Editor: Chronic viral replication during HIV-I infection results, among other effects, in the depletion of dendritic cell (DC) subsets,1, 2 and decrease of natural killer cells (NK) frequency and function.3 An inverse correlation between chronic viral replication and numbers of mature NK or DC cells1,3 together with a lack of DC-associated functions has been reported.1,4 However, introduction of suppressive antiretroviral therapy (ART) is associated with a variable degree of recovery of frequency and function among innate effector cells.1 Following ART initiation, delayed kinetics of recovery of the innate compartment are observed in contrast to acute changes on T cell activation, suggesting a differential relationship between ART-mediated control of viremia and recovery of innate effector function.5 Although the effect of structured therapy interruptions (TI) in ART-suppressed subjects has been described for adaptive immune responses6 indicating an acute onset of T cell activation, no data are available regarding the effects of acute viral replication on innate immune system parameters following the absence of therapy. We therefore analyzed the effects of temporal viral replication on levels of innate cell immune reconstitution in ART-treated HIV-infected subjects who were participating on an observational study of the immune consequences of repeated therapy interruptions.
More precisely, we assessed changes in innate cell numbers and function during TIs in fresh blood samples from an observational cohort of 14 chronically HIV-1-infected patients (age ≥18 years) on suppressive ART (plasma HIV-1 RNA <50 copies/ml). ART initiation for all patients ranged from 1986 to 2000. All patients underwent two consecutive TIs of 6 and 18 weeks, with intervening ART resumption and resuppression to <50 copies HIV mRNA/ml for 4 weeks. All parameters were assessed at the start of each TI as well as at weeks 4 and 6 of the first TI, and weeks 4, 6 and 18 of the second TI. Informed consent was obtained according to the Human Experimentation Guidelines of the U.S. Department of Health and Human Services and of the authors' Institutions. The study protocol was approved by the Institutional Review Boards of the Wistar Institute and Philadelphia FIGHT.
Immunophenotyping characterization of NK, DC [plasmacytoid (PDC) and myeloid (MDC)], and T cell subsets was performed by same day whole blood four-color staining as previously described7 by using the following combinations of directly fluorochrome-conjugated antihuman cell surface monoclonal antibodies from Becton Dickinson (BD) Biosciences (San Diego, CA): (1) CD56-fluorescein isothiocyanate (FITC)/CD16-phycoerythrin (PE)/CD3-PerCP/CD161-allophycocyanin (APC) (NK cells), (2) CD56-FITC/CD161-PE/CD3-PerCP/HLADR-APC (activated NK cells), (3) Lin-1 (CD3/CD14/CD16/CD19/CD20/CD56)-FITC/CD123-PE/HLA-DR-PerCP/HLA-ABC-APC (PDC), (4) Lin-1-FITC/CD11c-PE/HLA-DR-PerCP/HLA-ABC-APC (MDC), and (5) CD28-FITC/CD4-PE/CD3-PerCP/CD38-APC (T cells). Definitions of total NK cells included all CD3−/CD161+/–/CD56+/–/CD16+/– NK subsets (with the exception of CD3−/CD161−/CD56−/CD16+/– NK subsets, which were not included) whereas definitions of total HLA-DR+ NK cells included all CD3−/HLA-DR+/CD56+/–/CD161+/–subsets (with the exception of CD3−/HLA-DR+/CD56−/CD161−, which were not included)]. PDC and MDC were defined as Lin-1−/HLA-DR+/CD123+ and Lin-1−/HLA-DR+/CD11c+, respectively, whereas CD8 cells were defined by gating on CD3+/CD4− events. Results were expressed as mean fluorescent intensity (MFI), percent positive (%), and cells/mm3. In the text cell numbers represent cells/mm3.
PDC function within fresh peripheral blood mononuclear cell (PBMC) preparations was assessed by measurement of spontaneous (no stimulation), CpG oligodeoxynucleotide class A (CpG-2216, 10 μg/ml, Integrated DNA Technologies, Coralville, IA), or heat-inactivated PR8 influenza virus (10 HA units/ml)-induced IFN-α levels following 18 h of culture. Cell-free supernatants were harvested and tested in duplicate for IFN-α using commercial ELISA plates (PBL Biomedical Laboratories, Piscataway, NJ). Sensitivity of the assay was approximately 9.7 pg/ml.
NK function was assessed measuring spontaneous (no stimulation) and CpG-2216 (10 μg/ml)-induced NK cell-mediated cytotoxicity by standard 51Cr release assay, as previously described, using fresh PBMC preparations as effectors cells against the tumor derived erythroblastoid cell line K562.8 Results were expressed as area under the curve (AUC) for effector:target ratios of 50:1, 25:1, 12:1, and 6:1 for both spontaneous and induced NK function.
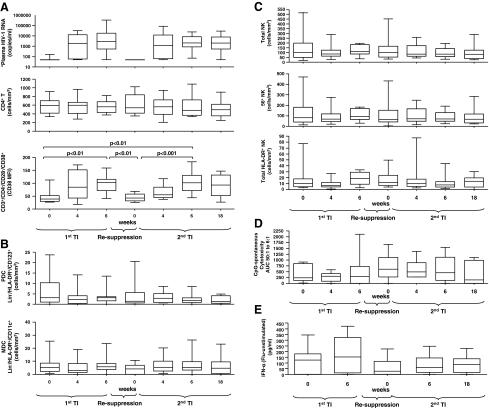
All statistical tests were performed using GraphPad Prism4 software. Briefly, a one-way ANOVA (Kruskal–Wallis test) was performed followed by Dunn's multiple comparison test. Differences were considered significant if p < 0.05. Short-term viremia following TIs did not significantly (p > 0.05) affect the number of CD4+ T cells, although as expected clear changes in the activation state of both CD4+ and CD8+ T cells were noted as indicated by cell surface CD38 expression (Fig. 1A) and numbers of CD38+ T cells. In contrast, short-term viremia had no effect on DC (Fig. 1B), NK, or activated HLA-DR+ NK cells (Fig. 1C), and retention of NK cytotoxicity (Fig. 1D) and flu-stimulated IFN-α production (Fig. 1E) was observed irrespective of viral rebound during TI periods or following suppression. Finally, no association between DC or NK cell numbers or function at baseline of each TI and resulting viremia was observed.
FIG. 1.
Retention of innate effectors cell numbers and function during subsequent therapy interruptions and in spite of acute viral replication. (A) Plasma HIV-1 RNA (copies/ml), CD4+ T cells (cells/ mm3), CD38 mean fluorescent intensity on CD3+/CD4−/CD28−/CD38+ T cells; (B) PDC (defined as Lin−/HLA-DR+/CD123+ cells/mm3), MDC (defined as Lin−/HLA-DR+/CD11c+ cells/mm3); (C) total NK cells, total 56+ NK cells, total HLA-DR+ NK cells; (D) CpG-2216-induced NK function expressed as area under the curve (AUC) for effector:target ratios of 50:1, 25:1, 12:1, and 6:1 [(CpG-2216-induced cytotoxicity) – (spontaneous cytotoxicity)]; and (E) flu-induced PDC function expressed as IFN-α levels (pg/ml) [(flu-induced) – (unstimulated)]. Data are shown in (A–D) as interquartile (IQR) boxes (median, and outliers) at baseline, week 4, and week 6 of the first therapy interruption (TI) and at baseline, week 4, week 6, and week 18 of the second TI in 14 HIV-1-infected chronically suppressed patients. Data are shown in (E) as IQR boxes at baseline and week 6 of the first TI and at baseline, week 4, week 6, and week 18 of the second TI in 14 HIV-1-infected chronically suppressed patients. *Although plasma HIV-1 RNA increased significantly during TIs, this is not illustrated in the figure for simplification.
Overall, our data show that acute viral replication during TIs of up to 18 weeks is not associated with acute changes in the number of innate effector cell subsets or the onset of immediate impairments of NK and DC cell-mediated responses. Our data support our and other groups' observations showing lack of an immediate immunological “cost” of short-term TIs, as exemplified by retention of recall responses9–14 and control of LPS levels during short-term TIs.15 As a result, short-term viremia is unlikely to acutely deplete the innate effector cell subsets at the same rate it affects changes in distribution and activation within the T cell compartment. Although delays in the levels of increase of innate cell immune reconstitution as compared to uninfected subjects have been described after ART initiation and may vary between ART-treated subjects,1,2,5 our data suggest that short-term viremia is unlikely to result in a sustained innate change. Therefore, a period longer than 18 weeks of viremia is likely required to significantly erode realized gains from previous innate cell immune reconstitution levels. Importantly, our data support the hypothesis that innate function can contribute to immune control mechanisms (i.e., opportunistic infections) in spite of short-term viremia, while also serving as a potential target for immunotherapy, which may boost innate mechanisms of viral control. As a result, therapeutic trials targeting an activation of antiviral innate and/or adaptive responses (e.g., five such trials are currently recruiting as listed in clinicatrials.gov) on therapy interruption would not be expected to be acutely countered by an impairment in innate function.
Acknowledgments
We thank the patients who participated in the study and their providers, Cecile Gallo for study assistance, as well as Jane Shull and the Board and Staff of Philadelphia FIGHT for providing patients samples. This work was primarily supported by grants to L.J. Montaner by the National Institute of Allergy and Infectious Disease (NIH AI48398 and U01AI065279). Additional support was provided by The Philadelphia Foundation (Robert I. Jacobs Fund), The Stengel-Miller family, AIDS funds from the Commonwealth of Pennsylvania and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health, as well as by a Cancer Center Grant (P30 CA10815).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chehimi J. Campbell DE. Azzoni L, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 2.Donaghy H. Pozniak A. Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 3.Azzoni L. Papasavvas E. Chehimi J, et al. Sustained impairment of IFNgamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: Evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 4.Knight SC. Macatonia SE. Patterson S. HIV I infection of dendritic cells. Int Rev Immunol. 1990;6:163–175. doi: 10.3109/08830189009056627. [DOI] [PubMed] [Google Scholar]
- 5.Azzoni L. Chehimi J. Zhou L, et al. Early and delayed benefits of HIV-1 suppression: Timeline of recovery of innate immunity effector cells. AIDS. 2007;21:293–305. doi: 10.1097/QAD.0b013e328012b85f. [DOI] [PubMed] [Google Scholar]
- 6.Azzoni L. Papasavvas E. Montaner LJ. Lessons learned from HIV treatment interruption: safety, correlates of immune control, and drug sparing. Curr HIV Res. 2003;1:329–342. doi: 10.2174/1570162033485212. [DOI] [PubMed] [Google Scholar]
- 7.Papasavvas E. Ortiz GM. Gross R, et al. Enhancement of human immunodeficiency virus type 1-specific CD4 and CD8 T cell responses in chronically infected persons after temporary treatment interruption. J Infect Dis. 2000;182:766–775. doi: 10.1086/315748. [DOI] [PubMed] [Google Scholar]
- 8.Chehimi J. Azzoni L. Farabaugh M, et al. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179:2642–2650. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- 9.Dybul M. Chun TW. Yoder C, et al. Short-cycle structured intermittent treatment of chronic HIV infection with highly active antiretroviral therapy: Effects on virologic, immunologic, and toxicity parameters. Proc Natl Acad Sci USA. 2001;98:15161–15166. doi: 10.1073/pnas.261568398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dybul M. Nies-Kraske E. Daucher M, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: Effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–396. doi: 10.1086/376535. [DOI] [PubMed] [Google Scholar]
- 11.Garcia F. Plana M. Ortiz GM, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F29–40. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz GM. Wellons M. Brancato J, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci USA. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxenius A. Price DA. Gunthard HF, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci USA. 2002;99:13747–13752. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papasavvas E. Kostman JR. Mounzer K, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papasavvas E. Pistilli M. Reynolds G, et al. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369–375. doi: 10.1097/QAD.0b013e32831e9c76. [DOI] [PMC free article] [PubMed] [Google Scholar]