorder in the PBD group. Within the PBD group, the mean doses of SGAs received at the end point of the first 4 weeks of acute symptom stabilization were: Risperidone 1.3 ± 0.30 mg (n = 5); aripiprazole 12.5 ± 3.0. mg (n = 5); quetiapine 385 ± 75 mg (n = 3). Benztropine was required in 4 cases with a mean dose of 1.2 ± 0.6 mg per day at the end of the first 4 weeks of SGA therapy, and was weaned off subsequently, along with the SGAs. None of the subjects was on SGA or any other psychotropic medications during the 6-week trial of fixed dose of lamotrigine at 200 mg. A repeated-measures ANOVA on YMRS scores at baseline and follow up revealed a significant interaction of group by testing time [F(1,29) = 14.68, p < 0.001], with significantly higher scores in patients than HC at baseline, but not at follow up (see Table 1). For the CDRS-R, the significant interaction of group by testing time [F(1,29) = 82.96, p < 0.001] revealed that CDRS-R scores were significantly higher in the patient group than HC at baseline and also at follow up (Table 1).
Table 1.
Demographic and Clinical Characteristics of Subjects with Pediatric Bipolar Disorder and Healthy Controls
| |
PBD (n = 17) |
HC (n = 14) |
Analyses |
|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | F(p <) |
| Age (years) | 14.3 (1.1) | 14.1 (2.4) | 0.02 (0.88) |
| WASI- FSIQa | 98.40 (6.74) | 104.5 (10.14) | 3.99 (0.07) |
| WRAT-3, Reading Subtestb | 40.56 (6.68) | 44.79 (6.97) | 2.95 (0.10) |
| Socioeconomic Statusc | 2.35 (.61) | 2.00 (.92) | 2.39 (0.13) |
| YMRS at baseline | 14.13 (6.73) | 0.86 (1.5) | 51.88 (0.00001) |
| YMRS at follow up | 4.5 (6.32) | 1.07 (1.94) | 3.80 (0.06) |
| CDRS-R at baseline | 52.19 (10.12) | 19.43 (2.28) | 139.90 (0.00001) |
| CDRS-R at follow up | 25.14 (5.71) | 19.00 (1.57) | 16.10 (0.0004) |
| n (%) | n (%) | Fisher p (two-tailed) | |
|---|---|---|---|
| Sex | 0.48 | ||
| Male | 6 (35%) | 7 (50%) | |
| Female | 11 (65%) | 7 (50%) | |
| Race | 1.00 | ||
| Caucasian | 8 (47%) | 7 (50%) | |
| Other | 9 (53%) | 7 (40%) | |
| Handedness | 1.00 | ||
| Right-handed | 16 (94%) | 14 (100%) | |
| Left-handed | 1 (6%) | 0 (0%) |
Wechsler Abbreviated Scale of Intelligence Intelligent Quotient (WASI IQ; Matrix Reasoning and Vocabulary Subtests).
Wide Range Achievement Test –Third Edition (WRAT-3).
Mean revised Hollingshead socioeconomic status.
Abbreviations: PBD = Pediatric bipolar disorder; HC = healthy control; YMRS = Young Mania Rating Scale; CDRS-R = Child Depression Rating Scale–Revised.
Behavioral data
Separate repeated-measures ANOVAs were carried out on RT and accuracy with the between-subjects factor of group (PBD, HC) and the within-subjects factors of testing time (baseline, follow up) and word valence (negative, positive, neutral) (Table 2). The main effect of group [F(1,27) = 7.22, p < 0.012] revealed that RT was overall slower for the PBD group (639 msec) relative to HC (535 msec). In addition, planned comparisons on the significant three-way interaction of group by testing time by word valence [F(2,54) = 4.026, p < 0.02] revealed that RT was significantly slower for the PBD than for the HC group for positive [F(1,27) = 8.28, p < 0.008] and neutral [F(1,27) = 6.35, p < 0.02] word valences at baseline, but not at follow up (p < 0.26 and p < 0.12, respectively). No other significant results were found. With regard to performance accuracy, the only significant finding was a group effect [F(1,27) = 8.90, p < 0.01], where accuracy in the PBD group (87%) was lower than that in HC (96%). Group differences did not vary significantly depending on word valence and/or testing time.
Table 2.
Response Time and Accuracy Measures for Subjects with Pediatric Bipolar Disorder and Healthy Controls on the Pediatric Color-Matching Task
| |
PBD (n = 17) |
|
HC (n = 14) |
|
|---|---|---|---|---|
| |
Mean (SD) |
|
Mean (SD) |
|
| Response time (in msec) | Baseline | Follow-up | Baseline | Follow-up |
| Negative words | 632 (149.8) | 640 (108.1) | 557 (120.7) | 535 (99.4) |
| Positive words | 710 (221.3) | 601 (134.2) | 525 (96.1) | 542 (139.9) |
| Neutral words | 637 (146.4) | 616 (143.2) | 517 (105.8) | 536 (129.3) |
| Accuracy | % (SD) | % (SD) | ||
| Negative words | 85 (22.3) | 88 (13.6) | 95 (7.9) | 96 (5.6) |
| Positive words | 88 (21.7) | 87 (13.8) | 96 (2.5) | 96 (5.8) |
| Neutral words | 84 (22.4) | 88 (13.6) | 95 (3.4) | 96 (7.2) |
Abbreviations: PBD = Pediatric bipolar disorder; HC = healthy controls; SD = standard deviation.
fMRI data
The interaction of group by time by condition was significant [F(1,29) = 5.57, p < 0.025] after correcting for multiple comparisons using a corrected p < 0.025 (see Methods). To further decompose the significant three-way interaction, our primary analyses involved t-tests only on significant clusters resulting from the three-way interaction as follows:
-
Baseline versus follow-up within PBD group comparisons (Table 3).
Negative Word Condition. Within the PBD group, greater activation was seen at baseline, relative to follow up, in the bilateral middle and superior frontal gyrus, bilateral posterior cingulate gyrus and superior temporal gyrus, right thalamus and inferior frontal gyrus, and left medial frontal gyrus, paralimbic/parahippocampal gyrus (Table 3). The PBD group did not have greater activation at follow up compared to baseline in any brain region. Within the HC group, greater activation was seen in bilateral medial frontal gyrus at baseline relative to follow up, whereas the middle frontal gyrus showed greater activation on follow up, relative to baseline.
Positive Word Condition. Within the PBD group, greater activation was seen at baseline, relative to follow up, in right medial frontal gyrus and thalamus, left inferior frontal gyrus and left premotor cortex (Table 3). Within the HC group, greater activation was seen in left medial frontal gyrus at baseline, relative to that on follow up. Also within the HC group, at follow up relative to baseline, increased activation was seen in right middle frontal gyrus, left dorsal cingulate cortex, and bilateral inferior parietal lobule.
-
Follow-up versus baseline between-group comparisons (Table 4).
PBD versus HC: Negative Word Condition. With treatment, PBD patients showed greater activation in left medial frontal gyrus and reduced activation in bilateral middle frontal gyrus, right posterior cingulate gyrus, parahippocampal gyrus, and inferior parietal lobule relative to change over time in the HC, for the negative word condition relative to neutral word condition. These results are summarized in Table 4 and Fig. 3A.
PBD versus HC: Positive Word Condition. After the 14-week treatment period, activation in the positive word condition relative to neutral word condition in the PBD group, compared to HC, showed a greater activation in right medial frontal gyrus and less activation in right middle frontal gyrus, precuneus and inferior parietal lobule. These results are summarized in Table 4.
As a secondary analysis we compared the two groups at baseline, to examine differences between patients and controls before treatment that may inform our interpretation of the interaction effect.
-
PBD versus HC at baseline (Table 5).
Negative Word Condition (Negative vs. Neutral Blocks). At baseline, the PBD group exhibited greater activation than the HC group in bilateral medial frontal gyrus (VMPFC) and precuneus, and in the right middle frontal gyrus (DLPFC), inferior frontal gyrus (VLPFC), dorsal anterior cingulate cortex, posterior cingulate gyrus, supramarginal gyrus, and inferior parietal lobule. The HC group did not exhibit greater activation than the PBD group in any region.
Positive Word Condition (Positive vs. Neutral Blocks). At baseline, the PBD group exhibited greater activation than the HC group in right medial frontal gyrus and inferior parietal lobule, left inferior frontal gyrus, and bilateral precuneus. HC did not show greater activation than PBD in any region.
Table 3.
Matching the Negative or Positive versus Neutral Words Within Pediatric Bipolar Disorder Group (n = 17) at Baseline versus Follow-Up
| Area | Talairach coordinates | BA | Volume (mm3) | t and p values for peak activation | Within- and between-group differences |
|---|---|---|---|---|---|
| A. PBD. Negative versus Neutral Words | |||||
| L medial frontal gyrus | −10, 44, 17 | 10 | 270 | 2.50, p < 0.025 | PBD baseline > follow up |
| R inferior frontal gyrus | 41, 35, 14 | 47,10 | 270 | 2.99, p < 0.025 | PBD baseline > follow up |
| R middle frontal gyrus | 53, 26, 32 | 9 | 972 | 3.07, p < 0.01 | PBD baseline > follow up |
| L middle frontal gyrus | −46, 23, 38 | 9 | 432 | 4.47, p < 0.01 | PBD baseline > follow up |
| R superior frontal gyrus | 2, 41, 47 | 8 | 6502 | 3.50, p < 0.005 | PBD baseline > follow up |
| L superior frontal gyrus | −10, 41, 49 | 8 | 4001 | 2.84, p < 0.01 | PBD baseline > follow up |
| R posterior cingulate cortex | 11, −49, 26 | 31 | 945 | 4.18, p < 0.004 | PBD baseline > follow up |
| L posterior cingulate cortex | −4, −61, 26 | 31 | 1026 | 3.80, p < 0.006 | PBD baseline > follow up |
| R thalamus | 5, −25, 17 | 1107 | 3.80, p < 0.007 | PBD baseline > follow up | |
| L parahippocampal gyrus | −28, −31, −1 | 27 | 918 | 3.00, p < 0.01 | PBD baseline > follow up |
| L superior temporal gyrus | −49, −4, −7 | 21 | 513 | 4.47, p < 0.01 | PBD baseline > follow up |
| R superior temporal gyrus | 50, −19, −7 | 21 | 378 | 3.73, p < 0.02 | PBD baseline > follow up |
| B. PBD. Positive versus Neutral Words | |||||
| R medial frontal gyrus | 2, 59, 26 | 10 | 378 | 3.39, p < 0.02 | PBD baseline > follow up |
| L inferior frontal gyrus | −42, 20, −16 | 47 | 270 | 3.56, p < 0.02 | PBD baseline > follow up |
| L premotor cortex | −16, 2, 56 | 6 | 540 | 2.80, p < 0.009 | PBD baseline > follow up |
| R thalamus | 5, −10, 11 | 513 | 3.07, p < 0.01 | PBD baseline > follow up | |
Talairach coordinates and t and p values of peak activation for regions (significant clusters at corrected p < 0.025 with contiguity threshold) for which the negative or positive versus neutral words comparison differed significantly between baseline and follow up within the PBD group (n = 17).
Abbreviations: R = Right; L = left; PBD = pediatric bipolar disorder; HC = healthy controls; BA = Brodmann area.
Table 4.
Matching the Negative or Positive versus Neutral Words in Pediatric Bipolar Disorder Group (n = 17) and Healthy Controls (n = 14): Degree of Change Over the Duration of Clinical Trial
| Area | Talairach coordinates | BA | Volume (mm3) | t and p value for peak activation | Within- and between-group differences |
|---|---|---|---|---|---|
| A. Follow up vs Baseline. Negative versus Neutral Words | |||||
| L medial frontal gyrus | −13, 50, 5 | 10 | 540 | 4.06, p < 0.01 | PBD > HC |
| R middle frontal gyrus | 41, 44, 29 | 9 | 12,069 | 4.24, p < 0.002 | HC > PBD |
| L middle frontal gyrus | −46, 23, 38 | 9 | 1,728 | 3.54, p < 0.02 | HC > PBD |
| R posterior cingulate cortex | 14, −58, 11 | 30 | 1,215 | 3.28, p < 0.008 | HC > PBD |
| R parahippocampal gyrus | 8, −43, 5 | 29 | 1,080 | 4.92, p < 0.005 | HC > PBD |
| R inferior parietal lobule | 56, −46, 41 | 40 | 783 | 4.43, p < 0.005 | HC > PBD |
| B. Follow up vs Baseline. Positive versus Neutral Words | |||||
| R medial frontal gyrus | 11, 59, −1 | 10 | 486 | 3.16, p < 0.005 | PBD > HC |
| R middle frontal gyrus | 41, 44, 29 | 9 | 513 | 3.29, p < 0.02 | HC > PBD |
| R precuneus | 38, −67, 35 | 39 | 1,485 | 3.62, p < 0.001 | HC > PBD |
| R inferior parietal lobule | 47, −52, 47 | 40 | 513 | 2.54, p < 0.004 | HC > PBD |
Talairach coordinates and t and p values of peak activation for regions (significant clusters at corrected p < 0.025 with contiguity threshold) for which the Negative or Positive versus Neutral Words comparison differed significantly between the PBD Group (n = 17) and HC (n = 14) for Follow up vs. Baseline (i.e., Degree of Change Over the Duration of Treatment Trial).
Abbreviations: R = Right; L = left; PBD = pediatric bipolar disorder; HC = healthy controls; BA = Brodmann area.
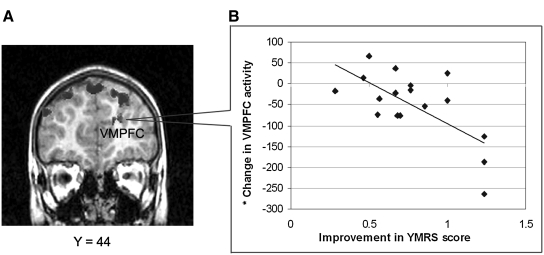
FIG. 3.
Lamotrigine treatment effects. (A) Differences in lamotrigine treatment over time within patients with pediatric bipolar disorder (PBD) (n = 17). The black areas at the top of the head indicate reduced activation for negative versus neutral condition at follow up relative to baseline. VMPFC = Ventromedial prefrontal cortex. (B) Graphic representation of the significant correlation, for the PBD group, between an index of improvement in the Young Mania Rating Scale (YMRS) scores (i.e., ratio between the difference of baseline and follow-up scores) and an index of the decrease in the left VMPFC activity with time. (*) Voxels showing a significant level of activation.
Table 5.
Baseline Comparisons of 17 Patients with Pediatric Bipolar Disorder and 14 Healthy Controls While Matching the Negative or Positive Words versus Neutral Words
| Area | Talairach coordinates | BA | Volume (mm3) | t and p value for peak activation | Within- and between-group differences |
|---|---|---|---|---|---|
| A. Negative vs Neutral: Baseline | |||||
| R medial frontal gyrus | 2, 62, 12 | 10 | 351 | 2.57, p < 0.025 | PBD > HC |
| L medial frontal gyrus | −2, 62, 12 | 10 | 270 | 2.35, p < 0.025 | PBD > HC |
| R inferior frontal gyrus | 41, 35, 14 | 47,10 | 297 | 3.38, p < 0.02 | PBD > HC |
| R middle frontal gyrus | 50, 29, 32 | 9 | 3078 | 3.46, p < 0.008 | PBD > HC |
| R dorsal anterior cingulate cortex | 2, 35, 20 | 32 | 378 | 3.40, p < 0.01 | PBD > HC |
| R posterior cingulate gyrus | 2, −55, 26 | 31 | 540 | 3.01, p < 0.009 | PBD > HC |
| R precuneus | 2, −64, 56 | 7 | 5238 | 3.02, p < 0.01 | PBD > HC |
| L precuneus | −13, −58, 35 | 7 | 1242 | 3.43, p < 001 | PBD > HC |
| R supramarginal gyrus | 47, −46, 29 | 40 | 351 | 3.81, p < 0.02 | PBD > HC |
| R inferior parietal lobule | 41, −61, 47 | 40 | 351 | 3.38, p < 0.01 | PBD > HC |
| B. Positive vs Neutral: Baseline | |||||
| R medial frontal gyrus | 5, 62, 14 | 10 | 378 | 2.99, p < 0.02 | PBD > HC |
| L inferior frontal gyrus | −40, 20, −16 | 47 | 432 | 4.55, p < 0.01 | PBD > HC |
| R precuneus | 32, −70, 35 | 19 | 486 | 2.74, p < 0.025 | PBD > HC |
| L precuneus | −10, −52, 65 | 7 | 432 | 2.93, p < 0.01 | PBD > HC |
| R inferior parietal lobule | 44, −55, 50 | 40 | 1323 | 2.92, p < 0.01 | PBD > HC |
Talairach coordinates and t and p values of peak activation for regions (significant clusters at corrected p < 0.025 with contiguity threshold) for which the Negative or Positive versus Neutral Words comparison differed significantly between the PBD Group (n = 17) and HC (n = 14) at baseline.
Abbreviations: R = Right; L = left; PBD = pediatric bipolar disorder; HC = healthy controls; BA = Brodmann area.
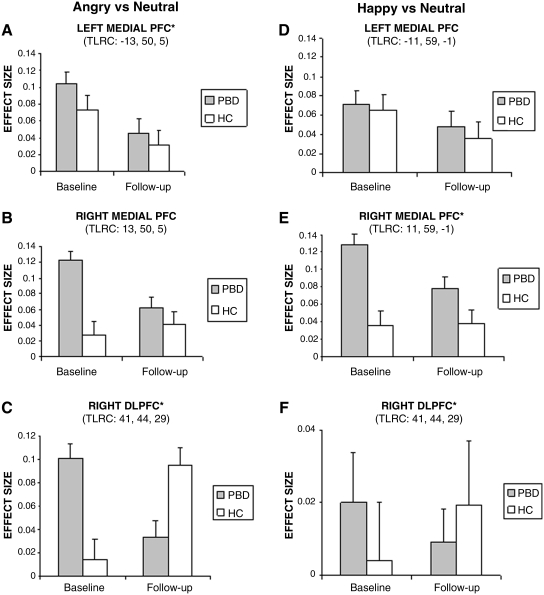
Figure 3 illustrates group activation data for each time point and each condition for left and right medial prefrontal cortex and DLPFC.
Correlations between symptom response and brain activation
Pearson correlation analyses (two-tailed) were carried out for the PBD group to examine the relationship between the improvement on the YMRS and the CDRS-R scores, and activation changes in right and left middle frontal gyrus and medial frontal gyrus for the negative versus neutral and the positive versus neutral conditions, over time. For both scales, an improvement index was obtained as the ratio between the difference of baseline and follow-up scores. Given that our correlation analyses were hypotheses driven, based on the AFNI ANOVA results, and were limited to a small number of comparisons, we report correlation results that were uncorrected for multiple comparisons. For the negative relative to neutral condition, there was a significant correlation between improvement in the YMRS scores and decreases in left medial frontal gyrus (left ventromedial prefrontal cortex) activation with time (r = −0.55, p < 0.02), showing that the greater the improvement in YMRS scores the greater the decrease in activation in this region (Fig. 4A,B). No other significant results were found for the YMRS or CDRS scores. For the positive condition relative to neutral condition, there was a significant correlation in the patient group between the improvement in the YMRS scores and decreases in activation in right medial frontal gyrus (r = −0.47, p < 0.05). A significant correlation was also found between the improvement on the CDRS-R scores and decreases in activation in the right medial frontal gyrus (r = − 0.46, p < 0.05).
FIG. 4.
Group activation data for each time point and condition. PFC = Prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; PBD = pediatric bipolar disorder; HC = healthy control; TLRC = Talairach coordinates for cluster peak activation. (*) Significant group differences for the cluster.
Discussion
This is the first pharmacological fMRI study to examine treatment effects on the neural systems supporting the interaction of cognitive and affective processing in PBD. Our central findings are two fold. First, there are baseline differences in mechanism of brain function between PBD and HC during cognitive performance under negative or positive emotional challenge. There is increased activation in both cognitive (e.g., right DLPFC, dorsal ACC, posterior cingulate gyrus), and emotional (e.g., right VLPFC and bilateral VMPFC) cortical regions in PBD relative to HC, suggesting greater effort expended by the affective and cognitive regions of the brain to accomplish the same task as HC. Second, on retesting after 14 weeks of treatment for PBD patients, PBD group showed normalization in emotional control region, i.e., VLPFC (inferior frontal gyrus) with no group differences between PBD and HC. However, PBD group showed reduced activity in DLPFC (middle frontal gyrus) while performing the cognitive task, while showing persistently increased activity in VMPFC (medial frontal gyrus), a region known to be recruited in emotional evaluation (Northoff et al. 2004; Deppe et al. 2005; Bermpohl et al. 2006; Kross et al. 2009), posttreatment, relative to baseline, when compared to HC group.
However, it is important to underscore that, given the nature of the fMRI studies (which, at any one time, could only demonstrate relative change between two groups, or two time points or type of conditions within a group), change within PBD group from baseline to posttreatment needs to be carefully scrutinized in addition to the comparison with HC. Indeed, pharmacotherapy resulted in improvement in manic symptoms on the YMRS that significantly correlated with a decrease in VMPFC activity, the region deployed in automatic emotional appraisal. This steep decrease in BOLD signal activity in VMPFC within PBD group is still not sufficient to reach the same level of activity as in HC, and therefore shows comparatively greater activity than in HC on follow up.
Our findings, therefore, illustrate that treatment led to decreases in manic and depressive symptoms in patients, accompanied by decreased BOLD signal activity in all the prefrontal regions, and the pattern of brain function in PBD remained distinct from that of HC. Behavioral data did not show improvement in patients or HC on follow up to account for the effects of treatment or familiarity with the retesting in either group.
Abnormal pattern of prefrontal activity at the interface of affective and cognitive circuitry in acutely Ill PBD patients
As mentioned above, overall, our study illustrated that untreated PBD patients, relative to HC, appear to expend greater effort in the prefrontal and parietal regions at the interface of affective and cognitive regions while performing the cognitive task under emotional challenge.
Affective neural system
While there was increased BOLD signal activity in acutely ill PBD patients in manic, mixed, or hypomanic phase, relative to HC, in higher cortical regions of affective control (i.e., VLPFC) (Rich et al. 2005; Heatherton et al. 2006; Kross et al. 2009; Posner et al. 2009) and emotional evaluation (i.e, VMPFC) (Northoff et al. 2004), there were no group differences in activation in the limbic or the paralimbic regions. In the studies on adult bipolar disorder (BD), VMPFC was shown to be dysfunctional in regulating subcortical limbic systems in response to emotional faces and words (Malhi et al. 2007; Phillips et al. 2008), and during emotional Stroop tasks that demand the ability to attend to a cognitive task while ignoring emotional information (Rubinsztein et al. 2001; Malhi et al. 2005; Lagopoulos and Malhi 2007). Furthermore, structural abnormalities in white matter tracts between the VMPFC and limbic regions have been reported in adult BD (Versace et al. 2008). The increased BOLD signal activity in the left VMPFC and right VLPFC in PBD patients, relative to the activity at follow up, may be an effort to modulate the increased activity in left parahippocampal gyrus, implicated in emotional arousal (Rich et al. 2005; Posner et al. 2009) in untreated patients.
Cognitive neural system
The main finding in cognitive neural operations in the acutely ill PBD group, relative to HC, is increased right DLPFC, dorsal anterior cingulate cortex, and posterior cingulate cortex activity during the negative word matching. It may be that the negative emotional stimuli may have greater impact on cognitive circuitry function in PBD because a similar pattern of increased cognitive effort was not noted during the positive word matching. Indeed, there was a greater decrease in DLPFC with negative words, relative to positive words during the color matching task in our treated euthymic sample (Pavuluri et al. 2008).
Impact of pharmacotherapy on affective and cognitive regions in PBD patients
Affective neural system
The present finding of an increase in VLPFC activity in PBD relative to HC at baseline is similar to that of other studies in medicated PBD patients that included both the bipolar type 1 and 2 subjects similar to this study (Rich et al. 2005; Dickstein et al. 2008). This increased activity in VLPFC in response to both negative and positive conditions appears to normalize with no group differences at the study end point, and may be an indicator of positive outcome of treatment. Previously, using a very similar paradigm, we reported decreased VLPFC in unmedicated and euthymic patients (Pavuluri et al. 2008). It is important to note that subjects in that study were limited to BD type 1 and treated with multiple medications. In contrast, the current study is similar to those by Dickstein et al. (2008), Passarotti et al. (2010), and Rich et al. (2005), which included BD type 2 in the sample where the top-down control is not taxed to the same extent as in the homogeneous sample of the BD type 1 (Pavuluri et al. 2008). Additionally, unlike our previous cross-sectional study of euthymic patients, the current longitudinal study examined the unmedicated patients being treated with a selective agent, lamotrigine, to maintain euthymic state and potentially improve cognition (Pavuluri et al. 2010) after initial stabilization with SGA.
With lamotrigine treatment, VMPFC activity remains increased in patients relative to HC in both the negative and positive conditions. However, the right VMPFC showed increased activity in the case of the positive condition and the left VMPFC showed increased activity in the negative condition in PBD relative to HC. Although this may be a laterality effect biased by the type of emotional stimuli, the sample is small and this laterality effect needs to be replicated to draw stronger conclusions.
This reduction in parahippocampal activity in response to negative emotions is similar to the results demonstrated by Chang et al. (2008) where there was a reduced activation of subcortical amygdala in depressed adolescents with BD receiving lamotrigine (Chang et al. 2008). Our findings are also consistent with those of the functional connectivity study using dynamic causal modeling in adult BD that demonstrated the top-down control with VMPFC modulating the parahippocampal activity in response to negative words (Almeida et al. 2009).
Cognitive neural system
In the current study, for the negative condition, decreased DLPFC activation was found with treatment in patients, relative to HC, with no decrease in performance accuracy in either group. Given that greater effort was required at baseline with higher DLPFC activity than HC, decrease in activity with treatment to accomplish the same task may reflect an improvement in cognitive efficiency. Therefore, findings from this longitudinal study design replicated our previous findings of decreased DLPFC activity in euthymic patients relative to HC from the cross-sectional study (Pavuluri et al. 2008) while performing the same color-matching task. Furthermore, in contrast to patients, HC may be demonstrating increased DLPFC activity on retest due to automaticity as they learn to better filter emotional information.
Although this longitudinal study design helps us to interpret the meaning of our findings in a different light than our previous cross-sectional study, these mechanistic explanations remain speculative because sample sizes are small and further work is needed to evaluate their heuristic value. Varying the degree of cognitive task difficulty may help evaluate the patient–control differences and treatment-related changes. But for now, we are limited to say that the current paradigm demonstrated generalized prefrontal abnormalities that changed with treatment, specifically in the DLPFC during a task dependent on integrated cognitive and affective processing. Furthermore, our results in HC, which are in line with the studies in healthy adults, showed that the tasks involving attention or strategic thinking led to an increased activity in DLPFC involved in cognition and decreased activity in VMPFC involved in emotional evaluation (Northoff et al. 2004; Deppe et al. 2005; Bermpohl et al. 2006; Kross et al. 2009). This pattern of activation may originate from the dynamic interaction between affective and cognitive operations. Our PBD patients did not follow that pattern.
Clinical ratings and brain activation
Change in left and right VMPFC activation correlated significantly with improvement in manic symptoms over time in both the negative and positive emotion-processing conditions, relative to the neutral condition. This is supportive of our prediction that mood stabilization can be demonstrated by improved affective circuitry function. In the positive word-matching condition, decrease in depressive symptoms was significantly associated with a decrease in the VMPFC activity. It may be that lamotrigine, known to be particularly effective for depressive symptoms (Chang et al. 2008), may enhance cognitive function while processing positive emotion.
This is a “proof of concept” study to examine the synergistic activity of affective and cognitive processes and the impact of pharmacotherapy on these processes in PBD. Limitations include the fact that we used a block design study that did not allow us to distinguish between correct and incorrect responses and to study the effect of affective response specifically to positive or negative words while performing the cognitive tasks. The task was successful in bringing out the emotionally specific BOLD signal activation in all groups, although there were no corresponding behavioral differences in either group. This underscores the greater sensitivity of the fMRI method in contrast to behavioral observations. However, future experiments using event-related designs are needed to separately evaluate brain activity for affective response to positive as compared to negative words, and for correct as compared to incorrect responses, during affective and cognitive processing independently and together. Varying cognitive task difficulty will probe the case–control differences and may yield a greater understanding about brain function changes that define PBD in response to pharmacotherapy. Larger samples and fMRI paradigms that target both affective and cognitive difficulties in double-blind cross-over design studies may be ultimately needed to move the field in the direction of mapping the brain circuitry in response to symptom-based treatments.
Conclusions
Findings in unmedicated PBD patients with mania and hypomania, relative to HC, demonstrated greater BOLD signal activity in affective (VMPFC and VLPFC) and cognitive (DLPFC) higher cortical regions of the brain while performing the same cognitive activity under emotional challenge. Relative to HC, on follow up, treated patients showed greater VMPFC activity and decreased DLPFC activity, illustrating a different and altered pattern of activity in patients. Within the patient group, pharmacotherapy resulted in lowered symptoms of mania and enhanced affective circuitry function accompanied by decreased BOLD signal activity in VMPFC, VLPFC, and paralimbic regions. Decreased DLPFC activity within PBD patients with treatment (which was also higher in PBD group when compared to HC at baseline) may represent partial recovery in cognitive circuitry operations.
Footnotes
This research was funded by National Alliance for Research on Schizophrenia and Depression (NARSAD), Marshall Reynolds Foundation, National Inisituties of Health (NIH) K23 RR018638, GlaxoSmith & Kline (Investigator Initiated Fund), and NIH-MO1-RR-13987.
Disclosures
Dr. Pavuluri's work unrelated to this manuscript is supported by the National Institute of Mental Health (NIMH), National Institute of Child Health and Human Development (NICHD), Dana Foundation, American Foundation for Suicide Prevention, Abbott Pharmaceuticals (study medication), and Johnson & Johnson (study medication). Dr. Sweeney, also unrelated to this work, received support from the National Institutes of Health (NIH) and Johnson & Johnson. The other authors have no financial relationships to disclose.
References
- Almeida JR. Akkal D. Hassel S. Travis MJ. Banihashemi L. Kerr N. Kupfer DJ. Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: Significant effects of gender and trait anxiety. Psychiatry Res. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. DSM-IV. [Google Scholar]
- Bermpohl F. Pascual-Leone A. Amedi A. Merabet LB. Fregni F. Gaab N. Alsop D. Schlaug G. Northoff G. Attentional modulation of emotional stimulus processing: An fMRI study using emotional expectancy. Hum Brain Mapp. 2006;27:662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM LP. Gainesville (Florida): The Center for Research in Psychophysiology: University of Florida; 1999. Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Chang KD. Wagner C. Garrett A. Howe M. Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Deppe M. Schwindt W. Kugel H. Plassmann H. Kenning P. Nonlinear responses within the medial prefrontal cortex reveal when specific implicit information influences economic decision making. J Neuroimaging. 2005;15:171–182. doi: 10.1177/1051228405275074. [DOI] [PubMed] [Google Scholar]
- Dickstein DP. Treland JE. Snow J. McClure EB. Mehta MS. Towbin KE. Pine DS. Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Dickstein DP. van der Veen JW. Knopf L. Towbin KE. Pine DS. Leibenluft E. Proton magnetic resonance spectroscopy in youth with severe mood dysregulation. Psychiatry Res. 2008;163:30–39. doi: 10.1016/j.pscychresns.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Eddy WF. Fitzgerald M. Genovese CR. Mockus A. Noll DC. Proceedings in Computational Statistics. Prat A. Heidelberg: Physica-Verlag; 1996. Functional image analysis software—computational olio; pp. 39–49. [Google Scholar]
- Geller B. Warner K. Williams M. Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ. Logie RH. Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1,944 words. Behav Res Meth Instrument. 1980;12:395–427. [Google Scholar]
- Haldane M. Jogia J. Cobb A. Kozuch E. Kumari V. Frangou S. Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with lamotrigine monotherapy. Eur Neuropsychopharmacol. 2008;18:48–54. doi: 10.1016/j.euroneuro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Wyland CL. Macrae CN. Demos KE. Denny BT. Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GS. Semantic power measured through the interference of words with color-naming. Am J Psychol. 1964;77:576–588. 576–588. [PubMed] [Google Scholar]
- Kross E. Davidson M. Weber J. Ochsner K. Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65:361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H. Francis WN. Carroll JB. Twaddell WF. Computational Analysis of Present-Day American English. Providence (Rhode Island): Brown University Press; 1967. [Google Scholar]
- Lagopoulos J. Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport. 2007;18:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- Malhi GS. Lagopoulos J. Sachdev PS. Ivanovski B. Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7(Suppl 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Malhi GS. Ivanovski B. Hadzi-Pavlovic D. Mitchell PB. Vieta E. Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- McClure EB. Treland JE. Snow J. Dickstein DP. Towbin KE. Charney DS. Pine DS. Leibenluft E. Memory and learning in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:461–469. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- Northoff G. Heinzel A. Bermpohl F. Niese R. Pfennig A. Pascual-Leone A. Schlaug G. Reciprocal modulation and attenuation in the prefrontal cortex: An fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21:202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM. Sweeney JA. Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN. Schenkel LS. Aryal S. Harral EM. Hill SK. Herbener ES. Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. O'Connor MM. Harral EM. Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. O'Connor MM. Harral EM. Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M. Passarotti AM. Harral E. Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML. Ladouceur CD. Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829. doi: 10.1038/mp.2008.65. 833–829, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J. Russell JA. Gerber A. Gorman D. Colibazzi T. Yu S. Wang Z. Kangarlu A. Zhu H. Peterson BS. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Hum Brain Mapp. 2009;30:883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E. Grossman J. Buchsbaum Y. Banegas M. Freeman L. Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio (Texas): Harcourt Brace & Company; 1999. [Google Scholar]
- Rich BA. Schmajuk M. Perez-Edgar KE. Pine DS. Fox NA. Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Rich BA. Vinton DT. Roberson-Nay R. Hommer RE. Berghorst LH. McClure EB. Fromm SJ. Pine DS. Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. Newbury Park (California): Sage; 1991. [Google Scholar]
- Rubinsztein JS. Fletcher PC. Rogers RD. Ho LW. Aigbirhio FI. Paykel ES. Robbins TW. Sahakian BJ. Decision-making in mania: A PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- Spearing MK. Post RM. Leverich GS. Brandt D. Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Talairach J. Tournoux P. Stuttgart. New York: Thieme Medical Publishers; 1988. Co-Planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- Versace A. Almeida JR. Hassel S. Walsh ND. Novelli M. Klein CR. Kupfer DJ. Phillips ML. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HA. Riederer SJ. Grimm RC. Ehman RL. Felmlee JP. Jack CR., Jr Prospective multiaxial motion correction for fMRI. Magn Reson Med. 2000;43:459–469. doi: 10.1002/(sici)1522-2594(200003)43:3<459::aid-mrm19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Young RC. Biggs JT. Ziegler VE. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]