Abstract
Background:
Sleep complaints are highly prevalent and associated with cardiovascular disease (CVD) morbidity and mortality. This is the first prospective study to report the association between commonly reported sleep symptoms and the development of the metabolic syndrome, a key CVD risk factor.
Methods:
Participants were from the community-based Heart Strategies Concentrating on Risk Evaluation study. The sample was comprised of 812 participants (36% African American; 67% female) who were free of metabolic syndrome at baseline, had completed a baseline sleep questionnaire, and had metabolic syndrome evaluated 3 years after baseline. Apnea-hypopnea index (AHI) was measured cross-sectionally using a portable monitor in a subset of 290 participants. Logistic regression examined the risk of developing metabolic syndrome and its components according to individual sleep symptoms and insomnia syndrome.
Results:
Specific symptoms of insomnia (difficulty falling asleep [DFA] and “unrefreshing” sleep), but not a syndromal definition of insomnia, were significant predictors of the development of metabolic syndrome. Loud snoring more than doubled the risk of developing the metabolic syndrome and also predicted specific metabolic abnormalities (hyperglycemia and low high-density lipoprotein cholesterol). With further adjustment for AHI or the number of metabolic abnormalities at baseline, loud snoring remained a significant predictor of metabolic syndrome, whereas DFA and unrefreshing sleep were reduced to marginal significance.
Conclusion:
Difficulty falling asleep, unrefreshing sleep, and, particularly, loud snoring, predicted the development of metabolic syndrome in community adults. Evaluating sleep symptoms can help identify individuals at risk for developing metabolic syndrome.
Citation:
Troxel WM; Buysse DJ; Matthews KA; Kip KE; Strollo PJ; Hall M; Drumheller O; Reis SE. Sleep symptoms predict the development of the metabolic syndrome. SLEEP 2010;33(12):1633-1640.
Keywords: Metabolic syndrome; sleep-disordered breathing; insomnia, cardiovascular risk
THE CLUSTERING OF CARDIOVASCULAR RISK FACTORS KNOWN AS THE METABOLIC SYNDROME IS STRONGLY AND PROSPECTIVELY LINKED WITH incident cardiovascular events, diabetes, and mortality.1 Given that prevalence rates of the metabolic syndrome are estimated at 20% in the adult population,2 identifying modifiable risk factors associated with the development of the metabolic syndrome is of critical public health importance.
Several prospective studies have documented an independent relationship between sleep disturbances, including sleep disordered breathing (SDB) and sleep duration, and increased risk of developing individual components of the metabolic syndrome, including obesity, hypertension, glucose intolerance, and diabetes.3–8 Additionally, a handful of cross-sectional studies have shown that a broader range of self-reported sleep disturbances, including snoring, sleep duration, difficulty initiating and maintaining sleep, and poor sleep quality, as well as polysomnographically assessed sleep architecture are associated with prevalent metabolic syndrome.9–13 However, to our knowledge, no study to date has prospectively examined the relationship between sleep disturbances that commonly present in clinical practice and the development of the metabolic syndrome. Given evidence that there may be added prognostic value of the metabolic syndrome, over and above its individual components, and the inability of cross-sectional studies to support the proposed direction of causality between sleep disturbances and metabolic dysregulation, prospective evidence is clearly needed to examine whether sleep disturbances predict the development of the metabolic syndrome.
Most epidemiologic studies of sleep and CVD risk have examined sleep symptoms rather than sleep disorders identified by diagnostic criteria, such as insomnia or obstructive sleep apnea syndrome (OSAS), which are the two most common adult sleep disorders.14 Understanding the relative impact of sleep symptoms versus sleep disorders may have important public health implications given that symptoms are much more prevalent than disorders,14 and the putative mechanisms linking sleep with cardiometabolic consequences may differ for symptoms and disorders. Moreover, there is considerable overlap in SDB and insomnia symptoms. Thus, to better understand the pathophysiology underlying links between sleep symptoms and cardiometabolic risk, it is critical to examine the independent effects of respective insomnia and SDB symptoms as well as more formally defined disorders.
The present study investigated the degree to which insomnia or SDB predicted the development of the metabolic syndrome and its component factors (hyperglycemia, central adiposity, hypertension, hypertriglyceridemia, and low high density lipoprotein cholesterol) over a 3-year period in a community sample. We used two different case definitions for both insomnia and SDB. For insomnia, we examined individual insomnia symptoms as well as insomnia syndrome, which included a sleep complaint along with reported daytime impairment. For SDB, we examined whether snoring, the most common symptom of OSA, predicted the development of the metabolic syndrome, independent of apnea-hypopnea index, a physiological indicator of OSA. Given racial and gender differences in the prevalence of sleep disorders and the metabolic syndrome, for significant effects, we explored whether relationships between sleep symptoms and the metabolic syndrome differed among men and women and among blacks and whites.
METHODS
Study Overview and Population
Participants were recruited from Heart SCORE, an ongoing, community-based prospective study (N = 2000) designed to examine the differential effects of race and gender on cardiovascular risk. Subject eligibility criteria for Heart SCORE included age 45 to 74 years, residence in the greater Pittsburgh metropolitan area, ability to undergo baseline and annual follow-up visits, and absence of known comorbidity expected to limit life expectancy to less than 5 years. For the present study, we further excluded a small number of participants (n = 67) who self-identified as race other than black or white (due to our interest in examining racial differences in the effects), those who did not have the metabolic syndrome evaluated at baseline (n = 86), those who were classified as having the metabolic syndrome or diabetes at baseline (n = 598), or those who were missing sleep or covariate data at baseline or metabolic syndrome at follow-up (n = 437). Thus, the final sample consisted of 812 participants who met study eligibility criteria and had key study variables assessed. Follow-up analyses which additionally adjusted for apnea-hypopnea index (AHI) were conducted in a subset of 294 participants who had volunteered to participate in the home apnea screening protocol at their annual visit, were not currently being treated for OSA, did not self-report a diagnosis of OSA, and had other key study variables available. In addition, n = 4 individuals were excluded due to having AHIs > 50 (> 3 SDs from the mean). As compared to those who had AHI data available, those who did not have AHI evaluated were older and less likely to self-report frequent loud snoring during sleep (P values < 0.05). All subjects provided written informed consent approved by the Institutional Review Board of the University of Pittsburgh.
Data Collection
Metabolic syndrome
At the baseline and subsequent annual visits, a 12-h fasting blood draw was collected and anthropometric measures were taken. At baseline, assays were performed using standard techniques in the clinical laboratory of the University of Pittsburgh Medical Center except for plasma lipids and lipoprotein subfractions which were quantified by a commercial laboratory using a vertical auto profile (VAP, Atherotech, Birmingham, AL). During annual follow-up visits, measurements were taken of fasting glucose and lipids (HDL, total cholesterol, and triglycerides, with calculation of LDL) using standard clinical laboratory techniques (Cholesetch LDX System). Waist circumference was measured using a measuring tape at the narrowest point between the iliac crest and the lowest rib.
The primary outcome was the presence or absence of the metabolic syndrome at the 3rd year follow-up visit, as quantified by the National Cholesterol Education Program's (NCEP) Adult Treatment Panel III report (ATP III) guidelines.15 Specifically, the syndrome is defined by presence of ≥ 3 of the following risk factors: fasting glucose ≥ 110 mg/dL, triglycerides ≥ 150 mg/dL, HDL-C < 40 mg/dL (for males) or < 50 mg/dL (for females), waist circumference > 102 cm (for males) or > 88 cm (for females), systolic blood pressure (SBP) ≥ 130 mm Hg, or diastolic blood pressure ≥ 85 mm Hg. If participants had blood pressure or glucose values within the normal range but were taking antihypertensive or glucose-lowering medications, they were classified as meeting criteria for blood pressure or glucose abnormalities, respectively.
Sleep symptoms/ insomnia syndrome
Sleep disturbances related to insomnia and SDB were assessed via the Insomnia Symptom Questionnaire (ISQ) and the Multivariable Apnea Prediction Questionnaire (MAP).16 The ISQ is a self-report instrument designed to screen for symptoms experienced in the past month associated with the diagnostic criteria for primary insomnia. The ISQ has recently been validated117 using classical test theory and item response theory (IRT). In addition, to assessing specific sleep symptoms, the ISQ includes additional criteria related to daytime impairments consistent with diagnostic criteria for insomnia (e.g., “Have your sleep difficulties affected your work? Social life?”). The presence of insomnia syndrome was coded as 1 for individuals endorsing at least one insomnia-related sleep complaint (difficulty falling asleep, difficulty staying asleep, frequent awakenings, feeling sleep is not sound, or feeling sleep is unrefreshing), with frequency criteria ≥ 3 times per week and the endorsement of at least one symptom of daytime impairment, rated as moderate to extremely severe.
The SDB symptoms (e.g., loud snoring, choking, or gasping during sleep) were derived from the MAP, which has previously been validated and shown good psychometric properties.16 Items on the ISQ and MAP were re-coded such that individuals who endorsed the symptom ≥ 3 times per week were coded as 1 and others coded as 0.
Covariates
Detailed demographic and medical histories were collected at the baseline visit. In addition, lifestyle characteristics including smoking history (current or former smoker versus never a smoker), and alcohol consumption (> 4 drinks per week versus ≤4 drinks per week) were measured by study-specific questionnaires. Physical activity was assessed by the Lipids Research Clinics Questionnaire18 and analyzed as a categorical variable (sedentary versus non-sedentary lifestyle). Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale19 (coded as a binary outcome using the clinical cutoff ≥ 16 to indicate the presence of clinically significant depressive symptoms). Nested within the cohort study was an intervention study that randomly assigned subjects to receive in the initial year a usual care (“advice only” regimen or a behavioral modification intervention to reduce CVD risk. Study randomization assignment (usual care or behavioral modification within 1 year), was also included as a covariate in all models; however, analyses demonstrated that randomization was unrelated to either sleep symptoms or metabolic syndrome incidence.
Apnea-hypopnea index (AHI)
AHI was evaluated in a subset of 294 participants using a previously validated portable monitor that measures airflow and snoring via a nasal pressure signal20 (ApneaLink, ResMed Corp). An apnea was defined as a decrease in airflow of ≥ 80% from baseline for ≥ 10 sec. A hypopnea was defined as a decrease in airflow for ≥ 30% but < 80% from baseline for ≥ 10 sec. AHI was evaluated for participants who had ≥ 4 h of data available from the portable monitor and was analyzed as a continuous covariate, with 4 extreme outliers (> 3 standard deviations from the mean) removed for a final N of 290. Portable monitoring was added to the HeartSCORE protocol following the baseline assessment, and was collected throughout the first 3 years of the study. Thus, AHI was included in secondary analyses as a covariate to examine the independent contribution of self-reported SDB and insomnia symptoms (assessed at baseline) and development of the metabolic syndrome. Given the inability to establish temporal precedence of AHI prior to the metabolic syndrome assessment, AHI was considered only as a cross-sectional covariate and not as a prospective risk factor.
Statistical Methods
Differences in baseline demographic, psychosocial, lifestyle characteristics, and sleep measures were compared between those who developed the metabolic syndrome over the follow-up period versus those who did not develop the metabolic syndrome by use of χ2 tests for categorical variables and student t-tests for continuous variables. Multivariate logistic regression was used to examine the relationship between individual sleep symptoms or insomnia syndrome and risk of developing the metabolic syndrome, with adjustment for covariates including age, sex, race (white/ black), marital status (married or partnered/ unmarried or unpartnered), study randomization assignment, smoking history, physical activity, alcohol consumption, and depressive symptoms. To reduce the number of statistical comparisons, relationships among sleep symptoms and individual components of the metabolic syndrome were restricted to the sleep symptoms which predicted the metabolic syndrome. For each metabolic syndrome component, we excluded participants who met criteria for that component at baseline. We also evaluated whether observed relationships between sleep symptoms and metabolic syndrome risk differed among blacks and whites or among men and women, by entering the race and gender interaction terms in separate logistic regression models. To determine the relative contribution of SDB or insomnia symptoms to the prediction of metabolic syndrome, follow-up logistic regression models simultaneously entered insomnia or SDB-related symptoms that were related to the metabolic syndrome and also examined whether relationships persisted after further adjustment for AHI (in the subset with AHI available). Finally, for significant effects of sleep symptoms on the development of the metabolic syndrome, we examined the results of models that statistically adjusted for the number of baseline metabolic abnormalities.
RESULTS
Table 1 presents the frequency of metabolic abnormalities at baseline and at 3-year follow-up. The frequency of meeting criteria for individual metabolic syndrome components increased from baseline to follow-up for all components. Over the 3-year follow-up period, 14% of the sample (n = 115) developed the metabolic syndrome. As shown in Table 2, African Americans and participants endorsing a sedentary lifestyle were more likely to develop the metabolic syndrome over the 3 years. Of the sleep symptoms, difficulty falling asleep (DFA), unrefreshing sleep, and loud snoring significantly predicted the development of the metabolic syndrome at follow-up in unadjusted models (Table 3). Insomnia syndrome was not related to incident metabolic syndrome in unadjusted models. AHI was significantly associated with development of the metabolic syndrome.
Table 1.
Frequency of metabolic abnormalities at baseline and 3-year follow-up (N = 812)
| Metabolic Syndrome Abnormalities |
||
|---|---|---|
| Baseline % (n) |
3-year follow-up % (n) |
|
| BP abnormal | 52 (420) | 60 (489) |
| HDL abnormal | 9 (69) | 18 (145) |
| Triglycerides abnormal | 10 (78) | 15 (118) |
| Waist circumference abnormal | 36 (295) | 40 (325) |
| Glucose abnormal | 3 (28) | 9 (71) |
| Count of Metabolic Abnormalities | % (n) | % (n) |
| None | 27 (217) | 21 (170) |
| 1 metabolic abnormality | 37 (300) | 35 (283) |
| 2 metabolic abnormalities | 36 (295) | 30 (224) |
| 3 or more metabolic abnormalities | - | 14 (115) |
Table 2.
Sample characteristics according to the presence or absence of the metabolic syndrome at 3-year follow-up (N = 812)
| Metabolic Syndrome |
|||
|---|---|---|---|
| Absent (86%) % (n) |
Present (14%) % (n) |
P-value | |
| Gender | 0.92 | ||
| Male | 86 (227) | 14 (38) | |
| Female | 86 (470) | 14 (77) | |
| Race | 0.06 | ||
| African American | 83 (244) | 17 (51) | |
| Caucasian | 88 (453) | 12 (64) | |
| Married/ Partnered | 0.92 | ||
| Yes | 86 (454) | 14 (76) | |
| No | 86 (241) | 14 (39) | |
| Randomized to intervention | 0.65 | ||
| Yes | 85 (82) | 16 (15) | |
| No | 86 (615) | 14 (100) | |
| Current/ past smoker | 86 (635) | 14 (103) | 0.60 |
| Consumed ≥ 4 alcoholic drinks/week | 87 (101) | 13 (15) | 0.77 |
| Sedentary lifestyle | 80 (214) | 20 (55) | 0.00 |
| Depression (CESD ≥ 16) | 84 (82) | 16 (16) | 0.54 |
| Mean (SD) | Mean (SD) | ||
| Age | 59 (7.51) | 59 (6.84) | 0.76 |
Sample restricted to those who were free of the metabolic syndrome at baseline.
Table 3.
Sleep characteristics according to the presence or absence of the metabolic syndrome at 3-year follow-up (N = 812)
| Metabolic Syndrome |
|||
|---|---|---|---|
| Absent (86%) % (n) |
Present (14%) % (n) |
P-value | |
| Difficulty falling asleep | 0.01 | ||
| Yes | 78 (87) | 22 (25) | |
| No | 87 (610) | 13 (90) | |
| Difficulty staying sleep | 0.82 | ||
| Yes | 85 (186) | 15 (32) | |
| No | 86 (510) | 14 (83) | |
| Frequent awaking from sleep | 0.36 | ||
| Yes | 84 (271) | 16 (50) | |
| No | 87 (425) | 13 (65) | |
| Sleep is not sound | 0.09 | ||
| Yes | 82 (148) | 18 (33) | |
| No | 87 (549) | 13 (82) | |
| Sleep is unrefreshing | 0.02 | ||
| Yes | 80 (128) | 20 (32) | |
| No | 87 (569) | 13 (83) | |
| Snoring/gasping during sleep | 0.23 | ||
| Yes | 82 (85) | 18 (19) | |
| No | 86 (612) | 14 (96) | |
| Loud snoring | 0.001 | ||
| Yes | 75 (73) | 26 (25) | |
| No | 88 (620) | 12 (88) | |
| Stop breathing/choking during sleep | 0.12 | ||
| Yes | 74 (17) | 26 (6) | |
| No | 86 (678) | 14 (109) | |
| Insomnia syndrome | 0.17 | ||
| Yes | 81 (63) | 19 (15) | |
| No | 86 (634) | 14 (100) | |
| Mean (SD) | Mean (SD) | ||
| AHI (n = 290) | 9.0 (8.55) | 12.67 (9.06) | 0.01 |
Sample restricted to those who were free of the metabolic syndrome at baseline. Unless otherwise noted, values represent n (%) in each metabolic syndrome category for those who endorsed the sleep symptom “frequently or always” or who met the defined criteria for insomnia syndrome. AHI refers to apnea hypopnea index.
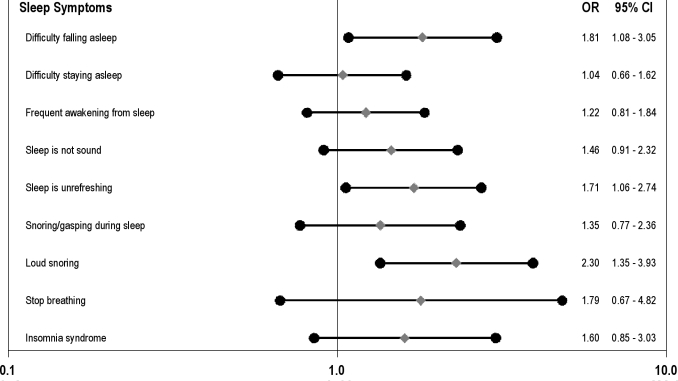
In the multivariate logistic regression models (Figure 1) which entered each symptom or insomnia syndrome in separate models, DFA, unrefreshing sleep, and loud snoring significantly predicted metabolic syndrome incidence (P < 0.05). There were no significant race or gender interactions for either DFA or loud snoring on metabolic syndrome incidence (analyses not shown). The remaining sleep symptoms and the syndromal definition of insomnia were unrelated to the development of the metabolic syndrome; however, all estimates were in the direction of being associated with higher risk of developing the metabolic syndrome. Analyses of the individual metabolic syndrome components revealed that loud snoring was a significant predictor of hyperglycemia and low HDL (Table 4); however, DFA and unrefreshing sleep did not predict any of the individual metabolic abnormalities.
Figure 1.
Odds of developing the metabolic syndrome according to sleep symptoms with covariate adjustment. Filled diamonds depict adjusted odds ratios of developing the metabolic syndrome; filled circles depict lower and upper 95% confidence limits. Odds ratios are adjusted for age, sex, race (white/black), marital status (married/unmarried), study randomization, smoking status (ever/never), alcohol consumption (0-3 drinks per week/4 or more drinks per week), sedentary lifestyle (yes/no), and presence of clinically significant depressive symptoms (yes/no).
Table 4.
Relationship between sleep symptoms and individual components of the metabolic syndrome
| Metabolic Syndrome Components |
|||||
|---|---|---|---|---|---|
| Central adiposity criterion OR (95% CI) N^ = 517 |
Glucose criterion OR (95% CI) N^ = 782 |
Blood pressure criterion OR (95% CI) N^ = 391 |
Triglycerides criterion OR (95% CI) N^ = 514 |
HDL criterion OR (95% CI) N^ = 742 |
|
| Difficulty falling asleep | 1.00 (0.49-2.04) | 1.00 (0.45–2.22) | 1.25 (0.64-2.43) | 1.25 (0.52- 3.01) | 1.11 (0.59–2.09) |
| Unrefreshing sleep | 1.01 (0.53–1.93) | 1.66 (0.88–3.10) | 1.39 (0.78-2.48) | 0.77 (0.41-1.44) | 1.29 (0.76–2.17) |
| Loud snoring | 0.80 (0.32–1.98) | 2.15 (1.09-4.24) | 0.64 (0.30-1.39) | 2.11 (0.85-5.26) | 1.92 (1.06–3.48) |
For each metabolic syndrome component, analyses are restricted to those who did not meet criteria for that abnormality at baseline. Covariates include: age, sex, race (white/black), marital status (married/unmarried), study randomization, smoking status (ever/never), alcohol consumption (0-3 drinks per week/ 4 or more drinks per week), sedentary lifestyle (yes/no), and presence of clinically significant depressive symptoms (yes/no).
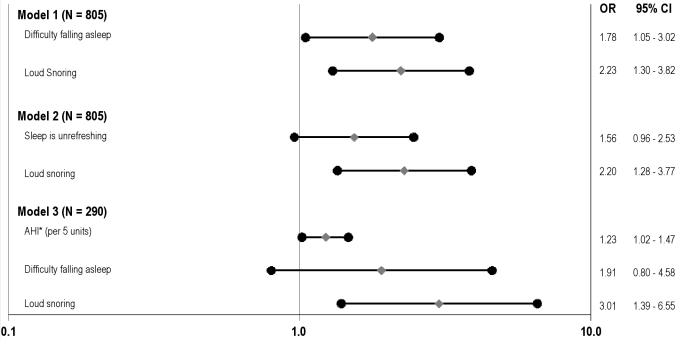
To examine the independent contributions of insomnia versus SDB-related symptoms, the next set of logistic models simultaneously entered DFA or unrefreshing sleep with loud snoring. As shown in Figure 2, Model 1, both DFA and loud snoring remained significant independent predictors of the metabolic syndrome (OR = 1.78; CI: 1.05, 3.02 and OR = 2.23; CI: 1.30, 3.82). Unrefreshing sleep was reduced to marginal significance (Model 2; OR = 1.56; CI: 0.96, 2.53) with additional adjustment for loud snoring.
Figure 2.
Effect of insomnia symptoms and loud snoring on metabolic syndrome incidence, with and without adjustment for AHI. Filled diamonds depict adjusted odds ratios of developing the metabolic syndrome; filled circles depict lower and upper 95% confidence limits. Odds ratios are adjusted for age, sex, race (white/black), marital status (married/unmarried), smoking status (ever/never), alcohol consumption (0-3 drinks per week/4 or more drinks per week), sedentary lifestyle (yes/no), and presence of clinically significant depressive symptoms (yes/no). AHI refers to Apnea Hypopnea Index (continuous, with n = 4 outliers removed). N for Models 1 and 2 = 805 due to missing data. N for Model 3 = 290 (subsample with AHI).
To examine the independent effects of DFA and loud snoring on the prediction of metabolic syndrome incidence, over and above the influence of AHI, a logistic regression model entered DFA, loud snoring, and AHI simultaneously, in addition to demographic, psychosocial, and lifestyle risk factors (Figure 2, Model 2). Once again, loud snoring remained an independent predictor of the metabolic syndrome incidence (OR = 3.01; CI: 1.39, 6.55), whereas DFA was reduced to marginal statistical significance (OR = 1.91; CI: 0.80, 4.58). AHI was also significantly associated with the metabolic syndrome in the subsample who had apnea link data (OR per 5 units = 1.23; 1.02, 1.47).
Finally, to examine whether DFA, unrefreshing sleep, or loud snoring predicted the development of the metabolic syndrome over and above baseline metabolic abnormalities, we conducted logistic regression models that statistically adjusted for the number of metabolic abnormalities present at baseline (0, 1, or 2). With adjustment for baseline metabolic abnormalities, the effects of DFA and unrefreshing sleep became marginally significant (OR = 1.64, CI: 0.95, 2.82 and OR = 1.54; CI: 0.94, 2.52, respectively), whereas the effect of loud snoring remained statistically significant (OR = 1.78, CI: 1.02, 3.12).
DISCUSSION
Previous research has shown that self-reported sleep duration and sleep-disordered breathing are prospectively linked with the development of individual components of the metabolic syndrome, including diabetes or glucose intolerance, hypertension, and obesity.3–8 To our knowledge, this is the first prospective study to evaluate the broader range of sleep symptoms commonly presented in clinical practice in relation to the development of the metabolic syndrome over a 3-year follow-up period. The endorsement of frequent loud snoring more than doubled the risk of developing the metabolic syndrome, and the endorsement of difficulty falling asleep increased the risk by 80%, even after adjusting for demographic, psychosocial, and lifestyle characteristics. These effects were similar among men and women and among whites and blacks in our community sample of middle-aged adults. Finally, our findings showed that in a population selected to be free of the metabolic syndrome at baseline, loud snoring, but not DFA or unrefreshing sleep, predicted the development of the metabolic syndrome even after accounting for the number of metabolic abnormalities present at baseline. This finding raises the possibility that loud snoring may in fact be a causal risk factor for cardiometabolic dysregulation.
Consistent with evidence suggesting that loud snoring may be implicated in the pathophysiology of CVD independent of its relationship with AHI,12,21 we found that self-reported loud snoring was associated with a 3-fold risk of developing the metabolic syndrome even after adjusting for DFA and AHI in our subsample with AHI evaluated. Moreover, our findings linking loud snoring with the development of hyperglycemia and HDL abnormalities, but not with the blood pressure criterion of the metabolic syndrome, suggests the possibility of alternative pathways linking loud snoring with metabolic dysregulation, other than that due to sympathetic activation resulting from hypoxia. In particular, Hedner et al.22 hypothesized that snoring-related vibrations may directly lead to atherogenesis via damage to the endothelial wall and subsequent triggering of the inflammatory cascade. Consistent with this hypothesis, in humans, objectively measured snoring in the laboratory was associated with a more than 10-fold increased risk of having carotid atherosclerosis, even after accounting for AHI and nocturnal hypoxemia.21 Snoring-related sleep fragmentation may also have a direct adverse impact on cardiometabolic profiles, by leading to weight gain, perhaps consequent to cortisol upregulation23 and physical inactivity.24 Alternatively, given limitations of the AHI assessment in the current study (smaller sample size, use of portable monitoring), loud snoring may have provided a more reliable measure of chronic exposure of SDB than one night of airflow monitoring.
Previous findings that have related sleep disturbances, such as inadequate sleep or difficulty initiating or maintaining sleep, to cardiometabolic risk factors or diabetes, are often interpreted as evidence of the physical health consequences of insomnia. These interpretations of sleep symptoms rather than the associated syndrome, per se, belie the reality that the evidence base linking insomnia with cardiovascular morbidity and mortality is far from conclusive.25
Indeed, our data suggest that DFA and unrefreshing sleep, which may be isolated sleep disturbances, or symptoms related to any one of several sleep disorders, such as insomnia, obstructive sleep apnea, or restless legs syndrome, are risk factors for the development of the metabolic syndrome; however, the syndromal definition of insomnia was unrelated to the metabolic syndrome. The symptom of DFA but not the symptom of unrefreshing sleep was an independent predictor of the metabolic syndrome even after accounting for the influence of loud snoring. This finding may reflect greater shared variance between the experience of unrefreshing sleep and loud snoring as compared to DFA and loud snoring. Indeed, there was a significant overlap in reporting the symptoms of unrefreshing sleep and loud snoring (χ2 = 10.24, P < 0.01), but there was not a significant overlap between reporting DFA and loud snoring (χ2 = 1.86, P > 0.10). Thus, sleep initiation problems may reflect a more “pure” measure of the core dysregulation of insomnia, whereas unrefreshing sleep may represent a more general symptom of sleep disturbance that is common to both insomnia and SDB.
Previous evidence has linked difficulty falling asleep with diabetes incidence7 and with mortality.28 Difficulty falling asleep is less contaminated with the expected age-related increase in wakefulness during the night.29 Prolonged sleep latency may reflect a state of emotional and physiological hyperarousal, which has been linked with heightened sympathetic activation and hypercortisolemia23—both of which have been implicated in the pathophysiology of insulin resistance and metabolic syndrome. Evidence suggests that experimentally induced sleep curtailment at the beginning of the night is associated with activation of a transcription factor (NF)-kB that serves a critical role in cellular inflammatory signaling.30 Given purported links between inflammatory processes and metabolic perturbations,31 inflammatory upregulation may serve as a pathway linking difficulty falling asleep with the development of the metabolic syndrome.
While difficulty falling asleep and unrefreshing sleep were predictors of the metabolic syndrome, neither of these symptoms predicted any of the individual factors comprising the metabolic syndrome. These findings are consistent with the notion that the metabolic syndrome indeed represents a syndrome, perhaps with a common etiology and with synergistic prognostic value, rather than the mere sum of its component factors. Notably, the analyses focusing on individual metabolic components may have lacked statistical power to detect significant differences, particularly for the central adiposity and blood pressure criterion, due to the fact that the sample was restricted to those individuals not meeting that specific criterion at baseline.
Among those classified as having insomnia syndrome using more stringent diagnostic criteria, which includes impact on daytime functioning as well as any sleep complaint, 35% reported having DFA and 40% reported having unrefreshing sleep. Despite this overlap, insomnia syndrome was not related to metabolic syndrome incidence. These findings suggest that specific symptoms, DFA, and unrefreshing sleep, may be stronger predictors of cardiometabolic outcomes than the syndrome of insomnia which includes a combination of at least one sleep complaint (including, but not limited to DFA or unrefreshing sleep) as well as a symptom of daytime impairment.
This lack of a statistically significant relationship between insomnia syndrome and the metabolic syndrome, may have important treatment implications, as worries about the health consequences of insomnia are a key perpetuating factor in the disorder.26 The experience of daytime consequences may be germane to the decision to treat insomnia and may be relevant to the psychiatric morbidity associated with insomnia, but may not be an adequate indicator of disease severity in terms of predicting cardiovascular risk. On the other hand, recent evidence from Vgontzas and colleagues27 suggests that the presence of insomnia in conjunction with a physiologic indicator of disease severity (polysomnographically determined short sleep duration) strongly and significantly predicts hypertension risk, suggesting that this phenotype of insomnia may represent a higher threshold of risk, relevant in the prediction of cardiovascular outcomes.
The current findings are strengthened by the large, representative sample of community-dwelling white and black men and women with thorough laboratory assessments of metabolic risk factors both at baseline and at 3-year follow-up. We also examined a wide range of sleep disturbances commonly presenting in clinical practice and assessed the relative contribution of SDB and insomnia symptoms using different case definitions. In addition, analyses controlled for a host of covariates (including AHI) that may have confounded the results.
Limitations
A limitation of the study is that we were unable to examine the relative contribution of AHI versus self-reported sleep symptoms in predicting incident metabolic syndrome, due to the cross-sectional nature of the AHI assessment and that AHI was assessed in a relatively small subsample of the study cohort. Moreover, AHI was determined via nasal airflow alone. Thus, we did not have a direct measure of intermittent hypoxemia. In addition, we did not have objective measures of sleep disturbances; however, self-reports of snoring and sleep latency are correlated with objective measurements.32 Finally, because the study did not include a measure of sleep duration we cannot determine the degree to which current findings may be influenced by short or long sleep durations—factors which have been reliably linked with cardiometabolic risk in previous investigations. To better understand the putative mechanisms linking sleep disturbances with cardiovascular risk, future prospective studies should examine the combination of subjective sleep complaints in conjunction with physiological indicators of poor sleep in relation to cardiovascular morbidity and mortality.
Summary
This study provides the first prospective evidence to support a directional link between commonly reported sleep symptoms, including difficulty falling asleep and loud snoring and development of the metabolic syndrome. These relationships persisted after accounting for the effects of AHI and other relevant covariates, and suggest that loud snoring, in particular, is independently implicated in cardiometabolic risk, rather than merely being a marker for OSA. Insomnia, defined using more stringent diagnostic criteria, was not a risk factor for the development of the metabolic syndrome. Given that in the general population, sleep complaints are considerably more prevalent than either insomnia or obstructive sleep apnea syndromes, these findings have far-reaching implications for public health, particularly given epidemic levels of obesity and its associated cardiometabolic consequences, which are associated with sleep disturbances. These findings reflect the utility of assessing for common sleep complaints in routine clinical practice as these individuals may be at elevated risk for the development of the metabolic syndrome.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Strollo has received research support from ResMed and Respironics. Dr. Drumheller has received research support from ResMed and Respironics. Dr. Buysse has consulted for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Somnus, Stress Eraser, Takeda, and Transcept; has received research support from Sepracor; and has helped to produce CME materials and has given paid CME lectures indirectly supported by industry sponsors. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funding for this research was provided by the Commonwealth of Pennsylvania Department of Health Contract ME-02-384), and the National Institutes of Health HL076852, HL076858, and CTSA/ N-CTRC #RR024153. Support for the first author was provided by the National Heart Lung Blood Institute (NHLBI) K23HL093220. Apnealink funding was providing by the non-profit ResMed Foundation. The Department and the National Institutes of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Dr. Steven E. Reis has had full access to the data, and takes responsibility for the integrity of the data and accuracy of the analysis.
REFERENCES
- 1.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type 2 diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 5.Gangswich JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami N, Takatsuka N, Schimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–3. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–9. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor GT, Caffo B, Newman A, B, et al. Prospective study of sleep-disordered breathing and hypertension. Am J Respir Crit Care Med. 2009;179:1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber A, Howrwood F, Sithole J, Ali NJ, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5:1–7. doi: 10.1186/1475-2840-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings JR, Muldoon MF, Hall M, et al. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 12.Leineweber C, Kecklund G, Åkerstedt T, Janszky I, Orth-Goméer K. Snoring and the metabolic syndrome in women. Sleep Med. 2003;4:531–6. doi: 10.1016/s1389-9457(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 13.Nock NL, Li L, Larkin EK, et al. Empirical evidence for ≪syndrome Z≫: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep. 2009;32:615–22. doi: 10.1093/sleep/32.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse DJ. Diagnosis and assessment of sleep and circadian rhythm disorders. J Psychiatr Pract. 2005;11:102–15. doi: 10.1097/00131746-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 17.Okun ML, Kravitz HM, Sowers MF, et al. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Jacobs DR, Leon AS. Validity and reliability of self-reported physical activity status: The Lipid Research Clinics Questionnaire. Med Sci Sports Exerc. 1993;25:92–98. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depresion scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Wang Y, Teschler T, Weinreich G, Hess S, Wessendorf TE, Teschler H. Validation of microMESAM as screening device for sleep disordered breathing. Pneumologie. 2003;57:734–40. doi: 10.1055/s-2003-812423. [DOI] [PubMed] [Google Scholar]
- 21.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Hedner J, Wilcox I, Sullivan C. Speculations on the interaction between vascular disease and obstructive sleep apnoea. In: Saunders NA, Sullivan C, editors. Sleep and Breathing. New York: Dekker; 1994. pp. 823–46. [Google Scholar]
- 23.Vgontzas AN, Chrousos GP, Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Bixler EO, Vgontzas AN, Bixler EO. Short sleep and obesity: are poor sleep, chronic stress, and unhealthy behaviors the link? [comment] Sleep. 2008;31:1203. [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor DJ, Lichstein KL, Durrence HH, Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1:227–47. doi: 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]
- 26.Harvey AG, Greenall E. Catastrophic worry in primary insomnia. J Behav Ther Exp Psychiatry. 2003;34:11–23. doi: 10.1016/s0005-7916(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. [see comment] Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 29.Feinsilver SH. Sleep in the elderly: What is normal? Clin Geriatr Med. 2003;19:177–88. doi: 10.1016/s0749-0690(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 30.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straznicky NE, Eikelis N, Lambert EA, et al. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–7. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 32.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]