Abstract
Background:
During sleep, sudden drops in pulse wave amplitude (PWA) measured by pulse oximetry are commonly associated with simultaneous arousals and are thought to result from autonomic vasoconstriction. In the present study, we determine whether PWA drops were associated with changes in cortical activity as determined by EEG spectral analysis.
Methods:
A 20% decrease in PWA was chosen as a minimum for a drop. A total of 1085 PWA drops from 10 consecutive sleep recordings were analyzed. EEG spectral analysis was performed over 5 consecutive epochs of 5 seconds: 2 before, 1 during, and 2 after the PWA drop. EEG spectral analysis was performed over delta, theta, alpha, sigma, and beta frequency bands. Within each frequency band, power density was compared across the five 5-sec epochs. Presence or absence of visually scored EEG arousals were adjudicated by an investigator blinded to the PWA signal and considered associated with PWA drop if concomitant.
Results:
A significant increase in EEG power density in all EEG frequency bands was found during PWA drops (P < 0.001) compared to before and after drop. Even in the absence of visually scored arousals, PWA drops were associated with a significant increase in EEG power density (P < 0.001) in most frequency bands.
Conclusions:
Drops in PWA are associated with a significant increase in EEG power density, suggesting that these events can be used as a surrogate for changes in cortical activity during sleep. This approach may prove of value in scoring respiratory events on limited-channel (type III) portable monitors.
Citation:
Delessert A; Espa F; Rossetti A; Lavigne G; Tafti M; Heinzer R. Pulse wave amplitude drops during sleep are reliable surrogate markers of changes in cortical activity. SLEEP 2010;33(12):1687-1692.
Keywords: Arousal, autonomic activation, electroencephalography, limited channel portable monitor, sleep breathing disorders
OBSTRUCTIVE SLEEP APNEA SYNDROME (OSAS) IS A SLEEP BREATHING DISORDER CHARACTERIZED BY INTERMITTENT UPPER AIRWAY NARROWING OR collapse during sleep that occurs in 2% to 5% of adults.1–4 Most OSAS patients complain of excess daytime sleepiness, cognitive impairment, and decreased psychological well-being.5–11 These daytime effects lead to an increased risk of motor vehicle accidents, work inefficiency, and have a negative social impact.12–15 Altered sleep architecture and decreased sleep efficiency are believed to result from sleep fragmentation caused by brief repeated arousals from sleep.16–18 Arousals have been found to be associated with central autonomic activation,19 which leads to increased sympathetic activity and resulting peripheral vasoconstriction. Repeated exposure to autonomic and hemodynamic stressors may be responsible for the significant association of OSAS with cardiovascular and cerebrovascular disorders.20–22 Autonomic activation concomitant with arousals from sleep induces acute measurable hemodynamic changes such as elevated arterial pressure and heart rate, and altered pulse transit time (PTT) and skin blood flow.23–31
Changes in PWA measured by finger plethysmography or photoplethysmography have been shown to be a reliable method of determining sympathetic activation.32–34 Finger plethysmography measures pulsatile blood volume in the fingertip using a peripheral arterial tonometry (PAT) device. Photoplethysmography is a noninvasive technique that measures the relative absorption of red light and infrared light across the finger. Arterial blood flow pulsation passing through finger arteries modulates light absorption and generates a pulse wave signal. This signal can be easily derived from conventional pulse oximeters and, unlike PAT, does not require a variable pressure at the fingertip. Photoplethysmographic pulse waves have also been used as markers of finger vasoconstriction.35
The gold standard diagnostic tool for OSAS is polysomnography (PSG), but for practical reasons limited-channel sleep recordings without EEG (type III portable monitors) are increasingly used as an alternative.36 While apneas are easily scored in the latter setting, hypopneas, which require, according to the 2007 AASM manual either a concomitant drop in oxygen saturation of 3% or an arousal (alternative definition),37 are more difficult to detect, because no electroencephalography (EEG) channels are available in these recordings. Moreover arousals are required to score respiratory effort related arousals (RERA).
The aim of this study was to determine whether PWA drops were associated with changes in cortical activity as measured by EEG spectral analysis, and whether these events could be considered as surrogates for arousals on limited-channel sleep recordings.
METHODS
Subjects and Recordings
Data from 10 consecutive PSG recordings (70% of men) were selected from the patient database of our sleep center (CIRS). All 10 patients were referred for a suspicion of sleep disordered breathing. None had prior diagnosis of a central nervous system disease or were using medications affecting the central nervous system. The study was performed in compliance with University of Lausanne institutional ethical guidelines.
Sleep Recordings
Overnight recordings were performed in individual bedrooms using Embla N7000 (Embla Systems, Broomfield, CO) acquisition systems. Four EEG electrodes (C3, C4, O1, and O2) were applied to the scalp using the International 10–20 System,38 together with 2 electro-oculogram (EOG; one to each outer canthus) and 2 surface electromyogram (EMG) electrodes over submental muscles. EEG and EOG electrodes were referenced to the linked earlobes (A1 + A2). Chest and abdominal movements, nasal air pressure, body position, sounds, and infrared video were also simultaneously recorded. Oxyhemoglobin saturation was recorded using a Nonin pulse oximeter (Nonin Medical, Inc., Plymouth, MN), using a sampling frequency of 10 Hz. All recordings were performed with the assistance of a registered PSG technologist.
Data Analysis
Data were visually analyzed using Somnologica software version 5.1 (Embla Systems, Broomfield, CO), which displays PWA signal in addition to the PSG signals described above.
First, an experienced investigator blinded to the PWA channel performed the EEG analysis for the whole night. Sleep stages were scored according to standard criteria.39 Sleep arousals were defined as an abrupt shift in EEG frequency, including a theta-alpha pattern and/or a frequency higher than 16 Hz (but not spindles), lasting ≥ 3 s with ≥ 10 s of stable sleep preceding the change (American Sleep Disorders Association 1999 criteria).40,41 If duration exceeded 15 s, the event was scored as an awakening.
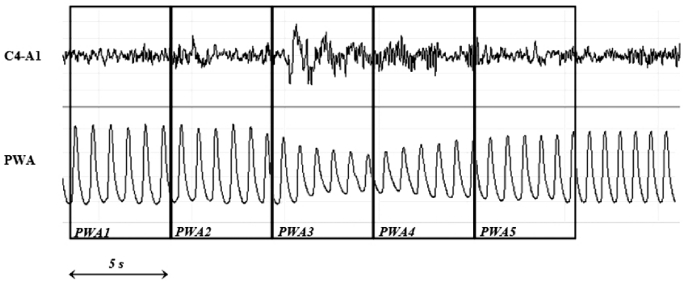
Two investigators (AD, FE), blinded to the corresponding EEG channels and arousal scoring subsequently, analyzed the PWA signal. For each PSG recording, more than 100 consecutive PWA drops and at least the first complete NREM/REM sleep cycle were analyzed. Finger PWA was measured for each cardiac cycle as the difference between the peak and nadir values of the pulse oximeter waveform. In order to calculate the percentage of decrease in each PWA, highest and lowest amplitudes for each PWA drops were measured using an electronic ruler provided by the software company. A 20% decrease in PWA was chosen as cutoff, as we considered it was the smallest identifiable drop on visual inspection. The recording period surrounding each PWA drop was divided into 5 epochs of 5 s: before (5-s epochs 1 and 2), during (5-s epoch 3), and after (5-s epochs 4 and 5) PWA drop (Figure 1). PWA drop was considered to be linked to visually scored arousal when the arousal occurred during the five 5-s epochs period (usually epochs 4 and 5).
Figure 1.
Example of PWA drop concomitant with EEG arousal. EEG spectral analysis was performed over each of the 5 consecutive 5-s epochs: before (5-s epochs 1 and 2), during (5-s epoch 3) and after (5-s epochs 4 and 5) the PWA drops.
A total of 1085 PWA drops from 10 consecutive PSG recordings were analyzed. EEG spectral analysis was performed for 5-s epochs 1-5 as defined above. The C4-A1 EEG channel was analyzed using a discrete fast Fourier transform (FFT) applied to each selected artifact-free EEG 5-s epoch with a frequency resolution of 0.2 Hz. Each 5-s epoch was first treated with a Hanning window prior to computing the power spectra (in μV2). The whole spectrum was divided into the following frequency bands: delta (0.5 to 4.0 Hz); theta (4.1 to 8.0 Hz); alpha (8.1 to 12.0 Hz); sigma (12.1 to 16.0 Hz), and beta (16.1 to 30.0 Hz). The power (in μV/Hz) of each frequency band was normalized and expressed as a percentage of total power.
Statistical Analysis
Data are reported as mean ± standard deviation (SD). Since data were not normally distributed, the difference in power densities between 5-s epochs 1–5 was determined using a one-way repeated measure ANOVA by rank. A post hoc Tukey test was used to determine significant differences between all 5-s epoch pairs using SigmaStat software version 3.0 (Systat Software, San Jose, CA). The difference in magnitudes between PWA associated and not associated with an arousal was calculated using an unpaired student t-test. Positive predictive value (PPV) was calculated by dividing the number of true positives by the sum of false positives and true positives. Sensitivity was calculated by dividing the number of true positives by the sum of false negatives and true positives. The negative predictive value and specificity were not calculated since true negative events (i.e., no PWA drop and no EEG arousal) could not be determined.
RESULTS
Anthropometric and pertinent clinical data of the 10 patients are shown on Table 1. Most of the subjects presented with moderate OSAS. None of them suffered from significant periodic limb movement.
Table 1.
Baseline characteristics and key polysomnographic results
| Mean | SD | |
|---|---|---|
| Age (year) | 41 | 17.31 |
| BMI (kg/m2) | 26.5 | 6.28 |
| Epworth score | 9.8 | 3.52 |
| PLM index (events/h) | 0.38 | 0.70 |
| AHI (events/h) | 9.7 | 6.50 |
| Arousal index (events/h) | 33.2 | 15.30 |
| Sleep efficiency (%) | 84.8 | 5.87 |
The total number of microarousals and PWA drops in the analyzed segments of the 10 recordings were 1188 and 1085, respectively. Of the 1085 PWA drops, 769 (70.9%) were associated with a visually recognized microarousal and 316 (29.1%) were not associated with EEG microarousal. The mean amplitude of the PWA drop was greater when a microarousal was associated than when no microarousal was detected (53.9% ± 11.9% vs 47.7% ± 10.6%, P < 0.0001). Overall, the positive predictive value and sensitivity of PWA drops for electroencephalographic microarousals were 71% and 65%, respectively. In NREM 1 and 2 sleep stages, the positive predictive value was higher (91.4% for both) than in other sleep stages (Table 2).
Table 2.
Number of microarousals and PWA drops by sleep stage
| NREM 1 | NREM 2 | NREM 3 | NREM 4 | REM | Total | |
|---|---|---|---|---|---|---|
| Microarousal (n) | 264 | 820 | 20 | 20 | 64 | 1188 |
| PWA drops (n) | 93 | 673 | 56 | 111 | 152 | 1085 |
| PWA drops without arousal (n) | 8 | 58 | 37 | 95 | 118 | 316 |
| PWA drops with arousal (n) | 85 | 615 | 19 | 16 | 34 | 769 |
| Sensitivity (%) | 32.2 | 75.0 | 95.0 | 80.0 | 53.1 | 64.7 |
| Positive Predictive Value (%) | 91.4 | 91.4 | 33.9 | 14.4 | 22.4 | 70.9 |
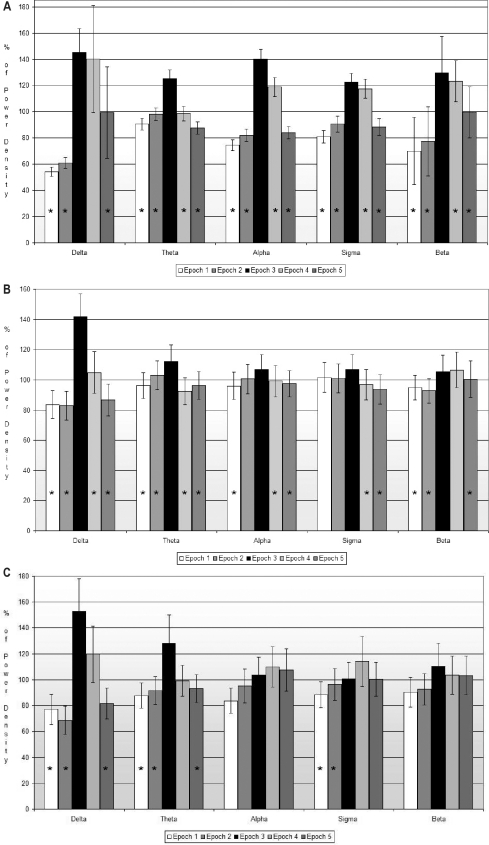
The global analysis of all PWA drops revealed a significant increase in EEG power densities involving all frequencies (global effect, P < 0.001). Power density in 5-s epoch 3 (during PWA drop) was greater in all frequency bands compared to 5-s epochs 1 and 2 (before drop), and 5-s epochs 4 and 5 (after drop). The most significant power density difference was found in the beta frequency between 5-s epoch 3 (during PWA drop) and the other four 5-s epochs. In all frequency bands, except for the delta band, power density showed a significant decrease during 5-s epochs 4 and 5 compared with 5-s epoch 3 (Figure 2A).
Figure 2.
Relative power density for each 5-s epoch by frequency band. The power of each 5-s epoch is expressed as percentage of the mean power density of the whole 25-s period surrounding the PWA drop. 2A: All PWA drops (n = 1085). 2B: PWA drops without EEG Arousal (n = 316). 2C: PWA drops during REM sleep (n = 149). *Significant (P < 0.05) difference in power density compared to 5-s epoch 3 (during PWA drop) as determined by the post hoc Tukey test.
A subgroup analysis restricted to all PWA drops that were not associated with an arousal also revealed a significant increase in EEG power in all frequency bands during PWA drops (global effect P < 0.001 except for alpha, P = 0.015). Pairwise comparisons between 5-s epoch 3 and the other 5-s epochs are shown in Figure 2B. During REM sleep, PWA drops were associated with significant cortical EEG changes in the delta and theta bands and not in alpha or beta bands (Figure 2C)
DISCUSSION
The main finding of this study is that PWA drops measured by finger photoplethysmography are tightly associated with an increase in EEG power density, indicative of a change in cortical activity. This increase in EEG power density is present even when PWA drop is not associated with an EEG arousal.
During PWA drops, EEG power density was not only increased in high-frequency bands such as alpha and beta but also in lower frequencies, including theta and delta bands, which may appear counterintuitive. This suggests that low-voltage, fast rhythmic EEG activation is not the only EEG sign of autonomic arousal. Similar observations have been previously reported at the end of respiratory events by Black et al.42 The authors reported a significant increase in delta power over a period from 6 s before to 2 s after esophageal pressure reversal (return to baseline level) in upper airway resistance syndrome (UARS). Pulse transit time (PTT) variations have also been shown to be associated with an increased delta power density43; however, contrary to our findings, Black et al. did not find significant differences in the other frequency bands. One possible explanation for the discrepancy with our findings is the time delay reported by the authors between EEG activation and the actual detection of a difference in PTT. Increased power density in low frequency bands associated with an autonomic reaction such as a PWA drop may represent a central nervous system mechanism to prevent arousal and promote sleep continuity. This is also suggested by the longer duration of the increase in delta activity when PWA drops were associated with arousal (no significant decrease in 5-s epochs 4 and 5), whereas delta power decreased significantly after PWA drops in the absence of arousal (Figures 2A and 2B).
We also found that PWA drops with a concomitant increase in EEG power density may occur even in the absence of standard arousal criteria.40,41 This is probably due to the fact that some PWA drops may be caused by subcortical brain activation,44 detectable in the EEG only with a quantitative method such as spectral analysis and not by visual inspection alone. However, the magnitude of PWA drop was significantly smaller in the absence of arousal (P < 0.0001). Black et al. also found that esophageal pressure reversal in UARS may occur without visually scored EEG arousal, but with significant EEG activation as detected by spectral analysis.42 The sleepiness commonly reported in UARS patients despite a low arousal index suggests that subtle changes in cortical activity may induce daytime vigilance impairment. Because the clinical relevance of subcortical arousals are still largely unknown, it remains to be investigated whether autonomic markers such as PWA signal represent a more reliable method of determining changes in cortical activity than traditional EEG visual inspection. Our findings warrant large prospective cohort studies to assess the impact of autonomic arousals on daytime sleepiness, traffic accidents, and cardiovascular morbidity.
Different markers of autonomic activation such as PTT, finger plethysmography, and heart rate variation have been proposed in previous studies.23–33 However, when arousals were experimentally induced by auditory tones in normal subjects, PWA drops yielded the best ROC curve result for detecting EEG-scored arousals compared with the other techniques.23 In another study, arousals following respiratory events were shown to induce a greater relative change in PWA than in heart rate.33 A proportional relationship between arousal duration and the magnitude of PWA drop was also reported by the same authors. Unlike the above studies analyzing autonomic responses following provoked or spontaneous arousal, we based our analysis on the autonomic signal (PWA signal) and concomitant EEG. This technique allows us to assess whether PWA drops can be used as a surrogate for EEG-defined arousals. Subtle respiratory events, such as UARS events or hypopnea ending with an arousal (but without significant oxygen saturation drop), may be underestimated in limited-channel recordings (type III portable monitors) because of the absence of EEG signal. The highly significant temporal association we found between PWA drops and EEG activation suggests that this technique can be used as a surrogate for EEG changes in cortical activity and could thus become a helpful tool for the detection of hypopnea or UARS events on limited-channel recordings. While the overall positive predictive value was only 71%, it was 91.4% in NREM stages 1 and 2 when most respiratory events usually occur.
PWA amplitude has previously been shown to improve inter-scorer reliability for the detection of arousals on PSG recordings and to increase the respiratory disturbance index (RDI) when PWA drops were considered arousal equivalents for respiratory events scoring.45 However, considering that in our study the number of PWA drops exceeded by about 30% the number of EEG-defined arousals, a possible overestimation of respiratory events using this technique cannot be excluded, since other stimuli than breathing disorders (limb movement, noise) can induce PWA drops. Before PWA drops can be routinely used as a scoring help to detect subtle respiratory events, a large prospective study comparing a scoring technique with PWA drops (without EEG-defined arousals) and a standard scoring with a full PSG recording will be needed.
There are a few limitations to this study. First, we chose an arbitrary cutoff of 20% to consider a PWA drop significant. As mentioned earlier, we used this cutoff for practical reasons, since it represents in our view the smallest identifiable PWA drop on visual inspection. Second, the method we used did not allow the detection of true negative events (no PWA drop and no EEG arousal). Therefore, specificity of PWA drops could not be calculated. We actually observed PWA drops not associated with visually scored arousals, but these events might relate to subtle EEG activations as suggested by spectral analysis. Additionally, we analyzed consecutive unselected PSG recordings for our study, resulting in a sample of patients with moderate and not severe OSAS. Nevertheless, our patients had a wide variety of respiratory events including upper airway resistance, hypopnea, and apnea, whereas severe OSA patients often show only obstructive sleep apnea. Mild to moderate OSA probably represents the population for which PWA drop may prove to be the most useful, since hypopnea may be missed with the conventional scoring of hypopnea in limited-channel sleep recordings. Finally, in the 2007 AASM manual, more emphasis is given to oxygen saturation drops in the definition of hypopnea than to arousals. Despite these changes, we still believe that an arousal surrogate is useful to score respiratory events in limited channel recordings since arousals can still be used to score hypopnea (alternative definition), and because they are mandatory to score respiratory effort related arousals (RERA).
CONCLUSION
Pulse wave amplitude drops observed on polygraphic sleep recordings are closely associated with increased EEG power density over a large frequency range. This suggests that drops in PWA could be considered as markers of changes in cortical activity, even in the absence of visually scored arousal. Increasing understanding of this phenomenon may possibly lead to its use as a surrogate for arousal in limited-channel recordings or as new method of quantification for sleep fragmentation.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Tafti has received research support from, is on the advisory board of, and has participated in speaking engagements for UCB-Pharma. Dr. Lavigne has consulted and/or participated in speaking engagements for Wyeth Canada, Pfizer Canada, and UCB Belgium. Dr. Heinzer has consulted for Pfizer and has received research support from ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
Work supported by: Swiss Pulmonary Society Fund for Research, Lancardis foudation and Lausanne University Young Investigator Grant.
All authors contributed to the design of the study. Data were collected by AD and FE. Data were analyzed by AD, FE, AR, and RH. The manuscript was written by AD, AR, and RH, and was reviewed by each author.
ABBREVIATIONS
- AHI
Apnea-hypopnea index
- CIRS
Centre d'Investigation et de Recherche sur le Sommeil
- EEG
Electroencephalography
- EMG
Electromyogram
- EOG
Electrooculogram
- FFT
Fast Fourier transform
- OSAS
Obstructive sleep apnea syndrome
- PPV
Positive predictive value
- PSG
Polysomnography
- PTT
Pulse transit time
- PWA
Pulse wave amplitude
- RDI
Respiratory disturbance index
- UARS
Upper airway resistance syndrome
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Bresnitz EA, Goldberg R, Kosinski RM. Epidemiology of obstructive sleep apnea. Epidemiol Rev. 1994;16:210–27. doi: 10.1093/oxfordjournals.epirev.a036151. [DOI] [PubMed] [Google Scholar]
- 3.Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing. Prevalence. Am J Respir Crit Care Med. 1995;152:711–716. doi: 10.1164/ajrccm.152.2.7633731. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth T, Hartse KM, Zorick F, Conway W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airways sleep apnea. Sleep. 1980;3:425–39. [PubMed] [Google Scholar]
- 6.Whyte KF, Allen MB, Jeffrey AA, Gould GA, Douglas NJ. Clinical features of the sleep apnoea/hypopnoea syndrome. Q J Med. 1989;72:659–66. [PubMed] [Google Scholar]
- 7.El-Ad B, Lavie P. Effects of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17:277–82. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH. Cognitive effects of sleep and sleep fragmentation. Sleep. 1993;16(Suppl 8):S65–S67. doi: 10.1093/sleep/16.suppl_8.s65. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 10.Béedard MA, Montplaisir J, Richer F, Malo J. Nocturnal hypoxemia as a determinant of vigilance impairment in sleep apnea syndrome. Chest. 1991;100:367–70. doi: 10.1378/chest.100.2.367. [DOI] [PubMed] [Google Scholar]
- 11.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with the sleep apnea/hypopnea syndrome. Arch Int Med. 1992;152:538–41. [PubMed] [Google Scholar]
- 12.Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea – II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 13.Teréan-Santos J, Jiméenez-Géomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 14.George CF, Nickerson PW, Hanly PJ, Millar TW, Kryger MH. Sleep apnoea patients have more automobile accidents. Lancet. 1987;330:447. doi: 10.1016/s0140-6736(87)90974-3. [DOI] [PubMed] [Google Scholar]
- 15.Findley LJ, Levinson MP, Bonnie RJ. Driving performance and automobile accidents in patients with sleep apnea. Clin Chest Med. 1992;13:427–35. [PubMed] [Google Scholar]
- 16.Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94:32–7. doi: 10.1378/chest.94.1.32. [DOI] [PubMed] [Google Scholar]
- 17.Roehrs T, Zorick F, Wittig R, Conway W, Roth T. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest. 1989;95:1202–6. doi: 10.1378/chest.95.6.1202. [DOI] [PubMed] [Google Scholar]
- 18.Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997;155:1596–601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- 19.Smith RP, Veale D, Péepin JL, Léevy PA. Obstructive sleep apnoea and the autonomic nervous system. Sleep Med Rev. 1998;2:69–92. doi: 10.1016/s1087-0792(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 20.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 21.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 22.Zou D, Grote L, Radlinski J, Eder DN, Lindblad U, Hedner J. Nocturnal pulse wave attenuation is associated with office blood pressure in a population based cohort. Sleep Med. 2009;10:836–43. doi: 10.1016/j.sleep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 24.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction – A new marker of obstructive sleep apnea. Sleep. 1999;22:939–46. [PubMed] [Google Scholar]
- 25.Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25:850–5. [PubMed] [Google Scholar]
- 26.Adachi H, Mikami A, Kumano-go T, et al. Clinical significance of pulse rate rise during sleep as a screening marker for the assessment of sleep fragmentation in sleep-disordered breathing. Sleep Med. 2003;4:537–42. doi: 10.1016/j.sleep.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000;111:1611–19. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 28.Lofaso F, Goldenberg F, d'Ortho MP, Coste A, Harf A. Arterial blood pressure response to transient arousals from NREM sleep in nonapneic snorers with sleep fragmentation. Chest. 1998;113:985–91. doi: 10.1378/chest.113.4.985. [DOI] [PubMed] [Google Scholar]
- 29.Pitson DJ, Stadling JR. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998;7:53–9. doi: 10.1046/j.1365-2869.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 30.Pitson DJ, Stradling JR. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:685–92. doi: 10.1183/09031936.98.12030685. [DOI] [PubMed] [Google Scholar]
- 31.Pitson D, Chhina N, Knijn S, van Herwaaden M, Stradling J. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci. 1994;87:269–73. doi: 10.1042/cs0870269. [DOI] [PubMed] [Google Scholar]
- 32.Grote L, Zou D, Kraiczi H, Hedner J. Finger plethysmography-a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003;136:141–52. doi: 10.1016/s1569-9048(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 33.Haba-Rubio J, Darbellay G, Herrmann FR, et al. Obstructive sleep apnea syndrome: effect of respiratory events and arousal on pulse wave amplitude measured by photoplethysmography in NREM sleep. Sleep Breath. 2005;9:73–81. doi: 10.1007/s11325-005-0017-y. [DOI] [PubMed] [Google Scholar]
- 34.Jaryal AK, Selvaraj N, Santhosh J, Anand S, Deepak KK. Monitoring of cardiovascular reactivity to cold stress using digital volume pulse characteristics in health and diabetes. J Clin Monit Comput. 2009;23:123–30. doi: 10.1007/s10877-009-9174-z. [DOI] [PubMed] [Google Scholar]
- 35.Johnson LC, Lubin A. The orienting reflex during waking and sleeping. Electroencephalogr Clin Neurophysiol. 1967;22:11–21. doi: 10.1016/0013-4694(67)90004-1. [DOI] [PubMed] [Google Scholar]
- 36.Collop NA. Portable monitoring for the diagnosis of obstructive sleep apnea. Curr Opin Pulm Med. 2008;14:525–9. doi: 10.1097/MCP.0b013e328312ed4a. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 38.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 39.Rechtshaffen A, Kales A. Bethesda, MD: US Dept of Health, Education, and Welfare, Public Health Services, Neurological Information Network; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 40.EEG arousals: scoring rules and examples. A preliminary report from Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 41.Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity and alternatives. J Clin Sleep Med. 2007;3:133–45. [PubMed] [Google Scholar]
- 42.Black JE, Guilleminault C, Colrain IM, Carrillo O. Upper airway resistance syndrome. Central electroencephalographic power and changes in breathing effort. Am J Respir Crit Care Med. 2000;162:406–11. doi: 10.1164/ajrccm.162.2.9901026. [DOI] [PubMed] [Google Scholar]
- 43.Poyares D, Guilleminault C, Rosa A, Ohayon M, Koester U. Arousal, EEG spectral power and pulse transit time in UARS and mild OSAS subjects. Clin Neurophysiol. 2002;113:1598–606. doi: 10.1016/s1388-2457(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 44.Haléasz P, Terzano M, Parrino L, Béodizs R. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 45.Zacharia A, Haba-Rubio J, Simon R, et al. Sleep apnea syndrome: improved detection of respiratory events and cortical arousals using oximetry pulse wave amplitude during polysomnography. Sleep Breath. 2008;12:33–8. doi: 10.1007/s11325-007-0126-x. [DOI] [PubMed] [Google Scholar]