Abstract
Events that occur during acute HIV infection likely contribute to the immune dysfunction common in HIV-infected individuals. During this early stage, there is high-level viral replication, loss in CD4+ T cell number and function, and an up-regulation of proinflammatory and immunoregulatory cytokines. The mechanisms responsible for this are not completely understood. We hypothesize that the HIV envelope glycoprotein, gp120, contributes to immune dysfunction during early HIV infection. Using a cohort of subjects enrolled during acute and early HIV infection, we determined the amount of gp120, TNF-α, IL-6, IL-10, IFN-α, and IFN-γ in plasma at baseline and 6 months. At matched time points, we also measured CD4+ T cell proliferation, T cell activation, and apoptosis. Plasma from 109 subjects was screened for gp120. Thirty-six subjects (33%) had detectable gp120 (0.5–15.6 ng/ml). Subjects with greater than 1 ng/ml of gp120 at baseline had similar levels at all time points tested, even when viral replication was undetectable due to therapy. Subjects with detectable gp120 had higher levels of plasma IL-6, IL-10, and TNF-α. There was no difference in the level of T cell activation, proliferation, or apoptosis in subjects with gp120 compared to those without. We conclude that persistent expression of gp120 occurs in a subset of individuals. Furthermore, the presence of gp120 is associated with higher levels of plasma IL-6, IL-10, and TNF-α, which may contribute to immune dysfunction during early HIV infection.
Introduction
Events that occur during the earliest stage of HIV infection are thought to contribute to the subsequent dysfunction of HIV-specific immune responses. In the weeks following infection, the virus replicates to peak levels, usually in excess of one million viral copies per milliliter of blood, before declining to a set point.1 During this early phase of infection, immune system dysfunction becomes apparent. There is a significant loss of CD4+ T cells, especially in the gut, and an up-regulation of proinflammatory and immunoregulatory cytokines, including IL-10, IL-6, and TNF-α.2,3 Additionally, virus-specific CD4+ T cell responses are typically weak or absent.4 The mechanisms that account for these defects are not completely understood. We hypothesize that the viral envelope protein, gp120, contributes to the immunological dysfunction that is observed during early HIV infection.
The HIV envelope glycoprotein is composed of two units, gp120 and gp41, which are held together by noncovalent interactions. This allows the gp120 subunit to readily be shed from virions and infected cells.5,6 Binding of gp120 to CD4 on the surface of T cells, dendritic cells, and macrophages in vitro results in the production of cytokines including interleukin (IL)-6, IL-10, interferon (IFN)-α, tumor necrosis factor (TNF)-α, IFN-γ, and IL-1β.7 Furthermore, it inhibits T cell functions through several mechanisms including down-regulation of the costimulatory molecule, CD40L, a decline in the production of IL-2, and decreased antigen-specific proliferation.7 HIV gp120− induced T cell dysfunction has also been observed in murine8 and nonhuman primate models.9 Furthermore, binding of gp120 to human CD4+ T cells has been indirectly observed ex vivo and correlated with decreased proliferative responses.10 The relevance of these observations to the pathogenesis of HIV infection has been debated.11 In particular, it is not known if the amount of gp120 in an infected individual is sufficient to cause immune system dysfunction. Given that virus replication is at its highest during acute infection, we hypothesize that the production of gp120 is sufficient during this time to contribute to the impairment of HIV-specific immune responses. The purpose of this study was to measure the concentration of gp120 in plasma during acute and early HIV infection and determine whether gp120 was associated with measures of immune dysfunction including production of proinflammatory and immunoregulatory cytokines, T cell activation and apoptosis, and lack of HIV-specific T cell proliferation.
Materials and Methods
Subjects and samples
Subjects were chosen from a cohort of individuals enrolled in an observational study of acute and early HIV infection at Massachusetts General Hospital (MGH). Acute HIV infection was defined by a negative HIV-1/2 enzyme-linked immunosorbent assay (ELISA) or a negative or indeterminate HIV-1 Western blot and the presence of detectable HIV-1 RNA. Individuals who did not meet these criteria but had recent infection as evidenced by a nonreactive detuned ELISA12 or a clinical history consistent with HIV infection within the last year were considered to have early HIV infection. One hundred and nine subjects were chosen based on the availability of samples prior to or around the time of seroconversion. Of these, 37 were in the acute phase of infection and 72 were in the early phase of infection. The MGH clinical laboratories performed all HIV RNA viral loads and CD4+ T cell counts. Ten uninfected subjects were enrolled onto a similar protocol and used as controls. This study was approved by the MGH Human Subjects Committee and all subjects gave informed consent prior to participation.
Isolation of cells
Peripheral blood mononuclear cells (PBMCs) were isolated from ACD-treated whole blood using a Ficoll-Hypaque (Sigma, St. Louis, MO) density gradient according to the manufacturer's instructions. The plasma layer was removed and stored in aliquots at −80°C.
gp120 ELISA
The presence of gp120 in plasma was measured by ELISA. Plates were coated with 1 μg/ml of a mixture of three human monoclonal antibodies against gp120 including 17b, A32, and EH21. These antibodies bind to discontinuous epitopes. The 17b epitope is conformationally sensitive and exposed on CD4 binding. The A32 epitope lies adjacent to the CD4 binding site. The EH21 epitope lies within C1–C4. This mixture of antibodies is known to cross-react with envelope glycoproteins from multiple Clade B isolates and likely binds to monomeric gp120.13–16 Plasma was added to the plate in duplicate and gp120 was detected using a 1/2000 dilution of the same mixture of biotinylated antibodies. The plate was then developed using standard methods. Two-fold serial dilutions of recombinant gp120 (HIVLAV) (Protein Sciences, Meriden, CT) were used as a standard for quantitation on every plate. Pooled HIV-negative plasma was included on every plate as a negative control. The assay is linear over a 100-fold range. Fifty-eight percent of 24 replicates of 250 pg/ml of gp120 tested above the highest OD450 value of 24 replicates with no gp120, while all 24 replicates of 500 pg/ml tested above this threshold. We therefore set 500 pg/ml as our cut-off for a positive result. Twelve independent HIV-negative plasma samples were tested and all fell below this cut-off. Preliminary experiments using plasma from HIV-negative donors indicated that plasma could interfere with the measurement of gp120. This matrix effect was dependent on the amount of exogenous gp120 that was spiked into the sample and varied by subject. We therefore conclude that values obtained from HIV-positive subjects may be an underestimation of the true concentration of gp120.
Cytokine ELISAs
IL-10, IL-6, IFN-α, IFN-γ, and TNF-α were measured by ELISA according to the manufacturer's instructions (eBioscience, San Diego, CA). These assays were performed on plasma at baseline and 6 months later from 13 subjects with detectable gp120, 15 subjects without detectable gp120, and 9 HIV-uninfected controls. These subjects were chosen based on sample availability. Cytokines were captured using antibodies specific to the cytokine of interest (IL-6 MQ2-13A5 and MQ2-39C3; IL-10 JES3-9D7, JES3-12G8; TNF-α Mab1, Mab11; IFN-α HTLa, rabbit polyclonal; IFN-γ N1B42, 4S.B3). Avidin-HRP was used for detection. A standard curve was generated from duplicate serial dilutions of the recombinant cytokine of interest. If plasma was not available at 6 months, the sample from the closest time point was used (range 42–242 days).
T cell activation and apoptosis
T cell activation and apoptosis were measured on cells obtained from the same subjects and time points that were used in the cytokine ELISA assays. Cryopreserved PBMCs were thawed in RPMI containing HEPES, l-glutamine, penicillin, streptomycin, and 10% human AB serum. The cells were then stained in calcium-containing buffer with antibodies to differentiate T lymphocyte subsets (CD3-Alexa700 BD Bioscience clone UCHT1, CD4-Q655 NIH Nonhuman Primate Reagent Resource clone T419Thy5D7, CD8-APC eFluor780 eBioscience clone RPA-T8), antibodies to identify activation markers, CD38 and HLA-DR (PE Biolegend clone HIT2 and PECy7 Biolegend clone L243, respectively), and markers of apoptosis including Annexin V (FITC eBioscience) and the dye 7AAD (Invitrogen). Cells were fixed in 4% formaldehyde and analyzed on a BD LSRII. Lymphocytes were gated based on forward and side scatter characteristics. CD3+ lymphocytes were then divided into CD4+ and CD8+ subsets. Fluorescence minus one controls were used to set the quadrant gates for CD38/HLA-DR and 7AAD/Annexin V. The frequency of CD38+HLA-DR+ cells and 7AAD+/Annexin V+ cells in each T cell subset is reported.
HIV-specific proliferative responses
Retrospective HIV-specific proliferation data were available on a subset of subjects (17 with detectable gp120, 53 without detectable gp120) that we tested for gp120. Freshly isolated PBMCs were incubated under standard conditions for 6 days with 5 μg/ml of recombinant p24 and Nef proteins or their controls as previously described.17 Replicate wells were pulsed with 1.0 μCi per well of tritiated thymidine for 6 h. Incorporation of the radionucleotide was measured using a scintillation counter (Packard Topcount, Packard Instruments). PHA was used as a positive control on each plate. A stimulation index (SI) was calculated by dividing the net mean counts per minute (cpm) of experimental wells by the net mean cpm of control wells. An SI greater than or equal to 5 was considered a positive response.
Statistics
A one-tailed Mann–Whitney test was used to determine if the CD4 count, log transformed viral load, median concentration of cytokine, or frequency of T cell activation or apoptosis differed between individuals with or without gp120 or between baseline and follow-up. Spearman correlation was used to determine if there was a relationship between gp120 concentration and log transformed viral load. Pearson correlation was used to determine if there was a relationship between gp120 concentration and CD4 count. Linear regression was used to determine if there was a correlation between T cell activation and apoptosis and viral load and CD4 count. All statistical calculations were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com).
Results
Detection of gp120 during acute and early HIV infection
The level of gp120 present during primary infection was measured by ELISA using plasma obtained around the time of seroconversion from 109 HIV-infected subjects. gp120 was detectable in plasma of 36 subjects (33%). The amount of gp120 in these subjects ranged from 0.5 to 15.6 ng/ml (∼4–130 pM). Thirty-seven of the patients were screened prior to seroconversion (Table 1). There was no difference in the number of subjects that screened positive for gp120 during acute infection compared to early infection (p > 0.05). The median viral load and CD4 count in subjects with detectable gp120 were similar to those without detectable gp120, even when stratified into acute and early infection. These data indicate that measurable levels of gp120 are present in the peripheral circulation in a minority of HIV-infected subjects during early infection.
Table 1.
HIV gp120 is Detectable During Acute and Early HIV Infectiona
| gp120 ng/ml median (range) | Viral Load RNA copies/ml median (range) | CD4 count cells/mm3median (range) | ||
|---|---|---|---|---|
| Acute | ||||
| gp120+ | n = 10 | 0.719 (0.553–4.18) | 529,500 (399–4.6 × 107) | 312 (165–958) |
| gp120− | n = 27 | 713,000 (14600–8.1 × 107) | 423 (155–677) | |
| ns*** | ns*** | |||
| Early | ||||
| gp120+ | n = 26 | 0.797 (0.5–15.56) | 24,178 (136–4 × 106) | 473 (281–1547) |
| gp120− | n = 46 | 67,600 (160–6.6 × 107) | 503 (231–929) | |
| ns* | ns** | ns*** | ns*** | |
Acute vs. early, not significant, Fisher Exact test.
Acute vs. early, not significant, Mann Whitney test.
gp120+ vs. gp120−, not significant, Mann Whitney test.
The concentration of gp120 in the plasma of Acute or Early HIV infected subjects was determined using an ELISA. In this assay, gp120 was captured using a combination of human monoclonal antibodies that are known to bind many Clade B isolates. The concentration was determined by comparison to a standard curve generated using 2-fold serial dilutions of HIVLAV.
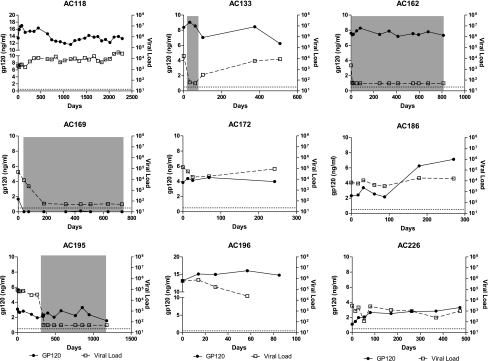
To further characterize its expression over time, we measured gp120 longitudinally in nine subjects with greater than 1 ng/ml of gp120 at baseline and five subjects who were negative at baseline. As shown in Fig. 1, if gp120 was detected at baseline it remained detectable at all time points tested in all but one subject. Furthermore, the concentration of gp120 remained relatively stable regardless of the level of viremia, except in one subject. In subjects AC162 and AC195, the concentration of gp120 failed to decline even after initiation of antiretroviral therapy and the decline of viral load below the limit of detection. However, in subject AC169, gp120 became undetectable when therapy was initiated. In subject AC186, the concentration of gp120 increased after 100 days, despite the viral load remaining constant. In those subjects who did not have detectable gp120 at baseline, gp120 was not detected at any time point tested, except in one subject. In subject AC190, 796 ng/ml of gp120 was detected at 108 days after baseline (data not shown). This did not coincide with any changes in viral load (data not shown). From these data, we conclude that the expression of gp120 is relatively constant and can be independent of plasma viral load or administration of antiretroviral therapy.
FIG. 1.
Expression of gp120 remains constant despite changes in viral load. HIV gp120 was measured longitudinally in subjects with greater than 1 ng/ml at baseline and compared to the viral load at each time point. Gray boxes indicated periods when a subject was on antiretroviral therapy.
Individuals with detectable gp120 have increased levels of proinflammatory and immunoregulatory cytokines
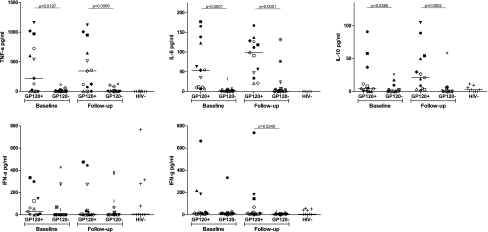
The above data provide evidence that there is a subset of HIV-infected individuals with gp120 in their plasma that is readily detectable at multiple time points during infection. This observation provides us with the opportunity to examine whether gp120 has an effect on the production of IL-6, IL-10, TNF-α, IFN-α, and IFN-γ, as has been shown in in vitro studies. To test this, we measured the concentration of each of these cytokines in a subset of subjects with detectable gp120 (n = 13) and compared it to those with undetectable gp120 (n = 15), matched by viral load and CD4 count, and HIV-uninfected controls (n = 9). The median concentration of gp120 in those subjects in which it was detected was 1.8 ng/ml (range 0.6–15.6 ng/ml) at baseline and 7.1 ng/ml (range 2.4–15 ng/ml) at follow-up. Subjects with detectable gp120 had significantly higher levels of plasma IL-6 (median of 53.8 pg/ml compared to 1.9 pg/ml, p < 0.0001), IL-10 (median of 4.8 pg/ml compared to 0 pg/ml, p = 0.0385), and TNF-α (median of 221.7 pg/ml compared to 1.5 pg/ml, p = 0.0197) at baseline compared to those without detectable gp120 (Fig. 2). There was no difference in the amount of IFN-α or IFN-γ (p > 0.05).
FIG. 2.
Proinflammatory and immunoregulatory cytokines are higher in subjects with detectable gp120. Plasma cytokines were measured by ELISA at baseline and a follow-up time point 6 months later. Subjects with detectable gp120 were compared to subjects without detectable gp120 who were matched by viral load and CD4 count. Each subject is represented by a unique symbol that is consistent throughout all graphs. HIV seronegative individuals were included as a control. The median concentration of TNF-α, IL-6, and IL-10 was higher in subjects with detectable gp120 compared to those without gp120 at baseline and follow-up. Additionally, the concentration of IFN-γ was higher at follow-up.
To determine whether these cytokines remained elevated over time, we measured them at a follow-up time point 6 months after baseline. As shown in Fig. 2, subjects with detectable gp120 had higher levels of IL-6 (98.4 vs. 0.6 pg/ml, p = 0.0001), IL-10 (21.1 vs. 0 pg/ml, p = 0.0002), TNF-α (344.6 vs. 2.9 pg/ml, 0.007), and IFN-γ (14.8 vs. 6.4 pg/ml, p = 0.01) at follow-up. There was no difference in IFN-α in the two groups. Cytokine responses in the two subjects who had a single time point when gp120 was detectable (AC169 at baseline and AC190 at follow-up) had low (<32 pg/ml) or undetectable levels of all five cytokines at each time point. The median amount of cytokine measured at baseline and follow-up did not differ for any cytokine tested for either group. We conclude from these data that the presence of gp120 is associated with the increased expression of IL-6, IL-10, TNF-α, and IFN-γ.
Individuals with detectable gp120 do not have increased frequency of activated T cells
The presence of gp120 or the cytokines associated with it may affect the level of activation of T cells. To test this, we measured the expression of CD38 and HLA-DR on CD4+ and CD8+ T lymphocytes at baseline and follow-up in the same subset of subjects as described above. Although HIV-infected subjects had a higher frequency of activated CD4+ and CD8+ T cells than seronegative controls at baseline, the frequencies of activated T cells were similar in subjects with and without detectable gp120 (data not shown). There was also no difference in T cell activation between the two groups at follow-up. It should be noted that CD8+ T cell activation was positively correlated with viral load (r2 = 0.3494, p = 0.0019) and negatively correlated with CD4 count (r2 = 0.2205, p = 0.0493) at baseline. CD4+ and CD8+ T cell activation was positively correlated with viral load (r2 = 0.6327, p < 0.0001 and r2 = 0.5482, p < 0.0001, respectively) and negatively correlated with CD4 count (r2 = 0.3443, p = 0.0215 and r2 = 0.3210, p = 0.0277, respectively) at follow-up. We conclude that the presence of gp120 in the plasma is not associated with the increased level of immune activation that is observed in HIV-infected individuals.
Individuals with detectable gp120 do not have increased frequency of apoptotic CD4+ T cells
At high concentrations or when cross-linked, gp120 can induce apoptosis of CD4+ T cells in vitro. Therefore, we next asked whether the presence of gp120 was associated with an increased frequency of apoptotic cell ex vivo. Cells obtained at baseline and follow-up from the same subset of subjects as described above were stained with 7AAD and Annexin V and the frequency of apoptotic CD4+ T lymphocytes was determined by flow cytometry. We found no difference in the frequency of apoptotic CD4+ T cells in gp120+ or gp120− individuals at baseline or follow-up (data not shown). There was also no correlation between apoptosis and viral load or CD4 count at baseline or follow-up. This suggests that detection of gp120 is not associated with increased levels of apoptosis.
Individuals with detectable gp120 can mount HIV-specific CD4+ T cell proliferative responses
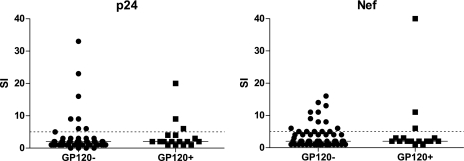
In vitro, gp120 can inhibit antigen-specific proliferation of CD4+ T cells. To determine whether the detection of gp120 was associated with a lack of HIV-specific proliferation, we measured HIV-specific proliferation of CD4+ T cells at baseline in individuals with and without detectable gp120. Stimulation with soluble p24 induced CD4+ T cell proliferation from 3 of 17 subjects with detectable gp120 and 8 of 53 subjects without detectable gp120 (Fig. 3). Similarly, three subjects with detectable gp120 and 15 subjects without gp120 responded to Nef. One subject with gp120 responded to both p24 and Nef. There was insufficient statistical power to determine whether the difference in proliferative responses was significant.
FIG. 3.
Proliferative responses to HIV may be affected by gp120. Proliferative responses to the HIV proteins, p24 and Nef were determined using a standard tritium incorporation assay. A stimulation index (SI) of greater than five (dashed line) was considered a positive response. Subjects with detectable plasma gp120 appeared less likely to respond to HIV antigens than subjects without detectable gp120; however, there was insufficient power to determine whether this difference was significant.
Discussion
The HIV envelope glycoprotein, gp120, has been shown to inhibit T cell function and induce the expression of proinflammatory and immunoregulatory cytokines in vitro. We thus hypothesized that gp120 contributes to the immune dysfunction that is characteristic of HIV infection. However, the extent to which gp120 plays a role in the pathogenesis and early immune defects common in HIV infection has been debated. In particular, the concern has been that the amount of gp120 used in in vitro experiments may not reflect physiologically relevant concentrations of the protein. We detected 4–130 pM (0.5–15.6 ng/ml) of gp120 in the plasma of 33% of the subjects tested. These results are slightly higher than the report by Gilbert et al., which described the detection of 2–20 pM of gp120 in 27% of chronic HIV-infected subjects,18 but lower than the amount found by Oh et al.19 A more recent report by Santosuosso et al. did not detect gp120 in the plasma of three chronic HIV-infected subjects, despite high levels of gp120 in cell-free extracts from spleen and lymph node.20 It is possible that we detected higher plasma gp120 concentrations because our samples were obtained during early rather than chronic HIV infection. Additionally, differences in the assays used to measure gp120 and the fact that plasma samples in the Santosuosso study were obtained postmortem may have contributed. A correlation between the amount of gp120 in plasma and lymphoid tissue has not been established. However, the concentration of gp120 appears to be higher in lymph nodes compared to matched plasma samples.9,20 In one study of four acute SHIV-infected macaques, the concentration of gp120 was greater than 100-fold higher in the lymph node.9 If this estimate were accurate, it would suggest that there is 500–1600 ng/ml of gp120 in the lymphoid tissue during primary HIV infection. This is consistent with the amount of gp120 used to induce cytokine secretion and apoptosis and inhibit T cell proliferation in vitro.7,21–23
It is not clear why gp120 would be detectable in some individuals and not others. Because we found no difference in the viral load of subjects with and without detectable gp120, factors other than viral replication are likely responsible for this result. One possibility is that plasma antibodies interfered with the detection of gp120 in some individuals. In principle, we may not detect gp120 if it were complexed with antibodies that target the same or overlapping epitopes as those used in our assay. However, we found no difference in the median concentration of gp120 or the frequency of individuals with detectable gp120 before and after seroconversion. This suggests that the antibody response generated around the time of seroconversion was unable to compete with the monoclonal antibodies used in our assay. Plasma antibodies that could interfere may be at too low a concentration, have a lower binding affinity, or target nonoverlapping epitopes. It is worth noting that disruption of immune complexes prior to measuring gp120 had only a minor effect on its detection in a study performed by Santosuosso et al.20 We were unable to test this using our assay because the antibodies we used bound conformational epitopes that would be destroyed by the conditions required for disruption of immune complexes. An alternative possibility is that different HIV isolates may shed gp120 at different rates. Thus individuals with detectable gp120 were infected with viruses that shed more gp120.
Although the lack of association between the level of gp120 and viral load seems counterintuitive, others have reported similar results. Specifically, p24 can still be detected in the plasma of subjects who maintain undetectable viral loads while on antiretroviral therapy24,25 and p24 and gp120 have been detected in the lymph nodes of treated individuals.20,26 Furthermore, several investigators have shown that HIV antigens get trapped within the follicular dendritic cell network of the lymph node and can remain there in the absence of viral replication.27–29 Thus, even when an individual is on therapy, infected cells are capable of producing gp120 that can be shed, bind to extracellular matrix proteins, and enter the peripheral circulation.
Cytokine dysregulation is one of the characteristic features of early HIV infection.2,3 In our study, individuals with detectable levels of gp120 in their plasma had significantly higher concentrations of IL-6, IL-10, TNF-α, and IFN-γ. Others have shown that gp120 can induce these cytokines in vitro using PBMCs from HIV-uninfected subjects.21 To our knowledge, this is the first study to find an association between gp120 concentration and cytokine levels in the plasma of HIV-infected individuals. The mechanisms by which gp120 induces cytokines has not been completely defined. It has been suggested that binding of gp120 to CD4 on T cells, macrophages, and dendritic cells induces a signaling cascade that up-regulates the production of these cytokines.7,21 Increased production of these cytokines in HIV-infected subjects with detectable levels of gp120 provides evidence that gp120 contributes to the cytokine dysregulation that is common during primary HIV infection.
We hypothesized that expression of gp120 may have additional downstream effects on cellular immune responses. However, we found no association between the detection of gp120 and HIV-specific T cell proliferation, T cell activation, or apoptosis. It is possible that an association exists, but we were unable to detect it due to limitations in our assays. It should be noted that we did find associations between T cell activation and viral load and CD4 count, as has been described previously.30
In conclusion, we have shown that there is a subset of HIV-infected individuals who express a detectable amount of gp120. Detection of gp120 is associated with an increased expression of the proinflammatory and immunoregulatory cytokines IL-6, IL-10, and TNF-α. The presence of gp120 and the perturbations in cytokine expression that are associated with it may contribute to the immune dysfunction that is typical of HIV infection. These results provide the impetus for reevaluating the role of gp120 in the pathogenesis of HIV infection.
Acknowledgment
This study was funded by NIH R01 AI071915.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mellors JW. Rinaldo CR., Jr Gupta P. White RM. Todd JA. Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272(5265):1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 2.Norris PJ. Pappalardo BL. Custer B. Spotts G. Hecht FM. Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses. 2006;22(8):757–762. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacey AR. Norris PJ. Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus Type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg ES. Altfeld M. Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 5.Gelderblom HR. Hausmann EH. Ozel M. Pauli G. Koch MA. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 6.Layne SP. Merges MJ. Dembo M, et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189(2):695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 7.Chirmule N. Pahwa S. Envelope glycoproteins of human immunodeficiency virus type 1: Profound influences on immune functions. Microbiol Rev. 1996;60(2):386–406. doi: 10.1128/mr.60.2.386-406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller SB. Tse H. Rosenspire AJ. King SR. CD4-independent inhibition of lymphocyte proliferation mediated by HIV-1 envelope glycoproteins. Virology. 1992;191(2):973–977. doi: 10.1016/0042-6822(92)90275-t. [DOI] [PubMed] [Google Scholar]
- 9.Stevceva L. Yoon V. Carville A, et al. The efficacy of T cell-mediated immune responses is reduced by the envelope protein of the chimeric HIV-1/SIV-KB9 virus in vivo. J Immunol. 2008;181:5510–5521. doi: 10.4049/jimmunol.181.8.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel V. Susal C. Weimer R. Zimmermann R. Huth-Kuhne A. Opelz G. Association of T cell and macrophage dysfunction with surface gp 120-immunoglobulin-complement complexes in HIV-infected patients. Clin Exp Immunol g. 1993;93(2):152–156. doi: 10.1111/j.1365-2249.1993.tb07958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klasse PJ. Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323(1):1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Janssen RS. Satten GA. Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280(1):42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Cham F. Zhang PF. Heyndrickx L, et al. Neutralization and infectivity characteristics of envelope glycoproteins from human immunodeficiency virus type 1 infected donors whose sera exhibit broadly cross-reactive neutralizing activity. Virology. 2006;347(1):36–51. doi: 10.1016/j.virol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Moore JP. McCutchan FE. Poon SW, et al. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68(12):8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JE. Elliott DH. Martin EA. Micken K. Rosenberg ES. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum Antibodies. 2005;14(3–4):115–121. [PubMed] [Google Scholar]
- 16.Tuen M. Visciano ML. Chien PC, Jr, et al. Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation. Eur J Immunol. 2005;35(9):2541–2551. doi: 10.1002/eji.200425859. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg ES. Billingsley JM. Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert M. Kirihara J. Mills J. Enzyme-linked immunoassay for human immunodeficiency virus type 1 envelope glycoprotein 120. J Clin Microbiol. 1991;29(1):142–147. doi: 10.1128/jcm.29.1.142-147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SK. Cruikshank WW. Raina J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5(3):251–256. [PubMed] [Google Scholar]
- 20.Santosuosso M. Righi E. Lindstrom V. Leblanc PR. Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis. 2009;200(7):1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 21.Ameglio F. Capobianchi MR. Castilletti C, et al. Recombinant gp120 induces IL-10 in resting peripheral blood mononuclear cells; correlation with the induction of other cytokines. Clin Exp Immunol. 1994;95(3):455–458. doi: 10.1111/j.1365-2249.1994.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirmule N. Kalyanaraman VS. Oyaizu N. Slade HB. Pahwa S. Inhibition of functional properties of tetanus antigen-specific T-cell clones by envelope glycoprotein GP120 of human immunodeficiency virus. Blood. 1990;75(1):152–159. [PubMed] [Google Scholar]
- 23.Foster S. Beverley P. Aspinall R. gp120-induced programmed cell death in recently activated T cells without subsequent ligation of the T cell receptor. Eur J Immunol. 1995;25(6):1778–1782. doi: 10.1002/eji.1830250644. [DOI] [PubMed] [Google Scholar]
- 24.Schupbach J. Gunthard H. Joos B, et al. HIV-1 p24 may persist during long-term highly active antiretroviral therapy, increases little during short treatment breaks, and its rebound after treatment stop correlates with CD4(+) T cell loss. J Acquir Immune Defic Syndr. 2005;40(3):250–256. doi: 10.1097/01.qai.0000181281.75670.56. [DOI] [PubMed] [Google Scholar]
- 25.Schupbach J. Tomasik Z. Knuchel M, et al. Optimized virus disruption improves detection of HIV-1 p24 in particles and uncovers a p24 reactivity in patients with undetectable HIV-1 RNA under long-term HAART. J Med Virol. 2006;78(8):1003–1010. doi: 10.1002/jmv.20655. [DOI] [PubMed] [Google Scholar]
- 26.Popovic M. Tenner-Racz K. Pelser C, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2005;102(41):14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alos L. Navarrete P. Morente V, et al. Immunoarchitecture of lymphoid tissue in HIV-infection during antiretroviral therapy correlates with viral persistence. Mod Pathol. 2005;18(1):127–136. doi: 10.1038/modpathol.3800267. [DOI] [PubMed] [Google Scholar]
- 28.Kuster H. Opravil M. Ott P, et al. Treatment-induced decline of human immunodeficiency virus-1 p24 and HIV-1 RNA in lymphoid tissue of patients with early human immunodeficiency virus-1 infection. Am J Pathol. 2000;156(6):1973–1986. doi: 10.1016/S0002-9440(10)65070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenner-Racz K. Stellbrink HJ. van Lunzen J, et al. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med. 1998;187(6):949–959. doi: 10.1084/jem.187.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeks SG. Kitchen CM. Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]