Abstract
Study Objectives:
A considerable amount of experimental evidence suggests that sleep plays a critical role in learning/memory processes. In addition to paradoxical sleep, slow wave sleep is also reported to be involved in the consolidation process of memories. Additionally, sleep deprivation can induce other behavioral modifications, such as emotionality and alternations in locomotor activity in rodents. These sleep deprivation-induced alterations in the behavioral state of animals could produce state-dependent learning and contribute, at least in part, to the amnestic effects of sleep deprivation. The aim of the present study was to examine the participation of state-dependent learning during memory impairment induced by either paradoxical sleep deprivation (PSD) or total sleep deprivation (TSD) in mice submitted to the plus-maze discriminative avoidance or to the passive avoidance task.
Design:
Paradoxical sleep deprivation (by the multiple platform method) and total sleep deprivation (by the gentle handling method) were applied to animals before training and/or testing.
Conclusions:
Whereas pre-training or pre-test PSD impaired retrieval in both memory models, pre-training plus pre-test PSD counteracted this impairment. For TSD, pre-training, pre-test, and pre-training plus pre-test TSD impaired retrieval in both models. Our data demonstrate that PSD- (but not TSD-) memory deficits are critically related to state-dependent learning.
Citation:
Patti CL; Zanin KA; Sanday L; Kameda SR; Fernandes-Santos L; Fernandes HA; Andersen ML; Tufik S; Frussa-Filho R. Effects of sleep deprivation on memory in mice: role of state-dependent learning. SLEEP 2010;33(12):1669-1679.
Keywords: Sleep, memory, state-dependency
MEMORY IS TYPICALLY DEFINED AS THE ABILITY OF RETAIN AND MANIPULATE INFORMATION PREVIOUSLY ACQUIRED BY MEANS OF NEURONAL PLASTICITY.1 This memory process is dramatically dynamic and can be modified by several factors. These factors include drugs,2–5 as well as experimental conditions such as sleep deprivation6–8 and stress,9,10 which interfere with acquisition, retention and/or retrieval processes.
It is well established that sleep plays a critical role in learning and memory formation. Indeed, a myriad studies have demonstrated that paradoxical sleep deprivation (PSD) in animals leads to memory deficits in several behavioral models such as avoidance,6,11,12 the Morris water maze,13,14 and the radial maze15 tasks.
It has been proposed that the deleterious effects caused by sleep deprivation are due to its effects on long-term potentiation (LTP) in the hippocampus, an area well known for the importance of encoding memories.16,17 Alternatively, some studies have also suggested that the memory impairments observed in sleep-deprived animals are induced by other behavioral alterations, such as decreased reaction time,18 motor function alterations,19 stress response, 13 and modification in exploratory and fear responses.20 As memory can be state dependent—when a response that has been acquired in a certain condition may not be retrieved when in another condition21,22—these behavioral alterations induced by sleep deprivation could contribute to its amnestic effects through a state-dependency phenomenon.
Although the consequences of paradoxical sleep deprivation in animal models have been studied for many years, the effects of total sleep deprivation (TSD) on learning/memory, as well as anxiety and locomotor activity in animal models have been overlooked. The aim of the present study was to investigate the participation of state-dependent learning in memory impairment induced by paradoxical sleep deprivation or by total sleep deprivation in the plus-maze discriminative avoidance task (PM-DAT—an animal model which evaluates learning/memory, anxiety-like behavior and locomotor activity concomitantly) or in the passive avoidance task (PAT—a classical animal paradigm for evaluating memory in rodents).
MATERIALS AND METHODS
Subjects
Three-month-old Swiss EPM-M1 male mice (outbred, raised, and maintained in the Centre for Development of Experimental Models in Medicine and Biology of Universidade Federal de São Paulo) were used. Animals weighing 30-35 g were housed under controlled temperature (22-23°C) and lighting (12 h light, 12 h dark; lights on at 06:45) conditions. Food and water were available ad libitum throughout the experiments. Animals used in this study were maintained in accordance with the National Institute of Health Guide for the care and use of laboratory animals (NIH Publications N° 80-23), revised 1996.
Sleep Deprivation Protocols
Paradoxical sleep deprivation
The method of PSD was adapted from the multiple platform method, originally developed for rats.23 Groups of 5-6 mice (PSD group) were placed in water tanks (41 cm x 34 cm x 16.5 cm), containing 12 platforms (3 cm in diameter) each, surrounded by water up to 1 cm beneath the surface. In this method, the animals are capable of moving inside the tank, jumping from one platform to the other. Homecage control animals (CTRL) were maintained in their cages in the same room. The animals were sleep deprived for 72 hours, and immediately after this period they were submitted to behavioral tasks.
We have previously demonstrated that 3-month-old Swiss EPM-M1 male mice subjected to the above-described protocol displayed a significant reduction in paradoxical sleep, dropping from 55.0 ± 4.6 min (mean ± S.E., baseline) to 2.1 ± 0.6 min on the first day of sleep deprivation, 2.9 ± 0.7 min on the second day, and 2.9 ± 1.0 min on the third day. Slow wave sleep was also significantly reduced, but at a much lower magnitude, falling from 558.5 ± 29.8 min (baseline) to 125.5 ± 9.5 min on the first day of the sleep deprivation protocol, 83.9 ± 9.9 min on the second day, and 101.5 ± 10.8 min on the third day.8
Total sleep deprivation
Mice were submitted to TSD through the gentle handling method, which consists of keeping the animal awake by tapping on the cage and, if necessary, by gently touching them with a soft brush if behavioral signs of sleep are observed. The animals were sleep deprived for 6 hours and immediately submitted to behavioral tasks.
Animal models for memory evaluation
Plus-maze discriminative avoidance task (PM-DAT):
The apparatus employed in the plus-maze discriminative avoidance task (PM-DAT) is a modified elevated plus-maze, made of wood, containing 2 enclosed arms with sidewalls, and no top (28.5 x 7 x 18.5 cm), opposite to 2 open arms (28.5 x 7 cm). A non-illuminated 100-W lamp was placed over the exact center of one of the enclosed arms (aversive enclosed arm). In the training session, each mouse was placed at the center of the apparatus; during a 10-min period, an aversive stimulus was administered every time the animal entered the enclosed arm containing the lamp and was continued until the animal left the arm. The aversive stimulus consisted of both the illumination of the 100-W light and the production of an 80-dB noise by a small machine placed under the aversive enclosed arm. In the test session (performed in the same room 10 days after the training session), mice were again placed in the center of the apparatus and were observed for 3 min; however, the mice did not receive an aversive stimulus when they entered the aversive enclosed arm (although the non-illuminated lamp was still placed on the middle of this arm to help distinguish between the aversive and non-aversive arm). In all experiments, the animals were observed in a blind manner, and the apparatus was cleaned with a 5% alcohol solution after each behavioral session. Total number of entries in any of the arms, percent time spent in the aversive enclosed arm (time spent in aversive enclosed arm/time spent in both enclosed arms), and percent time spent in open arms (time spent in open arms/time spent in both open and enclosed arms) were calculated. Learning and memory were evaluated by the percent time spent in the aversive enclosed arm in the training and in the test session, respectively. Anxiety-like behavior and motor activity was evaluated by the percent time spent in the open arms and the total number of entries in all the arms of the apparatus, respectively.
Passive avoidance task (PAT):
The method described by Denti and Epstein24 was used. The apparatus employed was a 2-way shuttle box with a guillotine door placed between the 2 modular testing chambers. One chamber was illuminated by a 40-W light, while the other remained dark. The animals were individually placed in the illuminated chamber facing away from the guillotine door. When the mouse entered the dark chamber, the door was noiselessly lowered and a 0.4 mA foot shock was applied for 1 sec through the grid floor. Prior to placing the animal into the apparatus for the multiple platform sleep deprivation experiment, the animal was placed for 15 min in a small box with sawdust to dry its paws. A test session was performed 10 days after conditioning. In both behavioral sessions (training and test), the latency in entering the dark chamber was recorded, with a cut-off duration of 300 sec.
Statistical Analysis
For the PM-DAT experiments, total number of entries in any of the arms, percent time spent in the open arms, and percent time spent in the aversive enclosed arm were calculated and compared using the t-test for independent samples in the training session and 2-way ANOVA followed by Duncan's test in the test session. For the PAT experiments, latency to step through was recorded in each session and compared by t-test for independent samples in the training session and by 2-way ANOVA followed by Duncan's test in the test session. For the passive avoidance experiments, the performance in the training vs. test session were also compared by a 2-way ANOVA with repeated measures for the session factor. A t-test for paired samples was used to compare within-subject differences. A probability of P < 0.05 was considered significant for all comparisons made.
Experimental Design
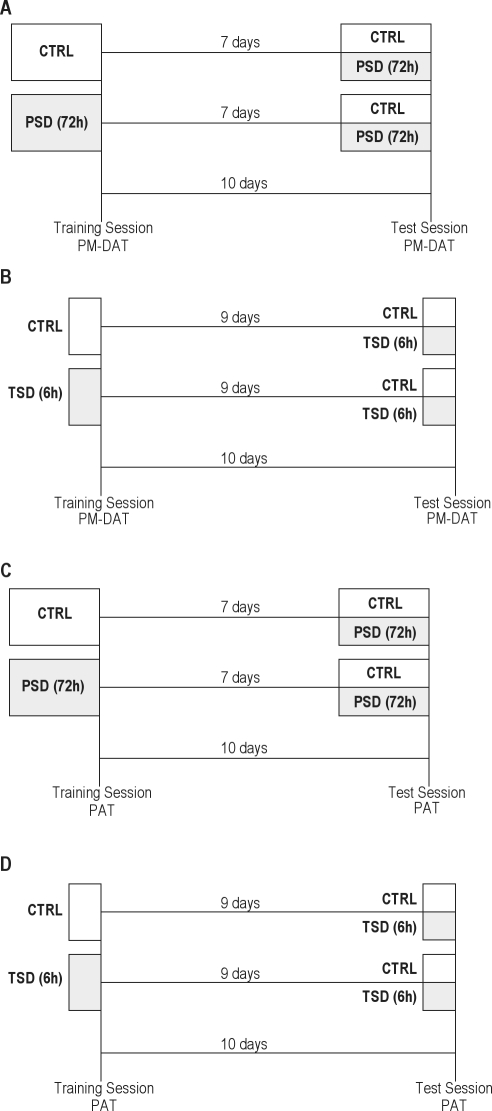
Experiment I: Role of state-dependency in memory impairment induced by paradoxical sleep deprivation in mice tested in the PM-DAT
Mice were randomly assigned to one of the following groups: home cage control (CTRL-CTRL, n = 12), pre-test paradoxical sleep deprivation (CTRL-PSD, n = 12), pre-training paradoxical sleep deprivation (PSD-CTRL, n = 12), or pre-training/pre-test paradoxical sleep deprivation (PSD-PSD, n = 11). All animals were submitted to a training session and 10 days later to a test session. Mice allocated in CTRL groups were kept in their homecages throughout the experiment. Mice allocated in the PSD groups were kept in a water tank during 72 h before the training session (PSD-CTRL group), before the test session (CTRL-PSD), or before both the training and test sessions (PSD-PSD group). Mice were submitted to the training and/or test sessions in the plus-maze discriminative avoidance task immediately after the end of the 72-h PSD period (Figure 1A).
Figure 1.
Experimental design. (A) Experiment I, (B) Experiment II, (C) Experiment III, and (D) Experiment IV. CTRL: control group; PSD: paradoxical sleep deprivation for 72 h; TSD: total sleep deprivation for 6 h; PM-DAT: plus-maze discriminative avoidance task; PAT: passive avoidance task.
Experiment II: Role of state-dependency in memory impairment induced by total sleep deprivation in mice tested in the PM-DAT
Animals were assigned to one of the following groups: control (CTRL-CTRL, n = 8); pre-test total sleep deprivation (CTRL-TSD, n = 10), pre-training total sleep deprivation (TSD-CTRL, n = 8), or pre-training/pre-test total sleep deprivation (TSD-TSD, n = 10). All animals were submitted to a training session and 10 days later to a test session. Mice allocated in CTRL groups were kept in their home cages throughout the experiment. Mice allocated in the TSD groups were gently handled for 6 h before the training session (TSD-CTRL group), before the test session (CTRL-TSD), or before both the training and test sessions (TSD-TSD group). Mice were submitted to the training and/or test sessions in the plus-maze discriminative avoidance task immediately after the end of the 6-h TSD period (Figure 1B).
Experiment III: Role of state-dependency in memory impairment induced by paradoxical sleep deprivation in mice tested in the PAT
Mice were assigned to the same groups (CTRL-CTRL, n = 11; CTRL-PSD, n = 11; PSD-CTRL, n = 11; and PSD-PSD, n = 11) as described in Experiment I. Immediately after the end of the 72-h PSD period, the animals were submitted to the training and/or to the test session of the passive avoidance task (Figure 1C).
Experiment IV: Role of state-dependency in memory impairment induced by total sleep deprivation in mice tested in the PAT
Mice were assigned to the same groups (CTRL-CTRL, n = 9; CTRL-TSD, n = 10; TSD-CTRL, n = 10 and TSD-TSD, n = 12) as described in Experiment II. Immediately after the end of the 6-h TSD period, the animals were submitted to the training and/or the test session of the passive avoidance task (Figure 1D).
In all experiments, training and testing sessions were performed between 14:00 and 15:40. Consequently, PSD began at the same time (between 14:00 and 15:20) 3 days earlier, and TSD began 6 h earlier (between 08:00 and 09:40). As previously described, the animals were maintained in a 12 h light/12 h dark cycle, with lights turning on at 06:45.
Experiment V: Effects of 66 hours of stress on memory impairment induced by total sleep deprivation in mice tested in the PAT
This experiment was designed to investigate the possibility that the differences between the amnestic effects of PSD and TSD were related to the differences in the duration of exposure to stress in each sleep deprivation protocol. Specifically, it could be argued that the amnestic effects of TSD were not related to state-dependent learning (see Results section) because this protocol was associated with a short-term stress exposure (6 h) instead of the prolonged stress exposure (72 h) associated with the PSD protocol. Thus, we have tried to match the duration of stress exposure during the TSD protocol to that of the PSD (72 h) protocol. For this reason, mice were submitted to 66 h of stress prior to the 6-h TSD procedure.
Mice were assigned to the following groups: CTRL-CTRL (n = 14) or St+TSD-St+TSD (n = 14). Mice allocated in the CTRL-CTRL group were kept in their homecages throughout the experiment. Mice allocated to the the St+TSD-St+TSD group were submitted to 66 h of stress (St) before the 6 h of TSD by gentle handling before both the training and test sessions. The stress consisted of 24 h of high population density (14 animals in a 30 x 19 x 13 cm box), 24 h in a soiled cage, and 18 h in a cage tilted 45°. Immediately after the end of the 6-h TSD period, the animals were submitted to the training and the test sessions of the PAT.
RESULTS
Experiment I: Role of State-Dependency in Memory Impairment Induced by Paradoxical Sleep Deprivation in Mice Tested in the PM-DAT
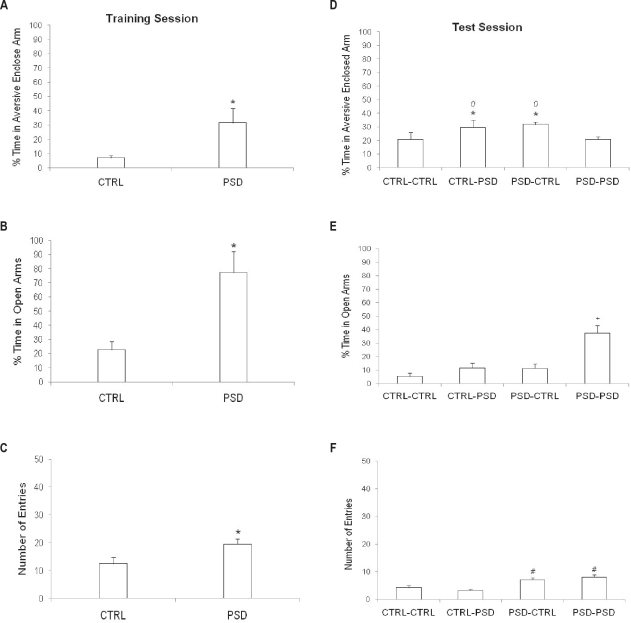
While both the CTRL-CTRL and CTRL-PSD groups performed the training session under the non-PSD condition (i.e., the home cage condition), both the PSD-CTRL and PSD-PSD groups performed it under the PSD condition. In the training session, data from animals in the CTRL-CTRL and CTRL-PSD groups were thus pooled into the CTRL group, whereas data from the PSD-CTRL and PSD-PSD groups were pooled into the PSD group. When compared with the CTRL group, the PSD group had an increased percent time spent in the aversive enclosed arm (t43 = 2.41, P < 0.05) (Figure 2A), an enhancement in the percent time spent in the open arms (t43 = 3.41; P < 0.005) (Figure 2B), and increased locomotor activity (t43 = 3.03; P < 0.005) (Figure 2C). Respectively, these data indicated that PSD produced acquisition deficits, anxiolytic effects, and hyperactivity in mice.
Figure 2.
Effects of paradoxical sleep deprivation (PSD) on training and test performances of mice in the plus-maze discriminative avoidance task (PM-DAT). Mice were kept in their home cages (CTRL) or paradoxical sleep-deprived for 72 h (PSD) before a 10-min training session and/or before a 3-min test session, performed 10 days later. Results are presented as mean ± SE of percent time spent in the aversive enclosed arm in the training (A) and test (D); percent time spent in the open arms in the training (B) and test (E) and total number of entries in all arms of the apparatus in the training (C) and test (F) sessions. *P < 0.05 compared to the CTRL group in the training (t-test for independent samples); 0P < 0.05 compared to the PSD-PSD group, +P < 0.05 compared to all the other groups and #P < 0.05 compared to the CTRL-CTRL and the CTRL-PSD groups in the test (2-way ANOVA and Duncan's test).
In the test session, 2-way ANOVA revealed a significant pre-training x pre-test interaction effects (F1,43 = 6.48; P < 0.05) for the percent time spent in the aversive enclosed arm. Indeed, both the CTRL-PSD and the PSD-CTRL groups presented higher percentages of time (i.e., impaired memory) when compared to the CTRL-CTRL and PSD-PSD groups, which did not differ from each other (Figure 2D). Concerning the exploration of the open arms (Figure 2E), 2-way ANOVA for percent time spent in the open arms revealed significant pre-training (F1,43 = 17.02; P < 0.001), pre-test (F1,43 = 17.79; P < 0.001), and pre-training × pre-test interaction (F1,43 = 6.71; P < 0.05) effects. Indeed, the PSD-PSD group presented an increase in the percent time spent in the open arms of the apparatus (i.e., decreased anxiety-like behavior) as compared to all the other groups. Still, when locomotor activity was analyzed (Figure 2F) in this session, 2-way ANOVA for the total number of entries revealed only significant effects for the pre-training condition (F1,43 = 30.44; P < 0.001). Thus, the PSD-PSD and the PSD-CTRL groups presented a greater number of total entries (i.e., hyperactivity) when compared to the CTRL-CTRL and the CTRL-PSD groups.
Experiment II: Role of State-Dependency in Memory Impairment Induced by Total Sleep Deprivation in Mice Tested in the PM-DAT
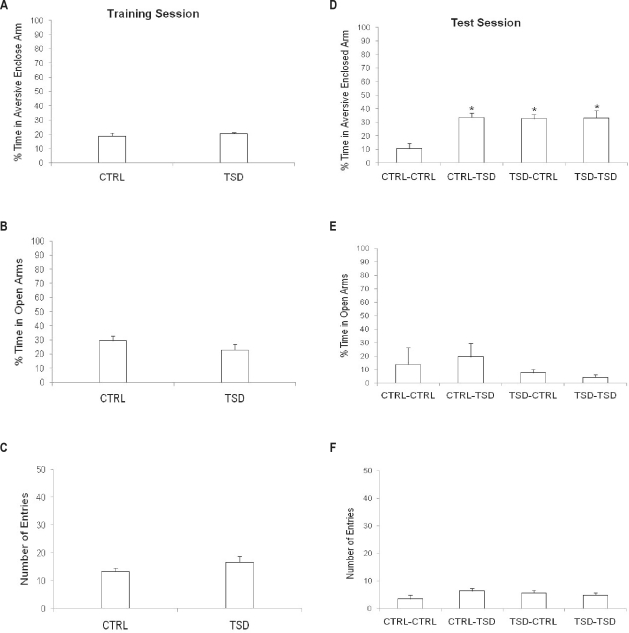
In the training session, data from animals of the CTRL-CTRL and CTRL-TSD groups were again pooled into a CTRL group, while data from mice of the TSD-CTRL and TSD-TSD groups were pooled into the TSD group. The t-test for independent samples did not show significant differences between groups with respect to the percent time spent in the aversive enclosed arm (Figure 3A), percent time spent in the opens arms (Figure 3B), or locomotor activity (Figure 3C).
Figure 3.
Effects of total sleep deprivation (TSD) on training and test performances of mice in the plus-maze discriminative avoidance task (PM-DAT). Mice were kept in their home cages (CTRL) or sleep-deprived for 6 h (TSD) before a 10-min training session and/or before a 3-min test session, performed 10 days later. Results are presented as mean ± SE of percent time spent in the aversive enclosed arm in the training (A) and test (D); percent time spent in the open arms in the training (B) and test (E) and total number of entries in all arms of the apparatus in the training (C) and test (F) sessions. *P < 0.05 compared to the CTRL group in the test (2-way ANOVA and Duncan's test).
In the test session, 2-way ANOVA revealed a significant pre-training condition effect (F1,32 = 6.99; P < 0.001), pre-test condition (F1,32 = 8.27; P < 0.05), and pre-training × pre-test interaction effect (F1,32 = 7.61; P < 0.05) for the percent time spent in the aversive enclosed arm. In fact, all groups showed an enhancement in the percent time spent in the aversive enclosed arm when compared to the CTRL-CTRL group, thus showing memory deficits (Figure 3D).
There were no differences among groups when the percent time spent in the open arms (Figure 3E), and total number of entries were analyzed (Figure 3F).
Comparison of the Amnestic Effects Produced by Paradoxical Sleep Deprivation and Total Sleep Deprivation Conditions in the PM-DAT
Within each sleep deprivation condition (PSD or TSD), the difference from mean CTRL-CTRL value was used as an adjustment measure in order to compare the effects of PSD and TSD on the percent time spent in the aversive enclosed arm of the PM-DAT apparatus during the test session (Table 1). Two-way ANOVA revealed a significant sleep deprivation condition (PSD × TSD) effect (F1,77 = 38.1; P < 0.001) and a significant interaction between sleep deprivation × group (CTRL-SD, SD-CTRL, or SD-SD) (F2,77 = 4.90; P < 0.05). Duncan's post hoc analysis revealed that the differences from the respective mean CTRL-CTRL values of both the PSD-CTRL and CTRL-PSD groups were greater than those of the PSD-PSD group. Conversely, the TSD-CTRL, CTRL-TSD, and TSD-TSD groups had a similar magnitude of memory deficits. These findings confirm that PSD-induced memory deficits, but not TSD-induced memory deficits, are critically related to state-dependent learning. In addition, Duncan's post hoc analysis revealed that the magnitude of memory deficits produced by the TSD condition in the PM-DAT was significantly greater than that produced by the PSD condition. Indeed, the memory deficits shown by the TSD groups (TSD-CTRL, CTRL-TSD, and TSD-TSD) were significantly greater as compared to their respective PSD groups (PSD-CTRL, CTRL-PSD, and PSD-PSD).
Table 1.
Comparison of the amnestic effects of paradoxical sleep deprivation (PSD) and total sleep deprivation (TSD) conditions in the plus-maze discriminative avoidance task (PM-DAT) and in the passive avoidance task (PAT)
| Groups | Memory deficit |
|
|---|---|---|
| PM-DAT (PTAv) | PAT (Latency) | |
| CTRL-PSD | 9.06 ± 5.25* | −92.27 ± 29.59* |
| PSD-CTRL | 11.32 ± 1.70* | −83.27 ± 32.98* |
| PSD-PSD | 0.03 ± 1.84 | 16.09 ± 0.00 |
| CTRL-TSD | 23.00 ± 2.32*° | −67.73 ± 4.68* |
| TSD-CTRL | 22.05 ± 1.83*° | −46.73 ± 13.53* |
| TSD-TSD | 22.54 ± 3.99*° | −57.33 ± 11.64* |
Results are mean ± SE of the differences from the respective mean CTRL-CTRL value in each sleep deprivation condition (percent time spent in the aversive enclosed arm (PTAv) during the test session for the PM-DAT data and latency (s) to enter the dark chamber during the test session for PAT data). For PAT data, the more negative the data, the greater the memory deficit.
P < 0.05 compared to the PSD-PSD group.
P < 0.05 compared to the respective PSD group.
Experiment III: Role of State-Dependency in Memory Impairment Induced by Paradoxical Sleep Deprivation in Mice Tested in the PAT
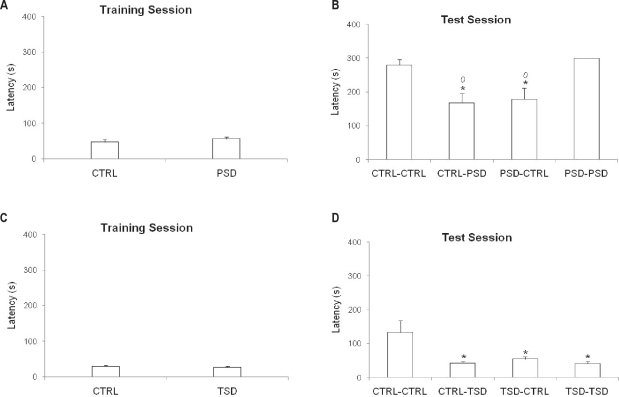
In the training session, a t-test for independent samples did not show significant differences between the groups in relation to the latency in entering the dark chamber of the apparatus (Figure 4A).
Figure 4.
Effects of paradoxical sleep deprivation (PSD) or total sleep deprivation (TSD) on training and testing performances of pre-training and/or pre-test sleep-deprived mice in the passive avoidance task (PAT). Mice were kept in their home cages (CTRL), or deprived of paradoxical sleep for 72 h (PSD) or deprived of total sleep for 6 h (TSD) before a training session and/or before test session, performed 10 days later. Results are presented as mean ± SE latency to enter the dark chamber, in seconds, in the training session (A and C) and latency to enter the dark chamber, in seconds, in the test session (B and D) *P < 0.05 compared to CTRL-CTRL group; 0P < 0.05 compared to group PSD-PSD (2-way ANOVA and Duncan test).
In the test session, 2-way ANOVA showed significant interaction effects between pre-training x pre-test conditions (F1,43 = 8.38; P < 0.05). In this way, the groups CTRL-PSD and PSD-CTRL presented a decreased latency in entering the dark chamber when compared to the CTRL-CTRL and PSD-PSD groups, demonstrating memory impairment (Figure 4B).
In contrast to the PM-DAT model (in which aversive stimuli are applied during the training session), in the training session of the PAT model, the aversive stimulus is applied immediately after the task. Thus, in the latter model memory can be also evaluated by a within-subject comparison (i.e., training performance vs. test performance). This alternative statistical approach confirmed the results of the between-subject analyses described above. Indeed, a 2-way ANOVA with repeated measures as the second factor (training or test sessions) revealed a significant effect of group (F3,44 = 5.70; P < 0.001), and session (F1,44 = 287.70; P < 0.001). Importantly, a significant interaction between groups and sessions was also revealed (F3,44 = 4.76; P < 0.001). All of the groups showed a significant increase in the latency to enter the dark chamber in the test session when compared with the training session (as revealed by the t-test for paired samples). However, this increase was of a lesser magnitude in the PSD-CTRL and CTRL-PSD groups, indicating that they had memory deficits compared to the CTRL-CTRL and PSD-PSD groups. These within-subject differences among the groups were statistically demonstrated by comparing the test minus training performance of the different groups by ANOVA followed by Duncan's test (F3,44 = 5.72; P < 0.05).
Experiment IV: Role of State-Dependency in Memory Impairment Induced by Total Sleep Deprivation in Mice Tested in the PAT
In the training session, a t-test for independent samples did not show any differences among groups with respect to the latency in entering the dark chamber of the apparatus (Figure 4C).
In the test session, 2-way ANOVA revealed a significant pre-test condition effect (F1,37 = 8.20; P < 0.05) as well as pre-training × pre-test interaction effect (F1,37 = 4.45; P < 0.05). Thus, all the groups (CTRL-TSD, TSD-CTRL, and TSD-TSD), showed decreased latency in entering the dark chamber compared to the CTRL-CTRL group, demonstrating memory deficits (Figure 4D).
These results were confirmed by comparing training performance to test performance. Indeed, a 2-way ANOVA with repeated measures as the second factor (training or test session) revealed a significant effect for both the group (F3,44 = 3.33; P < 0.05) and session factors (F3,44 = 19.10; P < 0.001). Importantly, a significant interaction between groups and session was also revealed (F3,44 = 3.40; P < 0.05). All the groups showed a significant increase in the latency to enter to the dark chamber in the test session as compared with the training session (as revealed by t-test for paired samples). However, this increase was of a lesser magnitude in the TSD-CTRL, CTRL-TSD, and TSD-TSD groups, demonstrating that these groups had memory deficits compared to the CTRL-CTRL group. These within-subject differences among groups were statistically demonstrated by comparing the test minus training performance of the different groups by ANOVA followed Duncan's test (F3,44 = 3.88; P < 0.05).
Comparison of the Amnestic Effects Produced by Paradoxical Sleep Deprivation and Total Sleep Deprivation in the PAT
Within each sleep deprivation condition (PSD or TSD), the difference from mean CTRL-CTRL value was used as an adjustment measure to compare the effects of PSD and TSD on the latency to enter the dark chamber of the PAT apparatus during the test session (Table 1). Two-way ANOVA revealed a significant group (CTRL-SD, SD-CTRL, or SD-SD) effect (F2,71 = 4.90; P < 0.05) and a significant interaction between group × sleep deprivation condition (PSD or TSD) (F2,71 = 4.74; P < 0.05). Duncan's post hoc analysis revealed that the differences from mean CTRL-CTRL values of both the PSD-CTRL and CTRL-PSD groups were greater than those of the PSD-PSD group. Conversely, the TSD-CTRL, CTRL-TSD, and TSD-TSD groups had a similar magnitude of memory deficits. These findings confirm that PSD-induced memory deficits, but not TSD-induced memory deficits, are critically related to state-dependent learning. In addition, Duncan's post hoc analysis revealed that the magnitude of memory deficits produced by the PSD and TSD conditions were not different, in that there were no significant differences between the memory deficits shown by the PSD-CTRL, CTRL-PSD, TSD-CTRL, CTRL-TSD, and TSD-TSD groups.
Experiment V: Effects of 66 Hours of Stress on Memory Impairment Induced by Total Sleep Deprivation in Mice Tested in the PAT
A t-test for independent samples did not show any differences between groups with respect to the latency to enter the dark chamber of the apparatus during the training session.
For the test session, t-test for independent samples revealed that St+TSD-St+TSD group showed a decreased latency to enter the dark chamber compared to the CTRL-CTRL group, demonstrating the presence of memory deficits (t26 = 2.44; P < 0.05) (Table 2).
Table 2.
Effects of 66 h of stress (St) on memory impairment induced by total sleep deprivation (TSD) in mice tested in the passive avoidance task (PAT)
| Groups | PAT performance |
|
|---|---|---|
| Training session | Test session | |
| CTRL-CTRL | 30.71 ± 5.40 | 218.50 ± 29.49 |
| St+TSD-St+TSD | 47.86 ± 6.26 | 117.50 ± 28.92* |
Mice were subjected to 66 h of stress (24 h at high population, 24 h in a soiled cage, and 18 h in a cage tilt 45°) prior to 6 h of TSD immediately before the training and test sessions of PAT. Results are presented as mean ± SE latency (s) to enter the dark chamber in the training and test sessions.
P < 0.05 compared to CTRL-CTRL group (t-test for independent samples).
DISCUSSION
The major finding of the present study is that in two different animal models of memory, both pre-training and pre-test PSD and TSD induced memory deficits; however, in contrast to TSD-induced memory deficits, PSD-induced memory impairment was completely related to the state-dependency phenomenon.
In the PM-DAT, the avoidance of the aversive enclosed arm upon testing has been validated as a measurement of retention, because amnestic manipulation decreases this effect.2–5,7,25–29 In contrast, memory-improving treatments increase this effect.2,3,27,30 This behavioral model has been shown to be effective in concomitantly evaluating anxiety-related behaviors5,27,31 and locomotor activity.25,26,29,31
In the training session of Experiment I, pre-training PSD for 72 hours decreased learning levels, as demonstrated by the increased time spent in the aversive enclosed arm. Furthermore, in the training session, PSD also led to anxiolytic-like (increased percent time in the open arms) and hyperlocomotor effects (increased total number of entries in all arms of the apparatus). It is well known that PSD can induce alterations in the anxiety levels of rodents. Indeed, it has been demonstrated that rats spend an increased amount of time in the open arms of an elevated plus-maze after paradoxical sleep deprivation.32 Moreover, it has been suggested that locomotor activity is related to the development of PSD-induced dopaminergic supersensitivity.33–35 Our group has demonstrated that PSD potentiates both spontaneous and amphetamine-induced locomotor activity in an open-field paradigm in mice.36 Another interpretation of these data is that an intra-session habituation (i.e., the decrease in exploratory activity when a rodent is exposed to a new environment37,38) deficit may have also occurred in paradoxical sleep-deprived mice.
In our Experiment I, the pre-training or the pre-testing PSD groups (PSD-CTRL and CTRL-PSD) presented amnesia, characterized by an increase in the percent time spent in the aversive enclosed arm during the test session. These results are in agreement with other studies reporting pre-training paradoxical sleep deprivation-induced memory deficits in avoidance tasks6,8 and in the Morris water maze.13,14 Importantly, pre-test PSD promoted impairment during the retrieving the task (CTRL-PSD group). This finding replicates the data from our previous work,31 which demonstrated deleterious effects of PSD on discriminative avoidance task retrieval in rats. Even more importantly, pre-test PSD did not produce a deficit in the test performance in mice that were also paradoxically sleep-deprived before training (PSD-PSD group), and vice-versa, demonstrating that memory impairments induced by PSD are state-dependent. Within this context, PSD certainly produces a myriad physiological modifications that could change the state of both the CTRL-PSD and the PSD-CTRL groups during the test and the training sessions, thereby leading to state-dependent memory impairment.
Concerning the performance deficit induced by pre-training PSD, it is very interesting to note that pre-test PSD counteracts this effect despite the deleterious effect of pre-training PSD on the acquisition of the task (demonstrated by the increased percent of time in the aversive enclosed arm of the apparatus in the training session). This finding suggests that although pre-training PSD attenuates the acquisition of the task, the magnitude of this learning attenuation is not enough to account for the poor performance of the animals in the test session.
Concerning anxiety-like behavior, in both the training and the test sessions the PSD-PSD group presented a decrease in anxiety-like behavior. However, it should be noted that pre-test PSD did not modify the percent time spent in the open arms of the apparatus in the CTRL-PSD group. This unexpected finding could be related to the well-known phenomenon of one-trial tolerance.39–42 Specifically, there is a marked attenuation or even abolition of the effects of anxiolytic agents in rats and mice by a single previous experience of the elevated plus-maze.39–42 Thus, mice in the CTRL-PSD group may have exhibited a decreased level of anxious behavior, but this anxiety could not be quantified by the behavioral tasks we employed. If this was the case, the one-trial tolerance phenomenon did not occur in the PSD-PSD group, a finding that warrants further investigation.
Concerning the locomotor activity data exhibited by mice in Experiment I, it is interesting to note that whereas pre-training PSD induced hyperlocomotion in the training session, it did not increase the total number of entries in all arms of the apparatus in the test session (CTRL-PSD group). This finding supports the possibility that the PSD-induced hyperlocomotion is related to an intra-session habituation deficit. In line with this hypothesis, PSD would induce hyperlocomotion only in novel environments (training session). In addition, if PSD impairs intra-session habituation during initial exposure to an environment, it is expected that these animals present higher locomotor activity after a second exposure to this environment (as was the case of the PSD-CTRL and PSD-PSD groups in the test session).
In Experiment II, in contrast with PSD, TSD did not promote deficits on the acquisition of the discriminative avoidance task. In fact, during the training session, there was no significant difference between control and total sleep-deprived mice in the percent time spent in the aversive enclosed arm. Furthermore, no alterations were observed in anxiety levels or motor activity of the total sleep-deprived animals during the training session. Both pre-training and pre-test TSD induced memory impairment. However, pre-test TSD was not able to counteract the memory deficits induced by pre-training TSD and vice-versa, demonstrating the lack of participation of the state-dependency phenomenon in TSD-induced memory deficits in the PM-DAT. Finally, there were no alterations in the exploration of open arms or in motor activity of animals during the test session.
In order to verify if state-dependency is critically associated with memory deficits produced by paradoxical sleep deprivation in other animal models of memory, Experiment III was performed using the passive avoidance task. In this classical animal model of memory, pre-training PSD did not promote behavioral alterations in the latency for entering the dark chamber of the passive avoidance task apparatus in the training session. Such a result could, at first, suggest that PSD did not produce alterations in the acquisition of the task. However, opposite to the PM-DAT model, the passive avoidance task does not allow the evaluation of such a process. Regarding the test session, the animals that were submitted to pre-training (PSD-CTRL group) or pre-test PSD (CTRL-PSD group) presented clear retention deficits for this task. Importantly, these pre-training- and pre-test-induced memory deficits were completely abolished by the pre-training plus pre-test PSD experimental condition (PSD-PSD group). Thus, the data obtained in the passive avoidance task corroborate the participation of the state-dependency phenomenon in the performance impairment induced by PSD, as previously described for PM-DAT data.
The possible role of state-dependent learning in TSD-induced amnesia was also evaluated in the passive avoidance task paradigm (Experiment IV). As previously observed for PSD, there were no differences between the behaviors presented by the experimental groups (CTRL or TSD groups) during the training session. On the other hand, pre-training, pre-test TSD or the combination of both (pre-training/pre-test TSD) promoted retention deficits in the test session. Interestingly, the deleterious effects of pre-training TSD on memory, as evaluated in the PM-DAT model, were not counteracted by pre-test TSD and vice-versa (i.e., the deleterious effects of pre-test TSD were not counteracted by the pre-training TSD), further corroborating the absence of state-dependency involvement.
Taken together, our results suggest that pre-training or pre-test PSD induced performance deficits in the PM-DAT and PAT by producing different arousal conditions in the training and test sessions (PSD vs. not-PSD), thereby impairing memory retrieval in a state-dependent manner. Notwithstanding, paradoxical sleep seems to be important for memory consolidation, as we have demonstrated that post-training PSD induced memory deficits in both the PM-DAT and PAT.7 Concerning TSD, both pre-training and pre-test TSD induced memory impairment regardless of state-dependency. From a functional perspective, our data suggest that memory deficits induced by non-REM sleep deprivation (or the combination of NREM sleep deprivation and PSD) are not caused by different arousal levels during training and testing.
Alternatively, it could be argued that the difference between the effects of PSD and TSD is procedural (e.g., the difference between prolonged and short-term stress effects on hippocampal function). Indeed, mice were deprived of paradoxical sleep for 72 h but were completely deprived of sleep for only 6 h. To address this question, we performed an additional experiment in which mice were stressed for 66 h before the 6-h TSD (Experiment V). These animals were thus submitted to the same duration of stress (72 h) as the PSD protocol. To assure that the arousal level was the same during both sessions, the stress procedure was performed before both PAT training and testing. Under this procedure, the TSD-TSD group again presented memory deficits when compared to the CTRL-CTRL group, suggesting that the differences between the effects of PSD and TSD were not related to the different durations of stress in each procedure.
To directly compare the effects of the PSD and TSD protocols (with important procedural differences) on both the PM-DAT and PAT, the difference from their respective mean CTRL-CTRL value was used as an adjustment measure. This alternative statistical approach confirmed that PSD- (but not TSD-) induced memory deficits in both memory tasks are critically related to state-dependent learning. Importantly, the use of this adjustment measure also revealed that memory deficits induced by TSD had a greater magnitude than those induced by PSD in the PM-DAT model, but both had a similar magnitude in the PAT model. The latter finding demonstrates that even when the same magnitude of memory deficits is compared, only the memory deficits induced by PSD are related to state-dependent learning. In addition, the former finding suggests that the PM-DAT and the PAT memory models may have different sensitivities to different sleep deprivation protocols. It also highlights the importance of non-REM sleep on memory because a 6-h TSD protocol produced a greater memory deficit in the PM-DAT than did a 72-h PSD protocol.
When combined with unpublished data from our laboratory showing that post-training TSD also impairs PAT performance in mice, our data suggest that TSD negatively affected both consolidation of the training phase and retrieval during the test. In contrast to PSD-induced deficits, the memory deficits induced by TSD were not state-dependent, thus adding to the already extensive evidence that non-REM sleep plays a critical role in memory processing. Indeed, a recent review by Walker43 suggests that slow wave sleep (SWS) may be important for memory processing because it prepares the brain for initial memory encoding before learning and facilitates the off-line consolidation of new memories after learning. From another standpoint, it could be argued that the TSD-induced memory deficits found in this study were not purely state-dependent because both SWS and paradoxical sleep were suppressed. It has been repeatedly demonstrated that SWS and paradoxical sleep have complementary functions that combine to optimize memory consolidation.44
In conclusion, this study demonstrated that pre-training or pre-testing PSD and pre-training or pre-testing TSD induced memory deficits in the PM-DAT and PAT in mice. However, only the PSD deficits were completely state-dependent. Many studies in both animals and humans have reported memory deficits induced by pre-training7,8,13,31,45–51 or pre-test PSD,14,15,31,52–60 and many different mechanisms have been proposed to explain such memory deficits. The present results strengthen the importance of investigating the involvement of the state-dependency phenomenon as a causal factor in the PSD-induced memory deficits described in previous studies.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by fellowships from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and from Associação Fundo de Pesquisa em Psicobiologia (AFIP). The authors would like to thank Ms. Gisele M. Evangelista, Ms. Lízia M. Ferreira, Ms. Thaís Fernanda Trombin and Mr. Jairo M. C. Leite for their collaboration, and Ms. Teotila R. R. Amaral, Mr. Cleomar S. Ferreira and Mr. Antonio Rodrigues dos Santos Ferreira for capable technical assistance. Monica L. Anderson., Sergio Tufik, and Roberto Frussa-Filho are recipients of CNPq fellowships.
REFERENCES
- 1.Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42:1039–42. doi: 10.1016/s0028-3908(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 2.Claro FT, Silva RH, Frussa-Filho R. Bovine brain phosphatidylserine attenuates scopolamine-induced amnesia. Physiol Behav. 1999;67:551–4. doi: 10.1016/s0031-9384(99)00099-2. [DOI] [PubMed] [Google Scholar]
- 3.Silva RH, Felicio LF, Frussa-Filho R. Ganglioside GM1 attenuates scopolamine-induced amnesia in rats and mice. Psychopharmacol (Berl.) 1999;141:111–7. doi: 10.1007/s002130050814. [DOI] [PubMed] [Google Scholar]
- 4.Patti CL, Kameda SR, Carvalho RC, et al. Effects of morphine on the plus-maze discriminative avoidance task: role of state-dependent learning. Psychopharmacol (Berl.) 2006;184:1–12. doi: 10.1007/s00213-005-0238-6. [DOI] [PubMed] [Google Scholar]
- 5.Kameda SR, Frussa-Filho R, Carvalho RC, et al. Dissociation of the effects of ethanol on memory, anxiety and motor behaviour in mice tested in the plus-maze discriminative avoidance task. Psychopharmacol (Berl.) 2007;192:39–48. doi: 10.1007/s00213-006-0684-9. [DOI] [PubMed] [Google Scholar]
- 6.Bueno OF, Lobo LL, Oliveira MG, Gugliano EB, Pomarico AC, Tufik S. Dissociated paradoxical sleep deprivation effects on inhibitory avoidance and conditioned fear. Physiol Behav. 1994;56:775–9. doi: 10.1016/0031-9384(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 7.Silva RH, Chehin AB, Kameda SR, et al. Effects of pre- or post-training paradoxical sleep deprivation on two animal models of learning and memory in mice. Neurobiol Learn Mem. 2004;82:90–8. doi: 10.1016/j.nlm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Silva RH, Abílio VC, Takatsu AL, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 2005;95(5):205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 10.Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PF, Overstreet DH, Orbach J. Disruption of passive avoidance memory by REM sleep deprivation: methodological and pharmacological considerations. Pharmacol Biochem Behav. 1982;17:1119–22. doi: 10.1016/0091-3057(82)90105-8. [DOI] [PubMed] [Google Scholar]
- 12.Skinner DM, Overstreet DH, Orbach J. Reversal of the memory-disruptive effects of REM sleep deprivation by physostigmine. Behav Biol. 1976;18:189–98. doi: 10.1016/s0091-6773(76)92086-1. [DOI] [PubMed] [Google Scholar]
- 13.Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav. 1999;67:643–9. doi: 10.1016/s0031-9384(99)00120-1. [DOI] [PubMed] [Google Scholar]
- 14.Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav. 1997;61:249–56. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 15.Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–7. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- 16.Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–62. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- 17.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–65. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polzella DJ. Effects of sleep deprivation on short-term recognition memory. J Exp Psychol. 1975;104:194–200. [PubMed] [Google Scholar]
- 19.Gruat-Masso A, Nadal-Alemany R, Coll-Andreau M, Portell-Cortes I, Marti-Nicolovius M. Effects of pretraining paradoxical sleep deprivation upon two-way active avoidance. Behav Brain Res. 1995;72:181–3. doi: 10.1016/0166-4328(96)00082-4. [DOI] [PubMed] [Google Scholar]
- 20.Oniani TN. Does paradoxical sleep deprivation disturb memory trace consolidation? Physiol Behav. 1984;33:687–92. doi: 10.1016/0031-9384(84)90032-5. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo I, Perry ML, Dias RD, et al. Endogenous opioids memory modulation and state dependency. In: Martinez JL, Jensen RA, Messing RB, Rigter H, McGaugh JL, editors. Endogenous peptides and learning and memory process. New York: Academic; 1981. pp. 269–90. [Google Scholar]
- 22.Bruins Slot LA, Colpaert FC. Opiate states of memory: receptor mechanisms. J Neurosci. 1999;19:1052–9. doi: 10.1523/JNEUROSCI.19-23-10520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes GP, Jr, Tufik S. Validation of the modified multiple platform method (MPM) of paradoxical sleep deprivation in rats. Brain Res Bull. 1994;34:453–6. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 24.Denti A, Epstein A. Sex differences in the acquisition of two kinds of avoidance behaviour in rats. Physiol Behav. 1972;8:611–5. doi: 10.1016/0031-9384(72)90083-2. [DOI] [PubMed] [Google Scholar]
- 25.Silva RH, Abílio VC, Torres-Leite D, et al. Concomitant development of oral dyskinesia and memory deficits in reserpine-treated male and female mice. Behav Brain Res. 2002a;132:171–7. doi: 10.1016/s0166-4328(01)00409-0. [DOI] [PubMed] [Google Scholar]
- 26.Silva RH, Kameda SR, Carvalho RC, et al. Effects of amphetamine on the plus-maze discriminative avoidance task in mice. Psychopharmacology. 2002b;160:9–18. doi: 10.1007/s00213-001-0948-3. [DOI] [PubMed] [Google Scholar]
- 27.Silva RH, Frussa-Filho R. The plus-maze discriminative avoidance task: a new model to study memory-anxiety interactions. Effects of chlordiazepoxide and caffeine. J Neurosci Methods. 2000;102:117–25. doi: 10.1016/s0165-0270(00)00289-2. [DOI] [PubMed] [Google Scholar]
- 28.Silva RH, Frussa-Filho R. Naltrexone potentiates both amnestic and anxiolytic effects of chlordiazepoxide in mice. Life Sci. 2002;72:721–30. doi: 10.1016/s0024-3205(02)02298-1. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho RC, Patti CL, Takatsu-Coleman AL, et al. Effects of reserpine on the plus-maze discriminative avoidance task: Dissociation between memory and motor impairments. Brain Res. 2006;1122:179–83. doi: 10.1016/j.brainres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Silva RH, Bellot RG, Vital MAB, Frussa-Filho R. Effects of long-term ganglioside GM1 administration on a new discriminative avoidance test in normal adult mice. Psychopharmacol (Berl.) 1997;129:322–8. [PubMed] [Google Scholar]
- 31.Alvarenga TA, Patti CL, Andersen ML, et al. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem. 2008;90:624–32. doi: 10.1016/j.nlm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Suchecki D, Lobo LL, Hipolide DC, Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res. 1998;7:276–81. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 33.Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensivity of dopaminergic receptors in the rat brain: Pharmacology. 1978;16:98–105. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- 34.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacol (Berl.) 1981;72:257–60. doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- 35.Troncone LR, Ferreira TM, Braz S, Silveira-Filho NG, Tufik S. Reversal of the increase in apomorphine-induced stereotypy and aggression in REM sleep deprived rats by dopamine agonist pretreatments. Psychopharmacol (Berl.) 1988;94:79–83. doi: 10.1007/BF00735885. [DOI] [PubMed] [Google Scholar]
- 36.Frussa-Filho R, Gonçalvez MTM, Andersen ML, Araújo NP, Tufik S. Paradoxical sleep deprivation potentiates amphetamine-induced behavioural sensitization by increasing its conditioned component. Brain Res. 2004;1003:188–93. doi: 10.1016/j.brainres.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 37.Conceição IM, Maiolini M, Jr, Mattia NF, Chang YH, Smaili S, Frussa-Filho R. Effect of withdrawal from long-term nifedipine administration on open-field habituation in the rat. Braz J Med Biol Res. 1994;27:1363–7. [PubMed] [Google Scholar]
- 38.Silva RH, Felicio LF, Nasello AG, Vital MA. Frussa-Filho R. Effect of ganglioside (GM1) on memory in senescent rats. Neurobiol Aging. 1996;17:583–6. doi: 10.1016/0197-4580(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 39.File SE, Mabbutt PS, Hitchcott PK. Characterization of the phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology. 1990;102:98–101. doi: 10.1007/BF02245751. [DOI] [PubMed] [Google Scholar]
- 40.Pereira JK, Vieira RJ, Konishi CT, Ribeiro RA, Frussa-Filho R. The phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze is abolished by the introduction of a motivational conflict situation. Life Sci. 1999;65:PL101–7. doi: 10.1016/s0024-3205(99)00339-2. [DOI] [PubMed] [Google Scholar]
- 41.Frussa-Filho R, Ribeiro RA. One-trial tolerance to the effects of chlordiazepoxide in the elevated plus-maze is not due to acquisition of a phobic avoidance of open arms during initial exposure. Life Sci. 2002;71:519–25. doi: 10.1016/s0024-3205(02)01709-5. [DOI] [PubMed] [Google Scholar]
- 42.Calzavara MB, Patti CL, Lopez GB, Abílio VC, Silva RH, Frussa-Filho R. Role of learning of open arm avoidance in the phenomenon of one-trial tolerance to the anxiolytic effect of chlordiazepoxide in mice. Life Sci. 2005;76:2235–46. doi: 10.1016/j.lfs.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 43.Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5:20–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 45.Hairston IS, Little MT, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 46.Dang-Vu TT, Desseilles M, Peigneux P, Maquet P. A role for sleep in brain plasticity. Pediatr Rehabil. 2006;9:98–118. doi: 10.1080/13638490500138702. [DOI] [PubMed] [Google Scholar]
- 47.Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sci. 2006;78:2833–8. doi: 10.1016/j.lfs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Piérard C, Liscia P, Philippin JN, et al. Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav. 2007;88:55–63. doi: 10.1016/j.pbb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Kalonia H, Bishnoi M, Kumar A. Possible mechanism involved in sleep deprivation-induced memory dysfunction. Methods Find Exp Clin Pharmacol. 2008;30:529–35. doi: 10.1358/mf.2008.30.7.1186074. [DOI] [PubMed] [Google Scholar]
- 50.Yang RH, Hu SJ, Wang Y, Zhang WB, Luo WJ, Chen JY. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res. 2008;1230:224–32. doi: 10.1016/j.brainres.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 51.Tiba PA, Oliveira MG, Rossi VC, Tufik S, Suchecki D. Glucocorticoids are not responsible for paradoxical sleep deprivation-induced memory impairments. Sleep. 2008;31:505–15. doi: 10.1093/sleep/31.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrien A, Dujardin K, Mandai O, Sockeel P, Leconte P. Enhancement of memory by auditory stimulation during post-learning REM sleep in humans. Physiol Behav. 1989;45:947–50. doi: 10.1016/0031-9384(89)90219-9. [DOI] [PubMed] [Google Scholar]
- 53.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 54.Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 55.Rauchs G, Bertran F, Guillery-Girard B, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- 56.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–9. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Palchykova S, Crestani F, Meerlo P, Tobler I. Sleep deprivation and daily torpor impair object recognition in Djungarian hamsters. Physiol Behav. 2006;87:144–53. doi: 10.1016/j.physbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara M, Iaria G, De Gennaro L, et al. The role of sleep in the consolidation of route learning in humans: a behavioural study. Brain Res Bull. 2006;71:4–9. doi: 10.1016/j.brainresbull.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Fu J, Li P, Ouyang X, et al. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–92. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 60.Gais S, Köster S, Sprenger A, Bethke J, Heide W, Kimmig H. Sleep is required for improving reaction times after training on a procedural visuo-motor task. Neurobiol Learn Mem. 2008;90:610–5. doi: 10.1016/j.nlm.2008.07.016. [DOI] [PubMed] [Google Scholar]