Abstract
AIDS vaccine and pathogenesis research will benefit from a more diverse array of cloned SIV challenge stocks from which to choose. Toward this end, 20 envelope genes were cloned from an extensively used, primary stock of uncloned SIVmac251. Each of the 20 clones had a unique sequence. Their translated sequences differed by as many as 26 amino acids from one another and by as many as 45 amino acids from the commonly used clone SIVmac239. Envelope sequences up to and including the membrane-spanning domain were exchanged into the infectious pathogenic SIVmac239 clone and virus stocks were produced by HEK293T cell transfection. Seventeen of the 20 recombinants were replication competent. The infectivities per ng p27 of the 17 new replication-competent recombinants in C8166-SEAP cells and in TZM-bl cells ranged from minus 32-fold to plus 7.6-fold relative to SIVmac239. A range of sensitivities to neutralization by sCD4 and by sera from SIV-infected macaques was observed but none was as sensitive to these neutralizing agents as SIVmac316, the highly macrophage-competent derivative of SIVmac239. Four strains that were most sensitive to sCD4 inhibition were also among the most sensitive to antibody-mediated neutralization. None of the new recombinant viruses replicated as well as SIVmac316 in primary alveolar macrophage cultures from rhesus monkeys but three of the strains did exhibit significant levels of delayed replication in these primary macrophages, reaching peak levels of virus production of ≥50 ng/ml p27 compared to 600–800 ng/ml p27 with SIVmac316. These new SIV clones are being contributed to the NIH AIDS Reagent Repository and are available to the scientific community.
Introduction
An important feature of retooled efforts to develop an effective AIDS vaccine is greater reliance on monkey models.1 Significant advantages accrue to the use of molecularly cloned SIV stocks of defined sequence produced by HEK293T cell transfection. When virus stocks are produced by transfection of cloned DNA into HEK293T cells, there is no virus replication. Consequently, such virus stocks contain no sequence heterogeneity and the sequence of the virus exactly reflects the sequence present in the cloned plasmid DNA that was used for transfection. Use of cloned virus allows for greater definition of assay conditions and for greater precision in the interpretation of results. For example, interpretation of neutralizing antibody assays can be ambiguous when a complex mix of sequences present in an uncloned virus stock is used for infection. Production of cloned virus stocks in a carefully controlled manner facilitates reproducibility in the properties of virus stocks prepared one time to the next or one laboratory to another. Finally, and perhaps most importantly, use of cloned virus produced by transient transfection can eliminate concerns over the presence of adventitious agents, a major concern when peripheral blood mononuclear cells (PBMCs) from rhesus monkeys are used to produce virus.
Only two infectious, pathogenic, molecular clones of SIV have been used to any significant extent for monkey studies: SIVmac239 and SIVsmE543.2,3 Both typically result in peak viral loads around 3 × 107 copies of viral RNA per ml of plasma 10–20 days following infection, viral load set points of 105–106, and a reasonably consistent disease course in outbred Indian-origin rhesus monkeys.4,5 SIVmac316 is a derivative of SIVmac239 with eight amino acid changes in Env that impart high affinity for CD4, extreme sensitivity to inhibition by soluble CD4 (sCD4), sensitivity to neutralization by a wide range of monoclonal and polyclonal antibodies, and the ability to replicate efficiently in primary, differentiated, alveolar macrophage cultures from rhesus monkeys.6–8 It is generally believed that these four properties go hand in hand. The ability of SIVmac316 to use low concentrations of CD4 for efficient entry is likely responsible for its efficient tropism for alveolar macrophages, which express very low levels of CD4 on their surface.8
Stocks of uncloned SIVmac251 have been extensively used for a wide variety of AIDS vaccine studies.9–15 Individual stocks have been titered by both intravenous and mucosal routes.9,15,16 Here we describe the derivation and characterization of recombinant cloned SIV stocks containing SIVmac251 envelope sequences.
Materials and Methods
Preparation of virus samples and cell culture
HEK-293T cells and C8166-45 SIV- SEAP cells were maintained as previously described.7,17 Virus was prepared by the transient transfection of HEK-293T cells with plasmids containing the full-length SIV proviral genomes. Cells were seeded at 1.5 × 106 cells per 75-cm2 flask the day before tranfection and each flask was transfected with 5 μg of each plasmid using the calcium phosphate method according to the manufacturer's instructions (Promega, Madison, WI). The culture medium was changed on day 2 posttransfection, and the supernatants were harvested on day 3. Virus was quantified by determining the concentration of p27 capsid in the supernatant by an antigen capture assay (Advanced BioScience Laboratories, Inc., Kensington, MD).
Sequence amplification and cloning
Primers were purchased from Sigma-Genosys Biotechnologies, Inc. (The Woodlands, TX). All plasmids were sequenced using a Beckman Coulter CEQ8000 apparatus.
SIV RNA was isolated from the 1991 animal-titered stock of uncloned SIV25116 by affinity column purification using a High Pure viral RNA kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocol. Viral RNA was amplified by RT-PCR using a SuperScript III One-Step RT-PCR System with Platinum Taq High Fidelity kit (Invitrogen, Carlsbad, CA) with primers 412EYu (5′-GGCCTTCGAATGGCTAAACAG-3′) and 413EYd (5′-CCTGCCTTAACT TAGCTAGC-3′). The 2.8-kb PCR product was gel purified and used for cloning into the 239-FL plasmid using the restriction sites BstBI and NheI. Fragments were ligated using a Rapid DNA Ligation kit (Roche Applied Science, Indianapolis, IN). After the transformation of Escherichia coli Stbl2 cells (Invitrogen, Carlsbad, CA), single ampicillin-resistant colonies were grown overnight at 30°C for plasmid preparations, which were sequenced as described above.
Phylogenetic analysis
Nucleotide sequences were aligned using the HIValign tool as implemented on the Los Alamos National Laboratory HIV Sequence Database website (http://www.hiv.lanl.gov/content/sequence/HMM/HmmAlign.html), using the HIV-2/SIV Hidden Markov Model setting, and designating SIVmac239 (GenBank accession no. M33262) as a reference sequence. For comparison with sequences reported by Keele et al.18 61 SIVmac251 env sequences corresponding to GenBank accession numbers FJ578007–FJ578067 were downloaded and incorporated prior to alignment. Aligned sequences were converted to Nexus format and neighbor-joining trees were generated using PAUP v4.0a109 for Macintosh. The sequences described in this study have been deposited in GenBank and are available under the accession numbers HM165237–HM165256.
Infectivity assays
Viral infectivity was measured using two cell lines: C8166-45 SIV-SEAP and TZM-bl. C8166-45 SIV-SEAP cells harbor a Tat-inducible, secreted alkaline phosphatase (SEAP) reporter construct enabling SIV infection to be measured by SEAP production in the culture supernatant.19 TZM-bl cells are a HeLa cell line with luciferase expression driven by the HIV-1 LTR on infection of the cells with HIV-1 or SIV.20,21 For infectivity assays in the SEAP cell line, aliquots of virus stocks were subjected to serial twofold dilutions and SEAP activity from the supernatant was measured at day 3 postinfection using the Phospha-Light kit (Applied Biosystems, Inc., Foster City, CA) according to the manufacturer's recommendations, with the modifications as described previously.19 For infectivity assays in the TZM-bl cells, a 96-well plate was set up with each row containing an uninfected well and a set of nine serial twofold dilutions of virus. To these wells, 1 × 104 TZM-bl cells were added and the plate was transferred to a humidified CO2 incubator at 37°C. After 3 days, luciferase activity was measured in triplicate using the Britelite Plus kit (Perkin Elmer, Shelton, CT).
Neutralization
The neutralization sensitivity of each virus was tested using an SEAP reporter assay as previously described.19 Virus equivalents to 5 ng of p27 capsid protein for SIVmac316 and 2 ng of p27 capsid protein for all other viruses were chosen as inputs. SEAP activity was measured on day 4 when levels were sufficiently over background to give reliable measurements (at least 10-fold). To perform neutralization assays, 96-well plates were set up as follows: 25 μl of medium (RPMI, 10% fetal bovine serum) was added to the first three columns and 25-μl aliquots of successive twofold or fourfold dilutions of test sera in medium were added to each of the other columns. All sera were heat inactivated at 56°C for 30 min before use in neutralization assays. Each virus in a total volume of 75 μl was then added to each well in columns 3 through 12. Virus-free medium was added to columns 1 and 2 (mock). The plate was incubated for 1 h at 37°C. After incubation, 5000 target cells (C8166-45 SIV-SEAP) in a volume of 100 μl were added to each well. The plate was placed into a humidified chamber within a CO2 incubator at 37°C. SEAP activity was measured using the chemiluminescent Phosphalight SEAP assay system (Applied Biosystems, Inc., Foster City, CA) according to the manufacturer's recommendations, with modifications as described previously.19 Neutralization activity for all antibodies and plasma samples was measured in triplicate using a Victor V multilabel counter (Perkin Elmer, Shelton, CT) and reported as a percentage of SEAP activity or percentage neutralization. Inhibition of infectivity by sCD4 was measured in the same way as antibody-mediated neutralization assays. Soluble CD4 was obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD).
SIV-positive sera
A pool of sera from SIVmac239 infected monkeys was available from a previous study performed at the New England Primate Research Center. Sera from rhesus monkeys 184-99 and 309-01 were obtained 48 weeks postinfection with the 1991 animal-titered stock of uncloned SIV251, and were a gift from Dr. Paul Johnson at the New England Primate Research Center.
Assays of virus replication
We generated growth curves in rhesus PBMCs and macrophages from bronchoalveolar lavage (BAL) of rhesus macaques. PBMCs were isolated from fresh citrate blood of rhesus macaques by density gradient centrifugation (LSM Lymphocyte Separation Medium, MP Biomedicals, LCC, Solon, OH). For analysis of viral replication in culture, PMBCs were resuspended at 1 × 106 cells per ml in R20 (RPMI 1640 + 20% fetal bovine serum) and were activated for 72 h with 1 μg of phytohemmaglutinin (Sigma, St. Louis, MO) per ml R20. Cells were then washed and resuspended in R20 supplemented with 10% interleukin-2. In each well of a 24-well plate, 1 × 106 PBMCs in 2 ml were infected with 10 ng of p27 capsid protein. One milliliter of supernatant was replaced every 3–4 days and the concentration of p27 capsid protein in the supernatant was measured using an SIV p27 antigen capture assay (Advanced BioScience Laboratories, Inc., Kensington, MD).
Rhesus macrophages were obtained from BAL. Approximately 20 ml of saline was used to collect cells from BAL. The cells were pelleted by centrifugation at 1000 rpm for 10 min, resuspended in 5 ml of ACK lysing buffer, and left to stand at room temperature for 3 min. Then 20 ml of HBSS with 2% fetal bovine serum was added and the cells were again pelleted. The cells were washed two more times before being resuspended at 0.43 × 106 cells per ml in macrophage culture medium (IMDM supplemented with 10% human serum type AB, 5% fetal bovine serum, 100 μg/ml streptomycin, 100 U/ml penicillin, 20 μg/ml gentamycin, and 0.25 μg/ml amphotericin B). Then 0.25 ml of cells was added to each well on a 96-well plate and allowed to incubate at 37°C in a CO2 incubator for 2 h until macrophages adhered to the plate. The remaining lymphocytes were washed out by extensive flushing of the medium. A total of 250 μl of fresh medium was added to each well and the plate was incubated for 48 h at 37°C in a CO2 incubator before infecting with 4.3 ng p27 capsid protein per well. Then 100 μl of supernatant was collected and replaced with fresh media every 2–3 days and the concentration of p27 capsid protein in the supernatant was measured using an SIV p27 antigen capture assay (Advanced BioScience Laboratories, Inc., Kensington, MD).
Results
The derivation of the PBMC-grown stock of uncloned SIVmac251 that was used here for env cDNA cloning has been described.16 Its infectivity titer by the intravenous route16 and by the rectal route9 (unpublished data) is known. Vaccine studies that have used this particular stock include references9,10,22 and other vaccine studies have used SIVmac251 stocks derived from it. cDNA was prepared from virion RNA and amplified using primers that allowed easy insertional cloning into the unique BstBI and NheI sites of the SIVmac239 full-length infectious clone. These sites are located at nucleotide number 5875 just upstream of the initiating methionine of Env and 8742 at the end of the membrane spanning domain according to the sequence of Regier and Desrosiers.23 Twenty independent clones were selected for analysis.
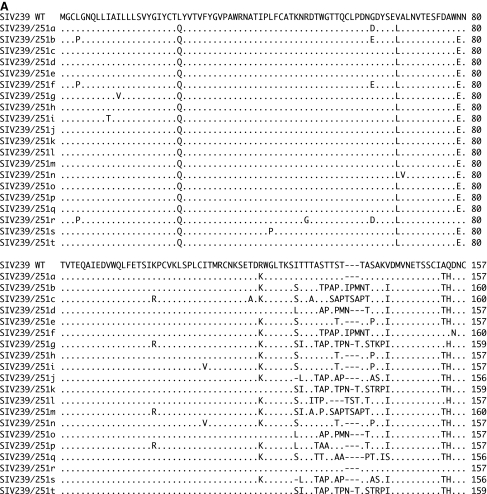
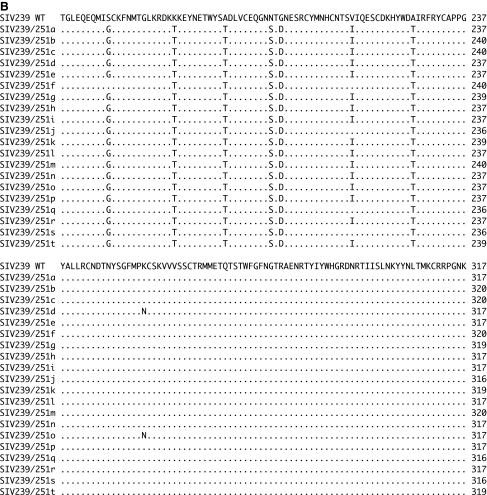
Sequencing of these 20 clones revealed that all of the clones differed from one another and differed from SIVmac239 (Fig. 1). Clones d and o had an identical predicted amino acid sequence but they differed from each other at one nucleotide position. Predicted amino acid differences ranged from 0 to 27 among the 20 clones and from 28 to 45 when each of the 20 clones was compared to SIVmac239 (Fig. 1). Some of the sequences did cluster with SIVmac239 or with other SIVmac251 clones on phylogenetic analysis (Fig. 2). Recently, Keele et al.18 described the envelope sequences in a stock of SIVmac251 that had been separately passaged from the SIVmac251 stock that we used here. These sequences from Keele et al.18 showed much less diversity than the envelopes sequences present in our 1991 stock and clustered with one branch of our phylogenetic tree (Fig. 2).
FIG. 1.
Alignments of SIV envelope sequences. (A–E) The alignments of SIV envelope sequences of 20 clones of SIV239/Env251 viruses. SIV239/Env251 clones are aligned to the first 716 amino acids of SIVmac239. The alignment includes only the sequences cloned from the virus stock; the remaining sequences are identical to SIVmac239. Periods indicate conservation with the SIVmac239 sequence. Dashes indicate deletions.
FIG. 2.
SIVmac251-derived env clones represent a diverse array of sequences. The unrooted neighbor-joining tree shows nucleotide diversity among the 20 cloned SIVmac251 envelope sequences characterized in this study (branches are labeled a through t). The red cluster depicts SIVmac251 sequences from an expanded SIVmac251 challenge stock characterized by Keele et al.18 The env sequence of SIVmac239 was included as a reference.
Infectivity
Virus stocks were produced by transfection of cloned DNA into HEK293T cells. All 20 plasmids yielded p27 capsid antigen in similar amounts. Seventeen of the 20 clones were infectious when scored on C8166-SEAP cells19 and on TZM-bl cells20,21 (Fig. 3). The three noninfectious clones (c, r, and s) were noninfectious for both cell lines. The use of normalized inputs based on p27 content allowed us to evaluate the relative infectivity of the different stocks. They ranged from minus 32-fold to plus 0-fold in C8166-SEAP cells relative to SIVmac239 and minus 1.7-fold to plus 7.6-fold in TZM-bl cells relative to SIVmac239. The highest infectivities were observed for clones a, e, and i in C8166-SEAP cells (Fig. 3A) and for clones a, b, j, m, o, k, and l in TZM-bl cells (Fig. 3B). The lowest infectivities were observed for clones f and t in C8166-SEAP cells (Fig 3A) and for clones f and t in TZM-bl cells (Fig. 3B). Infectivity for TZM-bl cells likely reflects use of CCR5 for entry into cells. The rank orders of infectivities are summarized in Table 1.
FIG. 3.
Comparative infectivity of SIVmac239 and 20 clones of SIV239/Env251. Virus stocks were obtained from transfection of HEK 293T cells. Stocks were normalized for the amount of p27 and used to infect the C8166-45 SIV-SEAP cell line or the TZM-bl cell line. (A) C8166-45 SIV-SEAP cells were infected and SEAP activity was measured on day 3 postinfection. The 20 SIV239/Env251 virus clones were divided into three sets. SIV239 was run as a control in each set. All viruses were assayed in triplicate. The SEAP counts were normalized to the values obtained for 1.25 ng of SIVmac239 virus per well. The relative standard deviation for the triplicate results with 1.25 ng input was calculated, and ranged from 3% to 42%, with an average relative standard deviation of 17%. Clones SIV239/251c, SIV239/251r, and SIV239/251s were not infectious in SEAP cells and were not included in this calculation. (B) TZM-bl cells were infected and luciferase activity was measured on day 3 postinfection. The graph shows RLU versus input of p27 in ng/well. The viruses were all assayed in triplicate. The relative standard deviation for the triplicate results with 1.25 ng input was calculated, and ranged from 1% to 21%, with an average relative standard deviation of 10%. Clones SIV239/251c, SIV239/251r, and SIV239/251s were not infectious in TZM-bl cells and were not included in this calculation.
Table 1.
Rank Orders of Infectivities
| Ranka | Infectivity in TZB-ml cells | Infectivity in C8166-SEAP cells |
|---|---|---|
| 1 | SIV239/251a | SIV239 |
| 2 | SIV239/251j | SIV239/251a |
| 3 | SIV239/251b | SIV239/251e |
| 4 | SIV239/251m | SIV239/251i |
| 5 | SIV239/251l | SIV239/251h |
| 6 | SIV239/251o | SIV239/251q |
| 7 | SIV239/251k | SIV239/251b |
| 8 | SIV239/251q | SIV239/251l |
| 9 | SIV239/251g | SIV239/251g |
| 10 | SIV239/251i | SIV239/251p |
| 11 | SIV239/251d | SIV239/251m |
| 12 | SIV239/251e | SIV239/251j |
| 13 | SIV239/251h | SIV239/251o |
| 14 | SIV239/251n | SIV239/251n |
| 15 | SIV239 | SIV239/251d |
| 16 | SIV239/251p | SIV239/251k |
| 17 | SIV239/251f | SIV239/251t |
| 18 | SIV239/251t | SIV239/251f |
| 19 | SIV239/251s | SIV239/251c |
| 20 | SIV239/251c | SIV239/251s |
| 21 | SIV239/251r | SIV239/251r |
Based on infectivity at 1.25 ng p27 per well. Viruses are ranked from most infectious to least infectious. Line spaces separate viruses that clustered separately from the others.
Replication in rhesus PBMCs
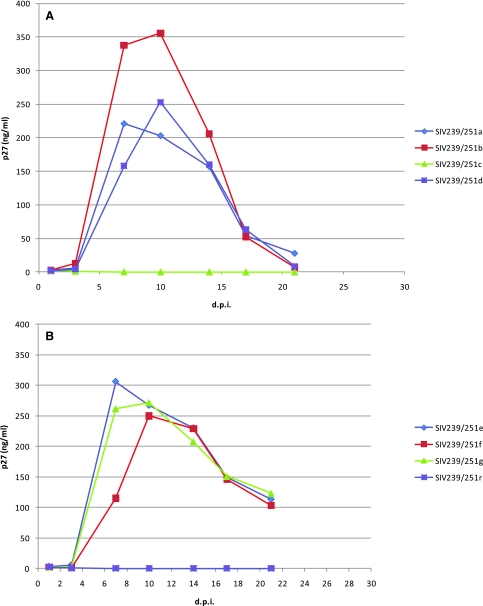
Cloned SIVs c, r, and s, which did not score in C8166-SEAP or TZM-bl cells above, also did not replicate detectably in primary rhesus PBMC cultures. The underlying basis for the lack of infectivity in cell lines and the failure to replicate in rhesus PBMCs for these three recombinant cloned viruses is not obvious from inspection of their sequences (Fig. 1). The 17 remaining SIVmac239/Env251 viruses replicated well in rhesus PBMCs. Although substantial differences were noted in the general ability of PBMCs from one animal to another to support SIV replication, only minor differences were noted among the 17 different SIVmac239/Env251 strains when tested head to head in cells from the same animal. Figure 4 shows representative replication curves to illustrate these points. SIVmac239/Env251 clone m replicated most poorly of the 17 replication-competent strains (Fig. 4C) and PBMCs from monkey Mm159-05 were the poorest of the monkeys tested in supporting replication of the recombinant SIVs (Fig. 4D).
FIG. 4.
Replication kinetics of SIV239/Env251 virus clones in rhesus PBMC cultures. (A) Viruses SIV239/251a, SIV239/251b, SIV239/251c, and SIV239/251d grown in PBMCs from animal Mm 180-05; (B) viruses SIV239/251e, SIV239/251f, SIV239/251g, and SIV239/251r grown in PBMCs from animal Mm 311-00; (C) viruses SIV239/251k, SIV239/251l, SIV239/251m, and SIV239/251s grown in PBMCs from animal Mm 359-98; (D) viruses SIV239/251k, SIV239/251l, SIV239/251m, and SIV239/251s grown in PBMCs from animal Mm 159-05. Clones SIV239/251h, SIV239/251i, SIV239/251j, SIV239/251n, SIV239/251o, and SIV239/251p were also tested and exhibited similar growth kinetics (data not shown).
Sensitivity to soluble CD4
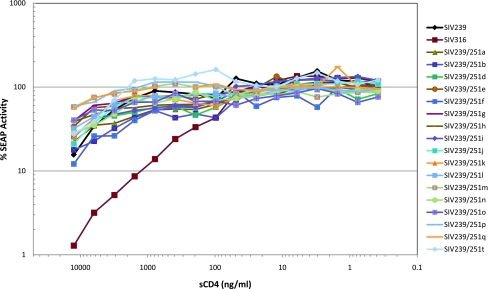
The IC50s for sCD4 against the 17 replication-competent recombinant clones were determined using C8166-SEAP cells. Controls for this experiment, SIV239 and SIV316, gave IC50s similar to what our laboratory has reported previously.6,24 SIV316 has a high affinity for CD4 and consequently requires much lower concentrations of sCD4 for inhibition.6,24,25 Consistent with this, SIV239 control yielded an IC50 of 3 μg/ml sCD4 and SIV316 control yielded an IC50 of 0.1 μg/ml. The 17 recombinant viruses had a sensitivity to sCD4 that was more similar to SIV239. The majority of the recombinant clones required somewhat higher concentrations of sCD4 for 50% inhibition than SIV239 (Fig. 5 and Table 2). Clones a, b, and f required 1 μg/ml for 50% inhibition and clones d, i, and n required 3 μg/ml for 50% inhibition by sCD4.
FIG. 5.
Comparative inhibition of SIV239, SIV316, and SIV239/Env251 clones by soluble CD4. Virus was incubated with sCD4 and then C8166-45 SIV-SEAP cells were added. SEAP activity was measured 3 days postinfection. The viruses were assayed in triplicate. The relative standard deviation for the 17 recombinant cloned viruses was calculated, and ranged from 1% to 56%, with an average relative standard deviation of 13%. Clones SIV239/251c, SIV239/251r, and SIV239/251s were not infectious in SEAP cells and were not included in this assay.
Table 2.
Neutralization Titers
| Virus | Concentration of sCD4 (μg/ml) reducing infectivity by 50%a | 70% neutralization titers using a pool of SIV239+ monkey serab | 70% neutralization titers using SIV251+ monkey sera 184-99b |
|---|---|---|---|
| SIV239 | 3 | 50 | 60 |
| SIV316 | 0.1 | 100,000 | 100,000 |
| SIV239/251a | 1 | 50 | 100 |
| SIV239/251b | 1 | 100 | 800 |
| SIV239/251d | 3 | 30 | 60 |
| SIV239/251e | 9 | 30 | 100 |
| SIV239/251f | 1 | 20 | 800 |
| SIV239/251g | 9 | 30 | 100 |
| SIV239/251h | 9 | 50 | 40 |
| SIV239/251i | 3 | 50 | 200 |
| SIV239/251j | 5 | 100 | 200 |
| SIV239/251k | 5 | 50 | 200 |
| SIV239/251l | 5 | 60 | 100 |
| SIV239/251m | >12.5 | 25 | 40 |
| SIV239/251n | 3 | 200 | 400 |
| SIV239/251o | 9 | 60 | 60 |
| SIV239/251p | >12.5 | 40 | 60 |
| SIV239/251q | >12.5 | 30 | 60 |
| SIV239/251t | 5 | 90 | 60 |
50% SEAP activity determined based on results from day 3 sCD4 inhibition curves.
Values represent the reciprocal dilution of sera needed to reduce infectivity by 70%. Results are based on day 4 neutralization curves.
Sensitivity to neutralization by sera from infected monkeys
The sensitivity of these 17 recombinant viruses to neutralization by sera from SIV-infected monkeys was evaluated. Three different sera were used. First, we used a pool of sera from SIV239-infected monkeys. We have many vials of this pool stored and we usually use it as our positive control in neutralization assays to gauge the consistency of performance against SIV239 (relatively neutralization resistant) and SIV316 (neutralization sensitive) as controls in each assay. We also used sera from rhesus monkeys 184-99 and 309-01 obtained 9 months after infection with the 1991 animal-titered stock of uncloned SIV251, the same stock that was used for the env cloning. We used SEAP activity from C8166-SEAP cells as the readout for the neutralization assay. Sera from uninfected SPF rhesus monkeys exhibited no neutralizing activity (not shown).
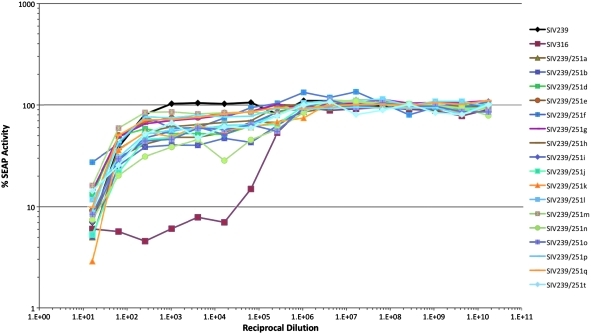
One curious feature of the neutralization profiles of the 17 new viruses was a “flattening” effect with the curves (see Figs. 5, 6, and 7). SIV infection (measured by SEAP activity) initially increased with increasing dilutions of sera at a typical rate, but extreme serum dilutions were required to eventually take the activity up to 100%. We have seen this curious effect with all 17 viruses using sCD4 and using each of the three positive monkey sera. We have seen it with independent virus stock preparations of these 17 viruses. We have seen the effect on both C8166-SEAP cells and TZM-bl cells. We never see it with SIV239 or with SIV316. Because of this, neutralization titers are reported as 70% neutralization values.
FIG. 6.
Comparative neutralization of SIV239, SIV316, and SIV239/Env251 clones by a pool of SIV-positive sera. Virus was incubated with serial 4-fold dilutions of sera and then C8166-45 SIV-SEAP cells were added. SEAP activity was measured 4 days postinfection. The viruses were assayed in triplicate. The relative standard deviation for the 17 recombinant cloned viruses was calculated, and ranged from 1% to 71%, with an average relative standard deviation of 12%. Clones SIV239/251c, SIV239/251r, and SIV239/251s were not infectious in SEAP cells and were not included in this assay.
FIG. 7.
Comparative neutralization of SIV239, SIV316, and SIV239/Env251 clones by sera from SIV251 + rhesus monkey 184-99. Virus was incubated with serial 4-fold dilutions of sera and then C8166-45 SIV-SEAP cells were added. SEAP activity was measured 4 days postinfection. The viruses were assayed in triplicate. The relative standard deviation for the 17 recombinant cloned viruses was calculated, and ranged from 3% to 64%, with an average relative standard deviation of 19%. Similar results were obtained using sera from SIV251 + monkey 309-01 (data not shown). Clones SIV239/251c, SIV239/251r, and SIV239/251s were not infectious in SEAP cells and were not included in this assay.
Neutralization curves with the SIV239-positive pool are shown in Fig. 6 and the 70% neutralization titers are given in Table 2. The 70% neutralization titer of this serum against SIV239 was 1:50 and against SIV316 was 1:100,000. These are similar to and consistent with all previous measurements of these combinations. 70% neutralization titers with this serum ranged from 1:20 to 1:200 against the 17 new viruses. We conclude that the neutralization sensitivity of these 17 new viruses with this particular serum pool is similar to or slightly greater than the sensitivity of SIV239. Strains b, n, and j were neutralized to the greatest extent by this serum pool.
Because these new viruses have env sequences derived from uncloned SIV251, it was important that we characterize the sensitivity to neutralization by sera from monkeys infected with this stock of uncloned SIV251. Neutralization curves with the SIV251-positive monkey serum 184-99 are shown in Fig. 7 and the 70% neutralization titers are given in Table 2. The 70% neutralization titers with this serum were 1:60 against SIV239 and 1:100,000 against SIV316. The 70% neutralization titers with this serum against the 17 new viruses ranged from 1:40 to 1:800. Nine of the 17 viruses exhibited 70% neutralization titers of 1:100 or greater. Thus, these new cloned viruses appear somewhat more sensitive on average to neutralization by this SIV251-positve serum than SIV239. Strains b, f, and n were neutralized to the greatest extent by this positive serum.
The same patterns and trends for neutralization that were observed with 184-99 serum were also observed with serum from 309-01, except the titers were lower (data not shown). As with serum from 184-99, the b, f, and n cloned viruses were the ones most sensitive to neutralization by serum from 309-01. This suggests that either these three cloned viruses are the most sensitive to antibody-mediated neutralization of the lot or that sequences in these cloned viruses are the most heavily represented in what grew out in these two animals.
Sensitivity to neutralization by selected monoclonal antibodies
We also tested the sensitivity of these 17 recombinant viruses to neutralization by selected monoclonal antibodies. The monoclonal antibodies that were used are 3.11H, 1.11A, 1.9C, and 1.10A. These antibodies represent competition groups IV, V, VI, and VII.26,27 We also included SIV239 and SIV316 as controls for these neutralization assays. None of the four mABs neutralized SIV239 detectably (IC50 > 10 μg/ml), consistent with previous analyses described in Johnson et al.26 All four monoclonal antibodies neutralized SIV316 at concentrations similar to what was reported previously by Johnson et al.;26 the IC50s were all less than 1 μg/ml with SIV316. The majority of mAB–SIV239/Env251 combinations showed no neutralization (IC50s > 10 μg/ml). Four of the SIV239/Env251 recombinant strains did appear to be weakly neutralized by the 1.10A mAB (IC50s of 1–5 μg/ml) (data not shown); these four strains were a, b, f, and n.
Replication in rhesus monkey alveolar macrophages
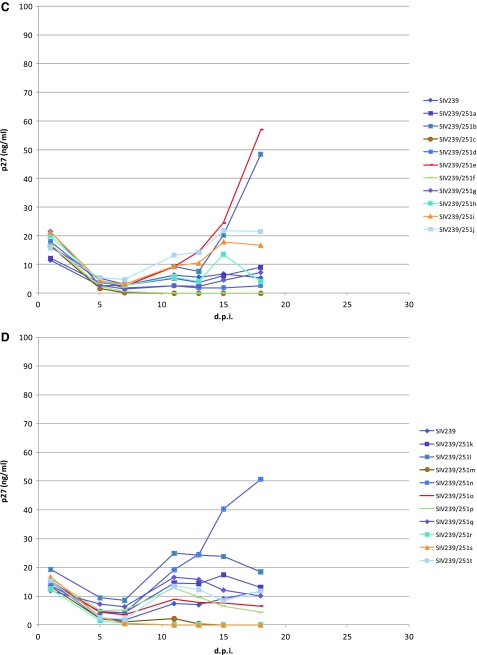
The recombinant viruses were tested for their relative ability to replicate in differentiated alveolar macrophages from rhesus monkey lung lavage as we have described previously.8,28 As controls, we included SIV316, SIV316TMstop, and SIV239. The TMstop derivative of SIV316 has a truncating mutation in the cytoplasmic domain of env that results in increased Env content in virions and increased infectivity.24 The SIV316 strains replicate extremely well in these CD4-low macrophages whereas SIV239 replicates poorly. Because enough cells were not obtained from a single monkey to test all recombinant strains, some strains were tested in macrophages from one monkey and the remainder of the strains were tested in macrophages from another monkey. The control SIV strains were used in both sets of cultures. Input inocula were normalized to contain the same amounts of p27. None of the recombinant SIV239/Env251 strains replicated as well as the SIV316 strains in these alveolar macrophage cultures. However, some of the recombinant strains exhibited significant levels of delayed replication well above the levels observed with SIV239 (Fig. 8). Recombinant strains b, e, and n achieved supernatant levels in excess of 50 ng/ml p27 and strains j and l achieved levels in excess of 20 ng/ml p27.
FIG. 8.
Replication kinetics of SIV239, SIV316open, 316TMstop, and SIV239/Env251 virus clones in rhesus macrophage cultures. Rhesus monkey macrophages were obtained from bronchoalveolar lavage and were infected with viruses after being cultured for 48 h. The p27 content in the supernatant was measured using an antigen capture assay. (A and C) Macrophages from Mm 222-06 were infected with SIV239, SIV316open, SIV316TMstop, and SIV239/251a–j and p27 amounts graphed with (A) and without (C) SIV316open and SIV316TMstop. (B and D) Macrophages from Mm 280-06 were infected with SIV239, SIV316open, SIV316TMstop, and SIV239/251k-t and p27 amounts graphed with (B) and without (D) SIV316open and SIV316TMstop.
Discussion
The range of envelope sequence diversity in the primary stock of SIVmac251 appears to be similar to what has been often observed with primary isolates of HIV-1.29–31 SIV had been in rhesus monkey #251 for several years prior to its isolation and consequently had ample time to diversify.32–34 SIVmac239 was derived from uncloned SIVmac251 by passage in monkeys.32–34 Consequently, the modest extent of envelope sequence differences between SIVmac239 and the parental SIVmac251 is not surprising. Nonetheless, SIVmac239 has quite clearly diverged beyond the range of diversity present in the SIVmac251 stock that we describe here. Sequence differences among clones not surprisingly occurred predominantly in previously defined variable regions, particularly in V1 (amino acids 110–150).35 The extent of envelope sequence diversity present in the 1991 SIVmac251 stock analyzed here appears to be considerably greater than that observed for a separately passaged stock of SIVmac251 analyzed recently by Keele et al.18 This observation has important implications for pathogenesis studies directed at the analysis of transmitted variants and for vaccine studies directed at protection against a heterogeneous swarm of viruses.
Seventeen of the 20 randomly selected envelope clones were compatible with replication competence. The three clones that were not replication competent (c, r, and s) are a bit of a curiosity as there is nothing obvious in their sequences to suggest what may be the cause. There are no stop codons or frame shifts in these clones. However, clone c does have a unique A at position 118, clone r a unique G at position 49, and clone s a unique P at position 42 that could potentially be responsible for their lack of function.
Some of the analyzed properties seemed to be linked. Clones a, b, f, and n showed the greatest sensitivity to inhibition by sCD4 and these same four cloned viruses were the only ones for which some level of neutralization by monoclonal antibodies was observed. Cloned viruses b, f, and n were also most sensitive to neutralization by sera from monkeys infected with the uncloned SIVmac251 stock. Sera from monkey 184-99 neutralized these three cloned viruses with 70% neutralization titers of 1:800, 1:800, and 1:400. Cloned viruses b and n, together with e, also exhibited the best replication of the 17 in primary rhesus monkey alveolar macrophages. These were the same trends in properties that we noted previously for SIVmac239 vs. SIVmac316,6,26 except that in the case of SIVmac316 the differences in properties are considerably more extreme.
Although the range of differences among clones was in some cases modest, the differences in characteristics may prove useful for some applications, for example in selecting a clone or clones for a particular study or for comparing different clones for biological outcomes. Relative infectivity varied over more than a 200-fold range and sensitivity to neutralization varied by as much as 20-fold. Mixtures of clones could be used for some applications, for example, for examining whether there is selective transmission of some strains on mucosal exposure. Although we do not yet know how these recombinant cloned viruses will perform in monkeys, they will almost certainly be helpful in continuing use of the SIV model.
Acknowledgments
This work was supported by the Reagent Resource Support Program for AIDS Vaccine Development, Quality Biological, Inc., Gaithersburg, MD (NIH contract N01-AI-30018), by PHS Grant P01AI071306 to R. Paul Johnson and RCD, and by PHS Grant RR00168 to the New England Primate Research Center. We thank Angela Carville and Keith Mansfield for providing blood and lung lavage samples. We also thank Paul Johnson for the gift of sera, Elosia Yuste for helpful technical tips, and David Evans, R. Paul Johnson, and Jeffrey Lifson for helpful discussion.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fauci AS. Johnston MI. Dieffenbach CW, et al. HIV vaccine research: The way forward. Science. 2008;321(5888):530–532. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 2.Kestler H. Kodama T. Ringler D, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch V. Adger-Johnson D. Campbell B, et al. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson WE. Lifson JD. Lang SM. Johnson RP. Desrosiers RC. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J Virol. 2003;77(1):375–381. doi: 10.1128/JVI.77.1.375-381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuwata T. Nishimura Y. Whitted S, et al. Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS Pathog. 2009;5(4):e1000372. doi: 10.1371/journal.ppat.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Means RE. Matthews T. Hoxie JA. Malim MH. Kodama T. Desrosiers RC. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: Correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol. 2001;75(8):3903–3915. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori K. Ringler DJ. Kodama T. Desrosiers RC. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori K. Rosenzweig M. Desrosiers RC. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol. 2000;74(22):10852–10859. doi: 10.1128/jvi.74.22.10852-10859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RP. Lifson JD. Czajak SC, et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: Inverse relation of degree of protection with level of attenuation. J Virol. 1999;73(6):4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyand MS. Manson KH. Garcia-Moll M. Montefiori D. Desrosiers RC. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70(6):3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz JE. Johnson RP. McClure HM, et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol. 2005;79(13):8131–8141. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidajat R. Xiao P. Zhou Q, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83(2):791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J. O'Brien KL. Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CJ. Li Q. Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosati M. Bergamaschi C. Valentin A, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci USA. 2009;106(37):15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis MG. Bellah S. McKinnon K, et al. Titration and characterization of two rhesus derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 17.Morrison HG. Kirchhoff F. Desrosiers RC. Evidence for the cooperation of gp120 amino acids 322 and 448 in SIVmac entry. Virology. 1993;195:167–174. doi: 10.1006/viro.1993.1357. [DOI] [PubMed] [Google Scholar]
- 18.Keele BF. Li H. Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206(5):1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Means RE. Greenough T. Desrosiers RC. Neutralization sensitivity of cell culture passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt EJ. Wehrly K. Kuhmann SE. Chesebro B. Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi Y. McClure MO. Pizzato M. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol. 2008;82(24):12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilyinskii PO. Desrosiers RC. Efficient transcription and replication of simian immunodeficiency virus in the absence of NF-kB and Sp1 binding elements. J Virol. 1996;70(5):3118–3126. doi: 10.1128/jvi.70.5.3118-3126.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regier DA. Desrosiers RC. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 24.Yuste E. Johnson W. Pavlakis GN. Desrosiers RC. Virion envelope content, infectivity, and neutralization sensitivity of simian immunodeficiency virus. J Virol. 2005;79(19):12455–12463. doi: 10.1128/JVI.79.19.12455-12463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannert N. Schenten D. Craig S. Sodroski J. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol. 2000;74(23):10984–10993. doi: 10.1128/jvi.74.23.10984-10993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson WE. Sanford H. Schwall L, et al. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol. 2003;77(18):9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole KS. Alvarez M. Elliott DH, et al. Characterization of neutralization epitopes of simian immunodeficiency virus (SIV) recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology. 2001;290(1):59–73. doi: 10.1006/viro.2001.1144. [DOI] [PubMed] [Google Scholar]
- 28.Mori K. Ringler DJ. Desrosiers RC. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodenow M. Huet T. Saurin W. Kwok S. Sninsky J. Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: Evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- 30.Saag MS. Hahn BH. Gibbons J, et al. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Gonzalez JF. Salazar MG. Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel M. Letvin N. Sehgal P, et al. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. IJC. 1988;41:601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- 33.Daniel MD. Letvin NL. King NW, et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 34.Mansfield KG. Lerch NW. Gardner MB. Lackner AA. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol. 1995;24(3):116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 35.Burns DPW. Desrosiers RC. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991;65:1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]