Abstract
Introduction
Methadone (ME) is a highly effective opioid agonist used for difficult pain syndromes. However, in the management of cancer pain with strong opioids, rotation to a different opioid (opioid rotation) may be required because of side effects or poor pain control. Rotation from methadone to another opioid has received limited study and therefore may be difficult because of the absence of a uniformly accepted dose conversion ratio.
Methods
Retrospectively reviewed consecutive medical records of patients undergoing an opioid rotation from methadone to an alternative opioid were evaluated. For inclusion, patients were required to have received methadone for at least 3 days and have reached stable dose of the alternative opioid(s) during the 7 days following. Stable dose was defined as a 30% or less change in opioid dose from one day to the next.
Results
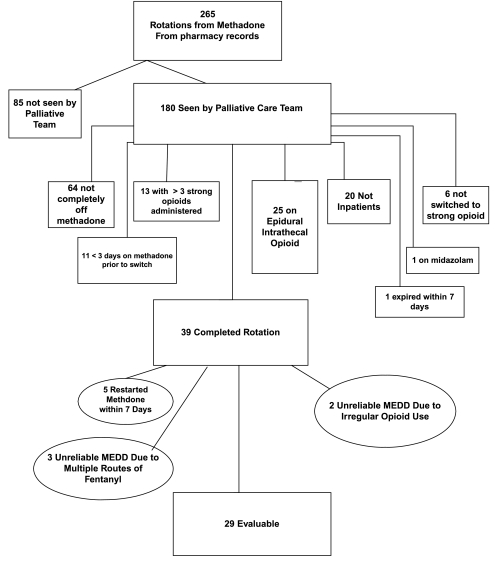
Records of 39 patients met inclusion criteria. Excluded from analysis were 5 patients who were restarted on methadone within 7 days, 2 with irregular opioid use resulting in negligible regular opioid doses post-switch, and 3 due to concerns about reliability of multiple routes used for fentanyl. Data from 29 patients, 10 female, mean age 48 ± 14.4 years, were evaluable. The mean dose ratio for oral methadone to oral morphine equivalent daily dose (MEDD) was 1:4.7 (95% confidence interval [CI], 3.0–6.5; n = 16), and for intravenous (IV) methadone to MEDD was 1:13.5 (95% CI, 6.6–20.5; n = 13), p = 0.06. Methadone dose was significantly correlated to stable MEDD after switching opioids for both methadone IV and oral (Spearman = 0.86, p = 0.0001 and Spearman = 0.72, p = 0.0024), respectively. Mean day of achieving stable dose was day 2.5 ± 0.2 for IV methadone and day 2.6 ± 0.3 for oral methadone.
Conclusion
These dose ratios are new findings that may assist in switching patients more safely to alternative opioids when side effects or pain problems occur when patients are receiving methadone. An important difference in analgesic potency appears to exist between IV and oral ME. Future research with prospective studies is required.
Introduction
Methadone is a synthetic opioid agonist that is used increasingly in the management of cancer pain.1,2 The strategy of opioid rotation, that is switching from one opioid drug to another, is substantiated by the medical literature and is commonly used in clinical practice to manage opioid side effects or inadequate analgesia.3–5 The use of equianalgesic dose ratios provides a method to determine equivalent and safe analgesic doses for opioids that differ in potency.2,6 Many studies have addressed the equianalgesic dose ratios for switching between morphine and hydromorphone, morphine and oxycodone, and when switching from morphine to methadone.7–10 only two small studies report on rotations involving methadone in the opposite direction, that is when switching from methadone to an alternate opioid.1,8 The direction of opioid rotation is important, as the conversion factors that result are not necessarily equivalent when switching the opioid in the opposite direction. One study by Lawlor et al.8 reports on six patients switched from methadone to morphine. The study by Moryl et al.1 reports that 12 of 13 patients were unable to complete a rotation from methadone to another opioid due to pain and adverse side effects. This is contrary to the experience of our group as we enjoyed successful pain control in switching patients from methadone to an alternative opioid.
Methods
Institutional Review Board (IRB) approval was obtained to perform a retrospective analysis of consecutive opioid rotations from methadone to an alternative opioid performed by the Department of Symptom Control and Palliative Care (eight board-certified palliative medicine specialists with significant expertise in the use of methadone and opioid analgesia supported by fellows and advance practice nurses that evaluate patients daily). Using the computerized pharmacy database, medical records were screened for inclusions and exclusion criteria. Opioid rotation was preformed empirically based on the experience of most physicians using a methadone: morphine conversion dose ratio of 1:5 or 1:10 as a benchmark. Standard practice had doses adjusted up or down by a minimum of 25%–30% until pain was controlled or side effects resolved. Breakthrough pain doses of 10% of the total daily opioid dose (or equivalent) were typically prescribed. Inclusion and exclusion criteria were determined by utilizing criteria from previous studies in order to obtain reasonable estimates of stable methadone dose prior to switch and to eliminate possible confounding factors such as delirium, discharge before stable dose, or death.
Patients were included if: they had cancer pain and were rotated from methadone to another strong opioid as an in-patient, had received methadone 3 days or more before the rotation, had reached stable dose of the new opioid 2 days or more before discharge or death, reached stable dose 1–7 days post-switch, and methadone was able to be completely stopped at time of rotation. Stable dose was defined as the dose maintained with 30% or less change for more than 24 hours.
Patients were excluded if: they were discharged from the hospital less than 48 hours after the opioid switch, were receiving opioids via intrathecal or epidural pump, if opioid rotation involved more than 3 strong opioids other than methadone, or if they were receiving sedation with midazolam.
Data collection included age, gender, race, primary tumor, pain score, reason for opioid switch, and date of death if available.
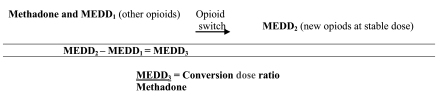
The dose of all opioids other than methadone were converted to morphine equivalent daily dose (MEDD) for purposes of analysis, using established conversion ratios for these opioids (these ratios are summarized at www.palliative.org/PC/ClinicalInfo/AssesmentTools, using parenteral MEDD).7,8,10–19 For patients receiving methadone and a second opioid prior to the switch, the MEDD of the second opioid was subtracted from the MEDD calculated for the day when stable dose was reached. The remainder was used to calculate the equianalgesic dose ratio with the previous methadone dose (Fig. 1).
FIG. 1.
Calculation of conversion ratio.
Summary statistics were generated to describe the demographic and clinical characteristics of the patients. Scatter plots of methadone dose on the day prior to switch versus stable MEDD dose were obtained. Regression modes with no Y-intercept were fit to predict the new opioid dose based on the observed methadone dose. Equinalgesic dose ratios were calculated. Statistical analysis of the relationship between dose ratio and methadone dose was performed. Linear, quadratic, and cubic models were fit to the data. Spearman's correlation coefficient was calculated to measure the strength of the relationship between the previous methadone dose and the stable opioid dose.
Results
The computerized pharmacy database determined 265 opioid rotations from methadone to other opioids. These were further screened for inclusions/exclusion criteria (Fig. 2).
FIG. 2.
Study outline.
Data from the 29 remaining patients were classified into two distinct subgroups: IV methadone switched to oral MEDD and oral methadone switched to oral MEDD (Table 1). Among the IV and oral methadone groups approximately equal numbers were switched to morphine (n = 6, n = 7, respectively) and hydromorphone (n = 5, n = 5). The remaining patients received oxycodone (n = 1, n = 2 respectively), a combination of morphine and hydromorphone (n = 1, n = 1), and a combination of morphine, hydromorphone and oxycodone (n = 1, oral methadone group). The majority of the cases represented the use of methadone plus other opioid(s) prior to the switch, who were then rotated to morphine (n = 13) or hydromorphone (n = 10).
Table 1.
Patient Characteristics
| Variable | IV methadone to oral MEDD n(%), n = 13 | Oral methadone to oral MEDD n (%), n = 16 | Total n (%), n = 29 |
|---|---|---|---|
| Gender | |||
| Female | 5 (38%) | 5 (31%) | 10 (34%) |
| Race | |||
| White | 8 (62%) | 11 (69%) | 19 (66%) |
| other | 5 (38%) | 5 (31%) | 10 (34%) |
| Age | |||
| Mean (SD) | 49 (12) | 48 (17) | 48 (14) |
| Reason for switching | |||
| Pain | 3 (23%) | 3 (19%) | 6 (21%) |
| Opioid toxicity | 3 (23%) | 5 (31%) | 8 (28%) |
| Other | 3 (23%) | 7 (44%) | 10 (34%) |
| Unknown | 4 (31%) | 1 (6%) | 5 (17%) |
| Methadone Dose | |||
| <10 mg/d | 4 (31%) | 1 (6%) | 5 (17%) |
| 10–19 mg/d | 1 (8%) | 5 (31%) | 6 (21%) |
| 20–34 mg/d | 2 (15%) | 4 (25%) | 6 (21%) |
| 35–57 mg/d | 4 (31%) | 3 (19%) | 7 (24%) |
| >57 mg/d | 2 (15%) | 3 (19%) | 5 (17%) |
| Site | |||
| Lung | 4 (31) | 4 (25) | 8 (28) |
| Hematologic | 3 (23) | 4 (25) | 7 (24) |
| Gastrointestinal | 3 (23) | 2 (13) | 5 (17) |
| Gynecological | 2 (15) | 1 (6) | 3 (10) |
| Other | 1 (8) | 5 (31) | 6 (21) |
IV, intravenous; MEDD, morphine equivalent daily dose; SD, standard deviation.
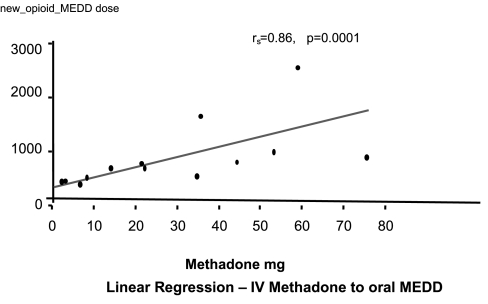
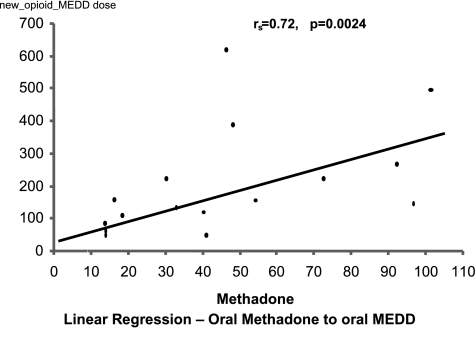
Plots of the linear regression for both IV methadone and oral methadone are displayed in Figures 3 and 4, from these are derived the estimated equianalgesic conversion dose ratios (Table 2). Analysis of the relationship between dose ratio and methadone dose did not yield a statistically significantly relationship for any of the models used.
FIG. 3.
Linear regression: intravenous methadone to oral morphine equivalent daily dose (MEDD).
FIG. 4.
Linear regression: oral methadone to oral morphine equivalent daily dose (MEDD).
Table 2.
Estimated Conversion Dose Ratios and Mean Dosages
| Variable | IV methadone oral MEDD | Oral methadone to oral MEDD |
|---|---|---|
| Methadone dose | ||
| Mean mg/d (SD) | 30 (25) | 35 (31) |
| Median mg/d (range) | 20 (2–75) | 26 (5–105) |
| Stable MEDD dose | ||
| Mean mg/d (SD) | 411 (552) | 191 (192) |
| Median mg/d (range) | 275 (43–2138) | 110 (15–610) |
| Estimated conversion | ||
| Dose ratio | ||
| Mean (95% CI) | 13.5 (6.6–20.5) | 4.7 (3.0–6.5), p = 0.06a |
| Median (range) | 15.0 (3.7–37.5) | 4.7 (0.5–15.3) |
p value reflects the difference between mean IV and oral methadone conversion dose ratios.
IV, intravenous; MEDD, morphine equivalent daily dose; SD, standard deviation; CI, confidence interval.
Methadone dose was significantly correlated to stable MEDD after switching opioids for both: methadone IV (Spearman = 0.86, p = 0.0001) and ME oral (Spearman = 0.72, p = 0.0024). The mean day of achieving stable dose was on day 2.5 ± 0.2 for intravenous ME, and on day 2.6 ± 0.3 for oral methadone.
Prior to the switch a pain score was documented in the records on only 12 of 29 patients (mean 3.9 ± 2.4 on 0–10 verbal rating scale): At the time of stable dose a pain score was available for only 8 of 29 patients (mean 3.9 ± 1.6 on 0–10 verbal rating scale).
Discussion
The term “equianalgesic dose ratio” refers to the ratio of the dose of two opioids required to produce the same analgesic effect.10 Equianalgesic dose ratios and “relative potency ratios” can cause confusion and are dangerous as they are inverse mathematical expressions to reflect the same relationships.10 In this study the terminology to indicate the direction of the opioid rotation (from methadone to other opioids) is used consistently as this is the novel feature of this study. To clarify and avoid dangerous misinterpretation, we have found intravenous methadone and oral methadone to be 13.5 and 4.7 times more potent than an equivalent oral MEDD of a strong opioid. Of interest we find a difference between the intravenous and oral methadone conversion dose ratios that obtained borderline statistical significance by p value, likely due to low numbers, and reached statistical significance based on confidence intervals. This was not expected based on methadone's high oral bioavailabity. This appears to be clinically important and likely due to presystemic (first pass) elimination.
These results run contrary to those of Moryl et al.1 who reported only one successful rotation from intravenous methadone to intravenous hydromorphone and 12 patients who were unsuccessful in switching to intravenous morphine, hydromorphone, fentanyl, and levorphanol from methadone. These patients were restarted on methadone. In contrast we have found that of 39 rotations from methadone that met inclusion criteria, only 5 (13%) needed to be restarted on methadone. Also, of our successful rotations the mean time to achieving stable dose was 2.5–2.6 days, indicating rapid stabilization of opioid requirements. It is unclear what may be responsible for the difference in findings between our report and those of Moryl et al.1 one important difference may have been the higher doses of IV methadone administered to the patients in the Moryl et al.1 study (2–80 mg/hr intravenous) compared to lower doses of intravenous and oral methadone administered to our patients prior to opioid rotation. This may explain the significant opioid induced neurotoxicity they report (dysphoria, confusion, sedation, myoclonus) as well as persisting pain which may possibly reflect the presence of hyperalgesia or allodynia.
Lawlor et al.8 has reported a subanalysis of 6 patients rotated from oral methadone to oral morphine. The reported median dose ratio was 8.25 with an interquartile range of 4.37–11.3. Our findings in 16 patients report a mean ratio of 4.7 (95% CI 3.0–6.5) and median ratio of 4.7 (range, 0.5–15.3; Table 2).
We were interested in determining if the conversion dose ratio would change based on methadone dose, as has been reported by Ripamonti et al.9 in a study of patients rotated in the opposite manner, i.e. from morphine to oral methadone. Statistical analysis of the relationship between dose ratio and methadone dose was performed. Linear, quadratic, and cubic models were fit to the data. None of the models yielded a statistically significant relationship. Therefore, unlike Ripamonti et al.9 who were able to conclude that there is an increasing dose ratio based on increasing doses of morphine when switching from morphine to methadone, we are unable to conclude that there exists a mathematical relationship that varies the dose ratio based on the dose of methadone, when rotating from methadone to another opioid.
To our knowledge this is the most extensive study of patients rotated from methadone to other opioids, an area of limited research but of increasing importance. Chronic, not single dose opioid use was investigated among a broad sampling of the cancer population. other strengths include a rigorous inclusion and exclusion criteria to minimize confounders and inclusion of data for both intravenous and oral methadone use. The use of opioid consumption rather than analgesic reporting has been established as an alternate viable method of establishing equianalgesic dose ratios.3,7,8 Multiple studies support the validity of the MEDD conversion ratios used in the methods section this study and in clinical practice.
Limitations of this study include: its retrospective nature, relatively low numbers, predominance of white males, lack of control for hepatic and renal impairment, lack of direct drug to drug dose comparison, no control for radiation, chemotherapy, hormonal therapy or other interventions that may influence pain control, and limited data available related to pain scores. A further limitation relates to methadone's long half-life. In this regard a design utilizing a longer period of methadone administration before rotation, and reporting of dosages of other opioids at longer follow-up may be beneficial in more accurately calculating the equianalgesic dose ratios.
Although the authors believe this study improves our knowledge of the relationship between analgesic requirements that occurs when switching from methadone to an alternative opioid, we urge caution in the use of these conversion dose ratios due to the limitations listed above. Further studies utilizing prospective design and larger numbers are required to confirm these results. We suggest that until further studies are completed, it would be judicious to rotate patients in clinical practice from methadone to other opioids using conversion dose ratios more conservative than described in this paper. It would be safer to under estimate the analgesic effect of methadone and use breakthrough opioid doses to “catch up,” than risk overdose with the new opioid due to a possible overestimation of methadone's analgesic potency.
Acknowledgments
The authors thank Edith M. Gipson and Stephanie Jones for assistance with the preparation of this manuscript.
Eduardo Bruera is supported in part by National Institutes of Health Grant #R01 NR0162-01A1, #R01 CA122292-01, and #R01 CA124481-01
This paper was presented in part as an abstract at the annual meeting of the American Society of Clinical Oncology, May 13–17, 2005, Orlando, Florida.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Moryl N. Santiago-Palma J. Kornick C. Derby S. Fischberg D. Payne R. Manfredi PL. Pitfalls of opioid rotation: Substituting another opioid for methadone in patients with cancer pain. Pain. 2002;96:325–328. doi: 10.1016/S0304-3959(01)00465-1. [DOI] [PubMed] [Google Scholar]
- 2.Jacox A. Carr DB. Payne R. Berde CB. Breitbart W. Cain JM. Management of cancer pain. Clinical practice guidelines No. 9. AHCPR Publication No. 94-0592. Rockville, MD: Agency for Health Care Policy and Research, US Department of Health and Human Services. Public Health Service. 1994 [Google Scholar]
- 3.Bruera E. Pereira J. Watanabe S. Belzile M. Kuehn N. Hanson J. opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. Cancer. 1996;78:852–857. doi: 10.1002/(SICI)1097-0142(19960815)78:4<852::AID-CNCR23>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S. opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86:1856–1866. doi: 10.1002/(sici)1097-0142(19991101)86:9<1856::aid-cncr30>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Ripamonti C. De Conno F. Groff L. Belzile M. Pereira J. Hanson J. Bruera E. Equianalgesic dose/ratio between methadone and other opioid agonists in cancer pain: Comparison of two clinical experiences. Ann Oncol. 1998;9:79–83. doi: 10.1023/a:1008263910494. [DOI] [PubMed] [Google Scholar]
- 6.Max MB. Payne R. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 4th. Glenview, IL: The American Pain Society Press; 1999. [Google Scholar]
- 7.Lawlor P. Turner K. Hanson J. Bruera E. Dose ratio between morphine and hydromorphone in patients with cancer pain: A retrospective study. Pain. 1997;72:79–85. doi: 10.1016/s0304-3959(97)00018-3. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor PG. Turner KS. Hanson J. Bruera ED. Dose ratio between morphine and methadone in patients with cancer pain: A retrospective study. Cancer. 1998;982:1167–1173. [PubMed] [Google Scholar]
- 9.Ripamonti C. Groff L. Brunelli C. Palaastri D. Starakis A. De Conno F. Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio? J Clin Oncol. 1998;16:3216–3221. doi: 10.1200/JCO.1998.16.10.3216. [DOI] [PubMed] [Google Scholar]
- 10.Pereira J. Lawlor P. Vigano A. Dorgan M. Bruera E. Equianalgesic dose ratios for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–678. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 11.Bruera E. Lawlor P. Watanabe S. Turner K. Hanson J. The effects of opioid rotation (OR), dose ratio (DR) on pain control and cognition in patients (P) with cancer pain. Presented at the A.S.C.O. Meeting in Denver, CO May 20, 1997. Proc ASCO. 1997;16:62a. [Google Scholar]
- 12.Charrois T. Lindsay MA. Bruera E. Utilizing a morphine equivalent daily dose for comparison of opioid use in two palliative care units in Canada. Presented at the 12th International Congress on Care of the Terminally Ill, Montreal, PQ September 13–17, 1998. J Palliat Care. 1998;14:117. [Google Scholar]
- 13.Gagnon B. Bruera E. Differences in the ratios of morphine to methadone in patients with neuropathic pain versus non-neuropathic pain. J Pain Symptom Manage. 1999;18:120–125. doi: 10.1016/s0885-3924(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 14.Lawlor P. Pereira J. Bruera E. Dose ratios among different opioids: underlying issues, an update on the use of the equianalgesic table. In: Bruera E, editor; Portenoy RK, editor. Topics in Palliative Care. Vol. 5. New York: Oxford University Press; 2001. pp. 247–276. [Google Scholar]
- 15.Lawlor PG. Quan H. Daeninck P. Hanson J. Bruera E. Dose Ratio of Oral to Subcutaneous Hydromorphone in Advanced Cancer Patients. MASCC and ISOO; Presented at 12th International Symposium-Supportive Care in Cancer; Mar 23–25;; Washington, DC: 2000. [Google Scholar]
- 16.Lawlor PG. Daeninck P. Doyle J. Quan H. Hanson J. Bruera E. American Pain Society; Ft. Lauderdale, FL: Oct 21–24, 1999. Dose ratio of oral to subcutaneous morphine in the treatment of cancer pain. [Google Scholar]
- 17.Lawlor P. Fan D. Hanson J. Bruera E. Equianalgesic conversion dose ratio of morphine (M) to hydromorphone (HM) in patients with cancer pain. Presented at the 11th International Congress on Care of the Terminally Ill; Montreal. Sep–Nov. 1996. [Google Scholar]
- 18.Pereira J. Bruera E. Watanabe S. Belzile M. Hanson J. Opioid rotation in patients with cancer pain: A retrospective comparison of dose ratios between methadone, hydromorphone, morphine. Presented at the 6th Canadian Palliative Care Conference “Setting our Sails, Advancing Care; Halifax, Nova Scotia. Oct–17; 1995. [Google Scholar]
- 19.Ripamonti C. De Conno F. Groff L. Belzile M. Pereira J. Hanson J. Bruera E. Equianalgesic dose/ratio between methadone and other opioid agonists in cancer pain. Comparison of two clinical experiences Ann Oncol. 1998;9:79–83. doi: 10.1023/a:1008263910494. [DOI] [PubMed] [Google Scholar]