Abstract
Study Objectives:
To perform a meta-analysis of the efficacy and safety of exogenous melatonin in advancing sleep-wake rhythm in patients with delayed sleep phase disorder.
Design:
Meta analysis of papers indexed for PubMed, Embase, and the abstracts of sleep and chronobiologic societies (1990–2009).
Patients:
Individuals with delayed sleep phase disorder.
Interventions:
Administration of melatonin.
Measurements and Results:
A meta-analysis of data of randomized controlled trials involving individuals with delayed sleep phase disorder that were published in English, compared melatonin with placebo, and reported 1 or more of the following: endogenous melatonin onset, clock hour of sleep onset, wake-up time, sleep-onset latency, and total sleep time. The 5 trials including 91 adults and 4 trials including 226 children showed that melatonin treatment advanced mean endogenous melatonin onset by 1.18 hours (95% confidence interval [CI]: 0.89–1.48 h) and clock hour of sleep onset by 0.67 hours (95% CI: 0.45–0.89 h). Melatonin decreased sleep-onset latency by 23.27 minutes (95% CI: 4.83 –41.72 min). The wake-up time and total sleep time did not change significantly.
Conclusions:
Melatonin is effective in advancing sleep-wake rhythm and endogenous melatonin rhythm in delayed sleep phase disorder.
Citation:
van Geijlswijk IM; Korzilius HPLM; Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. SLEEP 2010;33(12):1605-1614.
Keywords: Melatonin, delayed sleep phase disorder, meta-analysis
SLEEP-WAKE TIMING IS REGULATED BY THE BIOLOGIC CLOCK IN A CIRCADIAN RHYTHM: A RHYTHM CONSISTING OF APPROXIMATELY 24 HOURS. Entrainment of the biologic clock is achieved by environmental light. The endogenous rhythm of melatonin production by the pineal gland is regulated by the suprachiasmatic nucleus and is suppressed by exposure to bright light. Endogenous melatonin starts to rise in dim light (the so-called dim light melatonin onset [DLMO]), normally between 19:30 and 21:30 in adults and between 19:00 and 21:00 in children 6 to 12 years of age.1 This DLMO can be determined for each individual, and it characterizes the individual's circadian timing.
Delayed sleep phase disorder (DSPD) is a problem in which the circadian clock is entrained in the 24-hour rhythm but at a delayed phase angle.2 This can result in sleep- wake timing that is late with respect to societal norms.1 It has been estimated that approximately 10% of patients with chronic insomnia have DSPD.3
Treatment of DSPD relies on the use of chronotherapy or, in other words, the shifting of sleep-wake schedules4 using carefully timed “morning” light administration5 to phase advance the clock and “evening” melatonin treatment to advance the clock.6 Based on the principles of chronobiology, effective treatment is entirely dependent on the correct timing of light and melatonin in relation to the circadian clock (circadian phase).7
Some characteristic circadian clock times are wake time, defined as circadian time 0 and DLMO, which is classically defined as circadian time 14,8 the time at which a melatonin level of 10 pg/mL is attained in the blood. This level was chosen during a period in the past when blood melatonin levels lower than 10 pg/mL could not be detected. Later, when the lower limit of quantification dropped, it was possible to measure lower melatonin levels in both blood and saliva. Salivary melatonin levels appear to correspond with 30% to 40% of the melatonin level in the blood. Consequently, salivary DLMO has been defined as the time at which 3 pg/mL or 4 pg/mL is found in the saliva. Nowadays, it is possible to measure salivary melatonin levels of 0.5 pg/mL or even lower, which has led to different definitions of DLMO.9–11
All of the studies in which the effects of melatonin have been observed determined the shift of DLMO after an intervention; in these studies, this measure was not influenced by the method of determination. Nevertheless, in the included studies, the traditional method has been applied.
When exogenous melatonin is administered, it functions as a chronobiotic drug with hypnotic properties.12 Exogenous melatonin is currently under investigation as a potential treatment for DSPD.13 Previous studies have shown exogenous melatonin to shift the internal biologic clock8,14 in addition to eliciting direct soporific effects that occur mainly during the daytime when endogenous melatonin levels are low.15
The chronobiotic mechanism becomes apparent when depicting the shift of the biologic clock as a phase-response curve. Changes from baseline are plotted as an advance or delay of sleep. Studies that examine phase-response curves support the circadian-phase effectiveness of melatonin by showing a persistent effect upon the sleep profile after a washout period of 24 hours following cessation of melatonin administration.16 The cessation of melatonin therapy in adults with DSPD results in the delay of sleep onset and a return to pretreatment values within a few days to 1 year.17,18 In the children who had sleep-onset insomnia and who took melatonin, the drug holidays lasting 1 week resulted in the former sleep problem returning in more than 90% of the cases.19 This suggests that the chronobiotic effects of melatonin can only be sustained through continued use, although the need to advance sleep onset did disappear in 8% of the children who had received treatment19 during a 4-year period.
The greatest advancement can be observed when melatonin is administered 5 hours prior to both the traditionally determined DLMO (circadian time 9)20 and the threshold-determined DLMO.21 Delays are registered when melatonin is administered between 6 to 15 hours after DLMO.1,8,13,22,23
In addition to when the drug is administered, the dose of the drug may also play a role in the effectiveness of melatonin. When the dose is too low, no concrete effects will occur; when the dose is too high, the chronobiologic effects may be lost, and only the somnolent actions remain.24,25 Recently, an association between time of administration and dose of exogenous melatonin in relationship to to endogenous melatonin onset was demonstrated.21 Given that melatonin has a very short elimination half-life in most individuals (between 35 and 45 minutes),26 it is quite plausible that very low doses (i.e., 0.5 mg or less) administered relatively early (i.e., 5 hours prior to DLMO) will have already been cleared to subphysiologic levels before endogenous melatonin onset occurs, and, hence, no shift of DLMO will be observed.
A recent meta-analysis showed melatonin treatment to be ineffective for statistically significant adaptation of several sleep parameters, such as sleep-onset latency (SOL), sleep efficiency, wakefulness, total sleep time (TST), and percentage of rapid eye movement sleep.27 One plausible reason for this finding, however, is that the circadian timing of the melatonin treatment was not taken into consideration. Arendt,28 therefore, suggested that only those studies in which circadian timing was either measured or accurately predicted prior to treatment should be included in further analyses. In the current meta-analysis, only those studies in which the timing of melatonin treatment in relationship to the circadian clock was mentioned were analyzed.
METHODS
Data Sources
We searched in the databases PubMed and Embase and in the abstracts of sleep and chronobiologic societies that were published between January 1990 and September 2009 for randomized, placebo-controlled, double-blind, clinical trials that used melatonin in (circadian rhythm) sleep (onset) disorders. We did not include trials with melatonin agonists or other comorbidities (see online supplement for details).
Altogether, 182 articles were found. The full text for all articles thought to be potentially relevant was retrieved by 2 reviewers (the first and third authors), and additional publications were also sought.
Study Selection and Quality Assessment
All randomized controlled trials meeting the following criteria were selected for further analysis: had to involve individuals who had DSPD, whether or not they also had attention-deficit/hyperactivity disorder (ADHD); had to be reported in English; had to compare melatonin to a placebo; and had to report 1 or more of the following: DLMO, sleep onset (SOT), wake-up time (WUT), SOL (i.e., amount of time between lying down to sleep and onset of sleep), and TST (i.e., amount of time between SOT and WUT).
We included patients who had been diagnosed with ADHD because it is thought that part of the current ADHD epidemic might be attributed to DSPD that has either gone undiagnosed or been misdiagnosed as ADHD.29 Several theories linking ADHD and DSPD, previously published by others, have been discussed by Owens,30 such as a pathology that involves the prefrontal cortex, which regulates both sleep and attention or arousal and can, thus, result in shared symptoms. A compensation mechanism to daytime sleepiness (as a result of DSPD) could in turn lead to clinical manifestations thought to be ADHD. In those studies that have been conducted with both children and adults, the sleep-onset insomnia in individuals with ADHD is characterized as chronobiologic disturbances in sleep onset.31,32
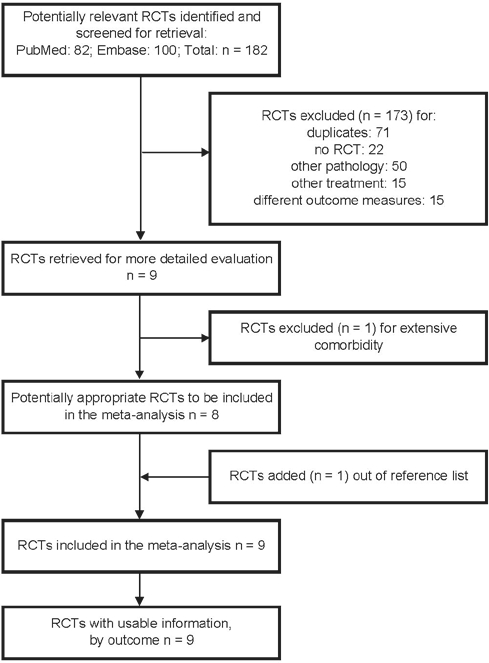
We excluded studies other than randomized controlled trials (such as reviews and case reports), trials with other patient groups or other indications (e.g., trials in dementia, trials or focused on epileptic patients or children with neurodevelopmental disorders other than ADHD, and trials for the treatment of jetlag or nightshift effects), trials with other treatment or combination or treatment (e.g., a melatonin agonist such as ramelteon, light therapy), and studies using other outcomes (such as the quality of sleep or life, biochemical measurements). Nine papers remained (Figure 1).
Figure 1.
Study selection
Two reviewers assessed the methodologic quality of these 9 studies independently by using the Jadad scale33 (quick scan) and the Downs and Black checklist.34 The concealment of treatment allocation was assessed by using the criteria of Schulz et al.35 Papers with a Jadad scale result of 3 or more were judged as suitable for this meta-analysis. The result of the more extensive Downs and Black list is depicted in Table 1 (quality assessment score). The discussion of what should be done with the results of quality assessment still remains open.36–38
Table 1.
Characteristics of placebo-controlled studies using melatonin in delayed sleep phase disorder
| AUTHOR, YEAR OF PUBLICATION | QA score | n | Baseline DLMO level, h:mina | Study design | Melatonin dose, mg | Duration, wks | Time of melatonin administration | Measures and method of data collection |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLMO | SOT | SOL | TST | WUT | ||||||||
| Adults | ||||||||||||
| Dahlitz et al. (1991)17 | 25.0 | 8 | — | X | 5 | 4 | 22:00 h | LOG | PSG | LOG | LOG | |
| Laurant et al. (1997)43 | 26.0 | 25 | 22:35 (0:54) | X | 5 | 2 | 5 h ā DLMO (mean 17:35) | PL/SAL | LOG/ACT | |||
| Nagtegaal et al. (1998)45 | 28.5 | 25 | 23:17 (2:18) | X | 5 | 2 | 5 h ā DLMO (mean 18:17) | PL | ACT | PSG | ||
| Kayumov et al. (2001)42 | 25.0 | 22 | — | X | 5 | 4 | 19:00–22:00 | PSG | PSG | |||
| Mundey et al. (2005)44 | 19.0 | 11 | 23:46 (1:62) | P | 0.3/3 | 4 | 1.5–6.5 h ā DLMO 15.00–21.30 (mean 17:15) | SAL | ACT | ACT | ACT | ACT |
| Children | ||||||||||||
| Smits et al. (2001)46 | 28.0 | 40 | 21:06 (1:16) | P | 5 | 4 | 18:00 | SAL | LOG/ACT | LOG | LOG | LOG |
| Smits et al. (2003)47 | 29.5 | 62 | 20:48 (0:59) | P | 5 | 4 | 19:00 | SAL | LOG | LOG | LOG | |
| Weiss et al. (2006)49 | 30.0 | 19b | — | X | 5 | 10 days | 20 min ā bedtime | LOG | ||||
| van der Heijden et al. (2007)48 | 31.0 | 105 b | 20:34 (0:55) | P | 3/6 | 4 | 19:00 | SAL | ACT | ACT | ACT | ACT |
Data are presented as mean (SD);
Includes children with attention-deficit/hyperactivity disorder.
X refers to crossover studies; P, parallel-group studies; QA, quality assessment; DLMO, dim-light melatonin onset; SOT, sleep-onset time; SOL, Sleep-onset latency; TST, total sleep time; WUT, Wake-up time; SAL, saliva; PL, plasma; LOG, diary; ACT, actigraphy; PSG, polysomnography.
Data Extraction
For this study, we extracted from each paper the number of patients in the placebo and in the melatonin groups (for the crossover studies this means seemingly the doubling of patients included in the study), the method of data collection (actigraphy, polysomnography or diary [also called somnolog or sleeplog]), the baseline DLMO (if available), study design, melatonin dose, duration of melatonin treatment, time of medication administration, and the available outcome measures after treatment (endpoints): DLMO, SOT, WUT, SOL, and TST. Extraction was checked by other authors.
For calculation reasons, clock times (applicable to DLMO, SOT, and WUT) were transformed to hour fractions, and, for clock times after 00:00, 24 was added; time spans (SOL and TST) were transformed to the number of minutes. The DLMO, SOT, and WUT results are therefore expressed in decimal hours, and SOL and TST results are expressed in minutes.
This meta-analysis was calculated with reported endpoints for melatonin treatment and placebo treatment for both crossover and parallel studies. This approach is different from the outcome measures in most of the original publications. All of the parallel and several of the crossover studies used the change of a parameter from baseline to treatment as the outcome measures for melatonin and placebo interventions. This subject will be further elucidated in the discussion section.
Data Analysis
Because all of the studies compared melatonin with placebo, we considered our study to be a direct head-to-head comparison of 2 treatments (placebo vs melatonin).
The meta-analysis was performed using the free software program MIX (Meta-analysis with Interactive eXplanations, version 1.739,40). The descriptive method was used; the input was the results calculated for the DLMO, SOT, WUT, SOL, and TST, as described above. All of these measures could therefore be defined as continuous (noted by decimal hours or minutes), by utilizing the random-effects method. To make the adjustment correct for the variance in the number of participants, we used the inverse variance method.41 The α level was set at 0.05 for each outcome.
We assessed, per parameter, the overall mean difference, the 95% confidence interval (CI), and the z score. First, those studies that had been conducted with adults17,42–45 and with children46–49 were separately assessed, whereas the crossover and parallel studies were done simultaneously. Second, studies were assessed according to the data collection (polysomnography, diary or actigraphy), and they were combined once again for the 2 types of studies (crossover and parallel), irrespective of age.
We present the results in standard forest plots containing the mean differences, the 95% CI, the weight of each study, and the pooled analysis. SPSS 15.0 (SPSS inc. 2006) was used for analyzing the DLMO changes in relationship to to the time of melatonin administration. Linear analysis of variance regression and curve fit were applied.
RESULTS
Study Characteristics
In total, 9 placebo-controlled studies on melatonin in DSPD met our inclusion criteria, 5 of which involved 91 adults17,42–45 and 4 of which involved 226 children, aged 6 years to adolescence46–49 (Table 1). The mean quality score was 4.0 out of 5 (range 3–5) based on the Jadad scale and 26 out of 32 (range 19–31) on the Downs and Black checklist. Concealment of allocation was adequate in all 9 studies. A funding source was described in 7 of the studies in which funding was received from public sponsors.
Four studies in adults and 1 study49 in children were crossover studies. In 2 crossover studies in adults, the participants received the trial medication during 2 subsequent periods of 4 weeks, separated by a 1-week washout period.17,42 The other 2 crossover studies in adults supplied the trial medication during 2 subsequent periods of 2 weeks, which were not separated by a washout period45 or the washout period was for only 1 day;43 in the crossover trial in children, the participants received melatonin for 2 ten-day periods, separated by 5 days of washout.49 The remaining study in adults44 and the 3 studies in children were parallel-group studies, and the duration of the treatment in all of the parallel-group studies was 4 weeks.
Dose
In 7 of the studies, a 5-mg dose of melatonin was administered. One study in adults compared the efficacy of 0.3 mg of melatonin with the efficacy of 3 mg of melatonin and with placebo.44 One study in children differentiated the dose according to bodyweight: children weighing less than 40 kg received 3 mg, those weighing 40 kg or more received 6 mg.48 In this meta-analysis, the dose was not taken into account.
Measurements
Dim-light melatonin onset
DLMO was determined by measuring the melatonin in plasma in 2 studies,43,45 in which 1 of these43 combined plasma samples with saliva samples; 4 studies exclusively used saliva for DLMO determination. Two studies evaluated endogenous melatonin production using an analysis of melatonin metabolites in urine,17,42 1 in combination with a 24-hour melatonin curve in plasma.17 All of the baseline DLMOs are depicted in Table 1.
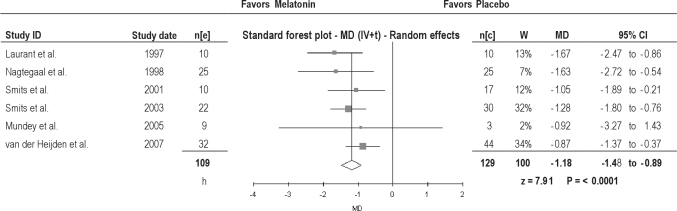
Melatonin significantly advanced DLMO: 1.69 hours in adults and 1.13 hours in children (Table 2); overall, 1.18 hours (Figure 2).
Table 2.
Study outcomes, differentiated between studies with adults17,42–45 and those with children46–49 with delayed sleep phase disorder
| Outcome variable | Adults |
Children |
||||
|---|---|---|---|---|---|---|
| Studies, no./ participants, no. | Mean difference (95% CI) | z score | Studies, no./ participants, no. | Mean difference (95% CI) | z score | |
| DLMO | 3/82 | −1.69 h (−2.31 to −1.07) | 5.34a | 3/155 | −1.13 h (−1.47 to −0.80) | 6.62b |
| SOT | 5/111 | −0.70 h (−1.04 to −0.36) | 4.08a | 4/193 | −0.64 h (−0.93 to −0.36) | 4.42a |
| WUT | 2/27 | −0.95 h (−3,25 to 1.36) | 0.8c | 3/168 | −0.16 h (−0.33 to 0.02) | 1.76c |
| SOL | 4/111 | −30.28 min (−63.29 to 2.74) | 1.80c | 4/206 | −16.04 min (−23.77 to −8.32) | 4.07a |
| TST | 3/67 | 0.77 min (−33.87 to 35.42 | 0.04c | 3/168 | 28.39 min (13.06 to 43.72) | 3.36b |
DLMO refers to dim-light melatonin onset; SOT, sleep-onset time; WUT, wake-up time; SOL, sleep-onset latency; TST, total sleep time.
P < 0.0001;
P < 0.001;
Not significant
Figure 2.
Dim Light Melatonin Onset in patients with delayed sleep phase disorder. MD, Mean difference (h); IV, inverse variance; t, estimate of the between study variance where the weight (W) given to each study is calculated by the inverse sum of the within study and between study variance estimates.
Sleep-onset time
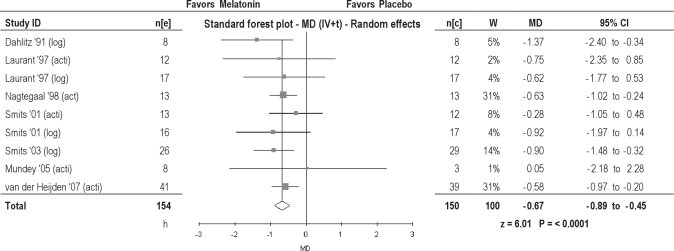
Three studies measured SOT using actigraphy, and 2 studies used data collected from a diary. Two studies applied both methods to assess SOT; the results of both measurements are used in this meta-analysis. Melatonin significantly advanced SOT: 0.70 hours in adults, and 0.64 hours in children (Table 2). Overall, an advance of 0.67 hours was found (Figure 3). The SOT outcomes assessed by actigraphy and diary were both statistically significant but differed clinically: 0.57 hours for actigraphy versus 0.94 hours using sleep-diary data (Table 3).
Figure 3.
Clock hour of sleep onset in patients with delayed sleep phase disorder. MD, Mean difference (h); IV, inverse variance; t, estimate of the between study variance where the weight (W) given to each study is calculated by the inverse sum of the within study and between study variance estimates.
Table 3.
Study outcomes, differentiated based on measures obtained with sleep diaries and with actigraphy of patients with delayed sleep phase disorder
| Outcome variable | Diary |
Actigraphy |
||||
|---|---|---|---|---|---|---|
| Studies, no./ participants, no. | Mean difference (95% CI) | z score | Studies, no./ participants, no. | Mean difference (95% CI) | z score | |
| SOT | 4/138 | −0.94 h (−1.37 to −0.52) | 4.39a | 5/166 | −0.57 h (−0.82 to −0.31) | 4.37a |
| SOL | 3/126 | −14.75 min (−24.21 to −5.29) | 3.06b | 2/91 | −11.05 min (−26.93 to 4.83) | 1.36c |
| TST | 3/104 | 12.98 min (−32.98 to 58.94) | 0.553c | 2/91 | 17.25 min (−0.602 to 35.10) | 1.89c |
SOT refers to sleep-onset time; SOL, sleep-onset latency; TST, total sleep time.
P < 0.0001;
P < 0.001;
Not significant
Wake-up time
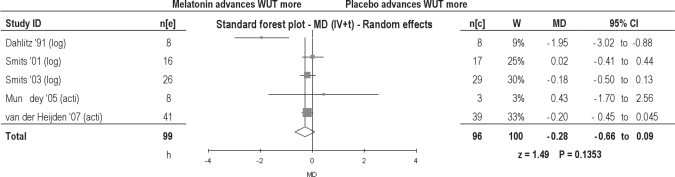
Two studies measured WUT by using actigraphy; 3 studies used data collected from a diary. Mean WUT was advanced in both adults (0.95 h) and children (0.16 h), but both were found to be statistically insignificant (Table 2). Overall, an insignificant advance of 0.28 hours was found (Figure 4).
Figure 4.
Wake up time in patients with delayed sleep phase disorder. MD, Mean difference (h); IV, inverse variance; t, estimate of the between study variance where the weight (W) given to each study is calculated by the inverse sum of the within study and between study variance estimates.
Sleep-onset latency
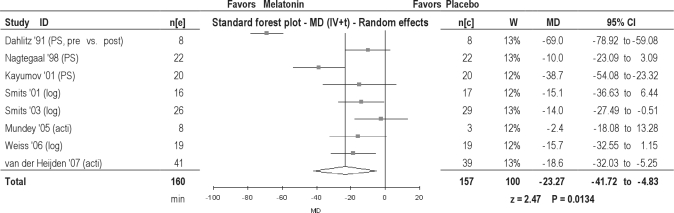
Three studies measured SOL using polysomnography, 3 studies used data collected from a diary, and 2 studies measured SOL by using actigraphy. The reduction in SOL with melatonin was statistically significant in children (by 16.04 min) but insignificant in adults (by 30.28 min) (Table 2). Overall, a statistically significant reduction of 23.27 minutes was found when data from adults and children were combined (Figure 5). When measured with data from only sleep diaries, the reduction in SOL was statistically significant (a reduction of 20.20 min); with actigraphy, a statistically insignificant reduction of 15.47 minutes was found (Table 3).
Figure 5.
Sleep onset latency in patients with delayed sleep phase disorder. MD, Mean difference (h); IV, inverse variance; t, estimate of the between study variance where the weight (W) given to each study is calculated by the inverse sum of the within study and between study variance estimates.
Total sleep time
One study measured TST with polysomnography, 2 studies used data collected from a diary, and 2 studies determined TST by actigraphy. Melatonin prolonged TST statistically significantly in children ((by 28.39 min) but insignificantly (statistically and clinically) in adults (by 0.77 min) (Table 2). Overall, a statistically significant prolongation of TST of 16.23 min was found (Figure 6). The TST outcomes of diary collection alone (by 17.54 min) or actigraphy alone (by 17.25 min) were all statistically insignificant (Table 3).
Figure 6.
Total sleep time in patients with delayed sleep phase disorder. MD, Mean difference (h); IV, inverse variance; t, estimate of the between study variance where the weight (W) given to each study is calculated by the inverse sum of the within study and between study variance estimates.
Safety and Adverse Events
Four studies did not report any adverse events.42–45 Headaches were reported in all 5 of the studies that reported patients experiencing adverse events: 2 out of 20 patients in the melatonin-treatment group had headaches46; 3 out of 53 in the melatonin-treatment group, irrespective of dose48; 1 out of 8 participants17 and 1 with severe migraine (treatment not specified) out of 19.49 One study reported headaches exclusively in the placebo group.47 In general, 6% or 7% of subjects reported having headaches during melatonin treatment. Other adverse events during melatonin treatment, and not during placebo treatment, were feeling cold (8 out of 5050 and unspecified numbers47), a mood dip (numbers not specified),47 and a decrease in appetite (numbers not specified).47 Dizziness during melatonin treatment was reported in 2 studies: 2 out of 53 participants48 and not quantified.47
In 1 patient, melatonin treatment was associated with an incidence of elevated alkaline phosphatase levels, although the elevation was almost reversed after 20 weeks of continued melatonin treatment.17 One patient developed a mild case of generalized epilepsy 4 months later after having started melatonin treatment and was treated with valproate.46
Timing of Melatonin Administration in Relationship to the Effect Size
We analyzed the relationship between the mean time of medication administration and the mean difference in after-treatment DLMO between melatonin and placebo groups. Due to the very limited amount of data available from our studies (n = 5, we excluded the study of Mundey et al.44 because of the very broad range of timing and the very small group size of placebo), the hypothesis that early administration enhances the DLMO shift properties of melatonin could not be statistically confirmed (P = 0.307). The resulting β coefficient of 0.578, representing the correlation coefficient for 2 variables, suggests that significance can be obtained by using an n of 12; indeed, more studies are needed.
When the pretreatment DLMO values were taken into account, as was the difference between the pretreatment and the posttreatment DLMO, the DLMO value (the DLMO shift) of the melatonin and placebo groups were evaluated in relation to the time of administration in all 6 studies, whereby the correlation coefficient increased to 0.614 but remained statistically insignificant (P = 0.065).
DISCUSSION
This meta-analysis concerns the effectiveness of exogenous melatonin in patients with DSPD, and it demonstrates that appropriately timed administration of exogenous melatonin does advance endogenous melatonin onset (DLMO) and sleep onset in both children and adults. Although melatonin decreased SOL in children, this was not the case in adults. Melatonin did extend sleep duration (TST) in children, but this was also not the case in adults. Finally, the WUT was not influenced.
Half of the studies assessed children in this review. We found differences between adults and children in melatonin efficacy in 2 measures: SOL and TST. This can be partly attributed to the disciplinary set routine in which children are brought to bed, whereas adults are more at liberty to choose their own bedtime, and, as a result, probably experience fewer problems regarding SOL and TST. The efficacy in children influenced the overall efficacy in this meta-analysis on both parameters.
Several methods of measurement were used to determine endpoints: polysomnography, actigraphy, and diary. Recently, Werner et al. reported rates of agreement among actigraphy, a diary, and a questionnaire for recording sleep patterns in children.51 The method chosen did not significantly influence the results in regard to the SOL and TST. Sleep-onset results, however, did differ: sleep-onset amelioration was much better when it was recorded in a diary than with actigraphy. This might be due to the direct hypnotic effects of melatonin, noticeable during low endogenous melatonin levels, and which can influence the subjective sense of being awake, while not influencing the more objective actigraphic measurement. Nevertheless, it reflects a positive feeling experienced by participants when falling asleep.
The use of melatonin was safe with respect to experienced adverse events in the treatment of DSPD, at least in the short-term treatment. This finding corresponds with the results of other studies that used melatonin.27 In a recently published evaluation of clinical experience in which melatonin treatment was prescribed by a pediatrician for 107 children,52 parents reported, after 1.8 ± 1.4 years of treatment, on the adverse effects found in 3 children: morning sleepiness, “fogginess,” and increased enuresis. There was no increase in the number of seizures that occurred in children with preexisting epilepsy nor were there any onset seizures.52
The evidence found in advancing the sleep-wake rhythm is contrary to the findings of Buscemi et al.,27 who could not demonstrate the efficacy of melatonin. The present study included approximately the same number of studies (9) and participants (317) as the meta-analysis of Buscemi et al.27 (9 and 297, respectively). Consequently, the number of studies and participants does not account for the difference between our findings and those of Buscemi et al. This difference in findings might be attributed to the properties of melatonin. In our studies, we focused on the chronobiotic properties of melatonin,12 which become evident when melatonin is administered only a few hours before endogenous melatonin onset. This chronobiotic effect was evidently expressed by the more than 1-hour advancement of endogenous melatonin onset and the advancement of the sleep-wake rhythm.
In the studies of Buscemi et al.,27 the time of administration was not taken into account. When there is a short interval between the administration of melatonin and the endogenous melatonin onset, the advancement of biologic clock can be expected to be statistically and clinically insignificant. Therefore, they may have assessed the hypnotic properties of melatonin, which might even have been absent in some studies, if melatonin was administered after the onset of endogenous melatonin.
Another reason for suboptimal effects of melatonin treatment can also be found in the close relationship between the dose, the timing, and the resulting melatonin levels found in the blood. When too high a dose is administered and when it is given too late with respect to endogenous melatonin onset, this might result in melatonin levels persisting through the early morning. This could even result in delaying DLMO instead of advancing it' If these thoughts are taken into account, one might even theorize about enhancing the efficacy of melatonin treatment by advancing the administration of melatonin every few days to prolong the advancing effect until the optimal rhythm has been reached. This is comparable to the strategy that Lewy et al. applied in the entrainment of the blind.8
A methodologic difference between our study and the meta-analysis of Buscemi27 is that Buscemi and coworkers used endpoints for the crossover studies with their own standard deviation (SD) and the changes from baseline for parallel studies with calculated SDs, when necessary.
The diagnosis DSPD remains a point of discussion. The clinical symptoms described as an “inability to fall asleep at conventional times” and in experiencing “difficulty in waking at conventional times in the morning” have not been precisely defined. The DLMO assessment might be helpful in diagnosing DSPD, as DLMO occurs late in this circadian-rhythm disorder. The American Academy of Sleep Medicine6 did not adopt DLMO assessment as a diagnostic tool because this measurement had probably not yet become widely available. In addition, a delayed DLMO, as such, could result from several other conditions, such as shift work or traveling. Furthermore, in healthy subjects, melatonin levels and melatonin onset vary with age,53 and DLMO can occur several hours apart among individuals who do not have any underlying pathology.
However, after a diagnosis of DSPD is made, assessment of the DLMO may be useful when evaluating the effects of initiated therapy. Furthermore, by establishing the most desirable timing for administering melatonin when considering the applied dose, DLMO assessment can also serve as a parameter for predicting the efficacy of a treatment using melatonin, thus preventing initiation in patients without anticipating any benefits.
The definition of DLMO is currently being discussed, as mentioned earlier.11 Consequently, a task force of the European Sleep Research Society was set up in 2006 to redefine DLMO. Once DLMO has been defined more precisely, it will be possible to agree on generally accepted standards.
A well-defined DLMO will not only help in diagnosing DSPD, but also will enable treatment to be more customized. A study in adults44 confirmed Lewy's22 finding that melatonin shifts the circadian rhythm the most when administered 5 to 6 hours before DLMO. Furthermore, a study in children20 showed that the earlier melatonin was administered with respect to DLMO, the more both SOT and the DLMO were advanced. This study applied high doses. The current meta-analysis, which consists of studies in which similarly high dosages were administered, cannot confirm this finding, but an analysis made of the DLMO shift suggests that the DLMO shift is enhanced when administered earlier.
This is the first meta-analysis of melatonin studies in which circadian timing was measured or accurately predicted before treatment. The quality of the studies was thoroughly assessed. Nevertheless, several limitations still need to be considered. The number of studies conducted in adults, as well as the number of participants, was rather low. Moreover, most of the studies conducted in adults were crossover studies. A carry-over effect in these studies cannot be excluded. However, most of the findings obtained from these studies correspond with those of the parallel-group studies carried out in children.
In the studies of Smits et al.,46,47 ADHD was diagnosed in 25% to 50% of the children, whereas, in the other 2 studies in children, ADHD was diagnosed in 100% of the participants. These individuals could fall under the category of circadian rhythm sleep disorder comorbid with ADHD. The separate DLMO and SOT results in children and adults were significant (Table 2). In children, the advancement of DLMO and SOT was less than in adults, which might be caused by the fact that the children represent a different clinical population than do the adults, who have a typical DSPD.
Because of the larger number of participants in the studies of children, the SDs were also lower, which therefore means that the children's groups contributed to a generally greater significant result for both DLMO and SOT (Figure 2 and Figure 4). For SOL, the general significant result (Figure 5) did not persist when data from children were excluded, as illustrated in Table 2 in the results of adults. Finally, 3 out of the 4 studies in children were performed by the same research group. This might indicate a sampling bias.
Only 3 studies used the gold standard, polysomnography, to establish the effects that melatonin had on sleep. However, the other methods, i.e., actigraphy and keeping a sleep diary, are reliable methods for establishing shifts in sleep-wake rhythm.54
This meta-analysis employed reported endpoints for melatonin treatment and placebo treatment. This differs from the original work conducted in all of the parallel and some of the crossover studies, in which the change of a parameter from baseline to treatment was used as the outcome measures for both melatonin and placebo interventions. For crossover studies, one can postulate that baseline corrections are not needed, since the same population underwent both treatments.
For the parallel studies, on the other hand, by reason of a presumed heterogeneity between both groups, it is common to depict the outcomes as changes from baseline. In reality, when pretreatment and posttreatment group results are used to determine this change instead of individual values, 2 SD values (SD of the pretreatment and posttreatment values in children) will determine the SD for the resulting change value.
In 1 paper, this value was given44; however, for the other 3 parallel studies, this value still needed to be calculated. This latter extensive method is comparable to the method of “adjusted indirect comparison of two different treatments versus placebo.”55 It assumes heterogeneity between study populations. If one should decide to compute the changes according to the extensive method, then one should also perform the same calculations for the resulting SD of the calculated difference for the crossover studies, since the baseline values of those studies are also characterized by a SD. For all of the crossover studies, this value needs to be calculated.
Although it is possible to compute these SDs by making a few assumptions, we decided it would be more straightforward to use all of the endpoints from the studies included, since these were all available, including the SD, assuming there is homogeneity in the treatment groups of the parallel studies. Therefore, our approach relies more on published data and depends less on calculations that inevitably introduce measurement error. However, we also analyzed the results using the extensive method and subsequently compared them to our method. We found no differences for the pooled DLMO result and only a small, but insignificant, difference in the pooled results of SOT and SOL. Nevertheless, the 2 methods showed different results regarding the effect of several studies on TST and WUT (although the significance of the pooled results of both methods was the same). This outcome was mainly attributable to very large baseline differences in 1 study using only 3 placebo patients.44
Another limitation was that the influence of melatonin on the daily health status was not assessed. More studies are needed to confirm the findings of the placebo-controlled trial in children46 and the open-label study in adults,56 thus suggesting that melatonin improves the quality of life.
The relatively small change in clock-hour sleep onset of 0.67 hours could raise questions about its clinical significance. However, the general treatment effect is limited by several factors. Firstly, the optimal dose has not yet been established, and some of the previous studies applied high doses. Secondly, the optimal timing of the administration was not applied in most of the trials, nor was it adapted (further advancing the administration time) during therapy.
We suggest that an optimal melatonin therapy be based on 3 basic principles, namely, by identifying the appropriate patients (with a delayed biologic timing); by melatonin administration being based on biologic clock time, i.e., 3 to 6 hours before DLMO (CT8-11); and by administering a small dose to avoid enhanced high melatonin levels during late night or early morning. To further optimize individual therapy, the results in which the administration time is advanced during the course of treatment should also be considered.
Currently, our findings can help to guide clinicians and patients in making decisions regarding the use of exogenous melatonin in the treatment of DSPD. The main conclusion we have been able to draw from our study is that, in cases in which a dose of melatonin has been administered in DSPD, it has been found to be effective, and that this dose should be kept as low as possible and administered as early as tolerable. Further research concerning the optimal timing of when to administer the dose in regard to DLMO shift would be warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
All of the authors contributed equally to this manuscript. No sponsorship was received, and no other outside source had any role in the design of the study or collection, management, or interpretation of the data, nor was there any external involvement in the decision to submit the manuscript for publication.
Ingeborg M. van Geijlswijk had full access to all of the data provided in the study, and she assumes responsibility for the integrity of the data and the accuracy of the data analysis.
We thank S.R. Pandi-Perumal and A.C.G Egberts for their helpful discussions and for critically reading the manuscript.
Footnotes
A commentary on this article appears in this issue on page 1581.
REFERENCES
- 1.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Weitzman ED, Czeisler CA, Coleman RM, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry. 1981;38:737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 3.Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry. 1995;152:602–8. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- 4.Czeisler CA, Richardson GS, Coleman RM, et al. Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4:1–21. doi: 10.1093/sleep/4.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13:354–61. [PubMed] [Google Scholar]
- 6.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner DR. Disorders of the circadian sleep-wake cycle. Neurol Clin. 1996;14:651–70. doi: 10.1016/s0733-8619(05)70278-4. [DOI] [PubMed] [Google Scholar]
- 8.Lewy AJ, Emens JS, Bernert RA, Lefler BJ. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: clinical implications. J Biol Rhythms. 2004;19:68–75. doi: 10.1177/0748730403259670. [DOI] [PubMed] [Google Scholar]
- 9.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 10.van Someren EJ, Nagtegaal E. Improving melatonin circadian phase estimates. Sleep Med. 2007;8:590–601. doi: 10.1016/j.sleep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Wirz-Justice A, Armstrong SM. Melatonin: nature's soporific? J Sleep Res. 1996;5:137–41. [PubMed] [Google Scholar]
- 13.Cardinali DP, Furio AM, Reyes MP, Brusco LI. The use of chronobiotics in the resynchronization of the sleep-wake cycle. Cancer Causes Control. 2006;17:601–9. doi: 10.1007/s10552-005-9009-2. [DOI] [PubMed] [Google Scholar]
- 14.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 15.Luboshizsky R, Lavie P. Sleep-inducing effects of exogenous melatonin administration. Sleep Med Rev. 1998;2:191–202. doi: 10.1016/s1087-0792(98)90021-1. [DOI] [PubMed] [Google Scholar]
- 16.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561:339–51. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337:1121–4. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 18.Dagan Y, Yovel I, Hallis D, Eisenstein M, Raichik I. Evaluating the role of melatonin in the long-term treatment of delayed sleep phase syndrome (DSPS) Chronobiol Int. 1998;15:181–90. doi: 10.3109/07420529808998682. [DOI] [PubMed] [Google Scholar]
- 19.Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res. 2009;47:1–7. doi: 10.1111/j.1600-079X.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijden KB, Smits MG, van Someren EJ, Gunning WB. Prediction of melatonin efficacy by pretreatment dim light melatonin onset in children with idiopathic chronic sleep onset insomnia. J Sleep Res. 2005;14:187–94. doi: 10.1111/j.1365-2869.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 21.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586:639–47. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 23.Lockley SW. Timed melatonin treatment for delayed sleep phase syndrome: the importance of knowing circadian phase. Sleep. 2005;28:1214–6. doi: 10.1093/sleep/28.10.1214. [DOI] [PubMed] [Google Scholar]
- 24.Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19:649–58. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- 25.Wise ME. Does melatonin improve sleep? Muddles with melatonin. BMJ. 2006;332:550. doi: 10.1136/bmj.332.7540.550-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourtillan JB, Brisson AM, Gobin P, Ingrand I, Decourt JP, Girault J. Bioavailability of melatonin in humans after day-time administration of D(7) melatonin. Biopharm Drug Dispos. 2000;21:15–22. doi: 10.1002/1099-081x(200001)21:1<15::aid-bdd215>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Buscemi N, Vandermeer B, Hooton N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–93. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendt J. Does melatonin improve sleep? Efficacy of melatonin. BMJ. 2006;332:550. doi: 10.1136/bmj.332.7540.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeinberg A, Borodkin K, Dagan Y. Melatonin treatment in adolescents with delayed sleep phase syndrome. Clin Pediatr. 2006;45:809–18. doi: 10.1177/0009922806294218. [DOI] [PubMed] [Google Scholar]
- 30.Owens JA. A clinical overview of sleep and attention-deficit/hyperactivity disorder in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2009;18:92–102. [PMC free article] [PubMed] [Google Scholar]
- 31.van der Heijden KB, Smits MG, van Someren EJ, Gunning WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22:559–70. doi: 10.1081/CBI-200062410. [DOI] [PubMed] [Google Scholar]
- 32.van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67:1091–6. doi: 10.1016/j.biopsych.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 34.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 36.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317:1185–90. doi: 10.1136/bmj.317.7167.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 38.Verhagen AP, de Vet HC, Vermeer F, et al. The influence of methodologic quality on the conclusion of a landmark meta-analysis on thrombolytic therapy. Int J Technol Assess Health Care. 2002;18:11–23. [PubMed] [Google Scholar]
- 39.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. MIX: comprehensive free software for meta-analysis of causal research data. Version 1.7. Available at: http://mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 41.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-analysis in Medical Research. Chichester, UK: Wiley Europe; 2000. [Google Scholar]
- 42.Kayumov L, Brown G, Jindal R, Buttoo K, Shapiro CM. A randomized, double-blind, placebo-controlled crossover study of the effect of exogenous melatonin on delayed sleep phase syndrome. Psychosom Med. 2001;63:40–8. doi: 10.1097/00006842-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Laurant M, Nagtegaal JE, Smits MG, Kerkhof GA, Coenen AML. Influence of melatonin on vigilance and cognitive functions in delayed sleep phase syndrome. Int Chronobiol. 1997;8:79–82. [Google Scholar]
- 44.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–8. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 45.Nagtegaal JE, Kerkhof GA, Smits MG, Swart AC, van der Meer YG. Delayed sleep phase syndrome: a placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7:135–43. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 46.Smits MG, Nagtegaal JE, van der Heijden J, Coenen AM, Kerkhof GA. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol. 2001;16:86–92. doi: 10.1177/088307380101600204. [DOI] [PubMed] [Google Scholar]
- 47.Smits MG, van Stel HF, van der Heijden K, Meijer AM, Coenen AM, Kerkhof GA. Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomized placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2003;42:1286–93. doi: 10.1097/01.chi.0000085756.71002.86. [DOI] [PubMed] [Google Scholar]
- 48.van der Heijden KB, Smits MG, van Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46:233–41. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 49.Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry. 2006;45:512–9. [PubMed] [Google Scholar]
- 50.Wasdell MB, Jan JE, Bomben MM, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res. 2008;44:57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 51.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children's sleep patterns. Arch Pediatr Adolesc Med. 2008;162:350–8. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- 52.Andersen IM, Kaczmarska J, McGrew SG, Malow BA. Melatonin for insomnia in children with autism spectrum disorders. J Child Neurol. 2008;23:482–5. doi: 10.1177/0883073807309783. [DOI] [PubMed] [Google Scholar]
- 53.Zhou JN, Liu RY, van Heerikhuize J, Hofman MA, Swaab DF. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J Pineal Res. 2003;34:11–6. doi: 10.1034/j.1600-079x.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 54.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 55.Gartlehner G, Moore CG. Direct versus indirect comparisons: a summary of the evidence. Int J Technol Assess Health Care. 2008;24:170–7. doi: 10.1017/S0266462308080240. [DOI] [PubMed] [Google Scholar]
- 56.Nagtegaal JE, Laurant MW, Kerkhof GA, Smits MG, van der Meer YG, Coenen AM. Effects of melatonin on the quality of life in patients with delayed sleep phase syndrome. J Psychosom Res. 2000;48:45–50. doi: 10.1016/s0022-3999(99)00075-6. [DOI] [PubMed] [Google Scholar]