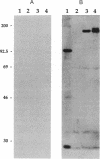
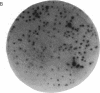
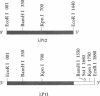
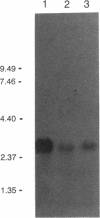
Abstract
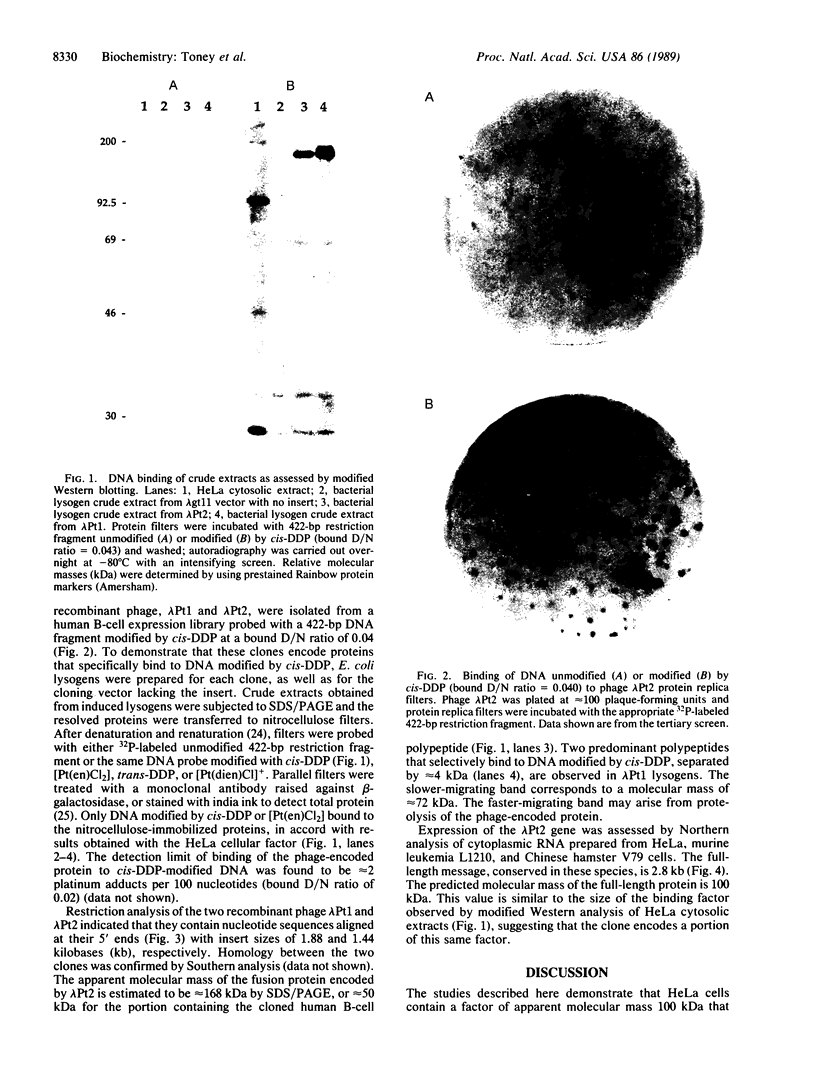
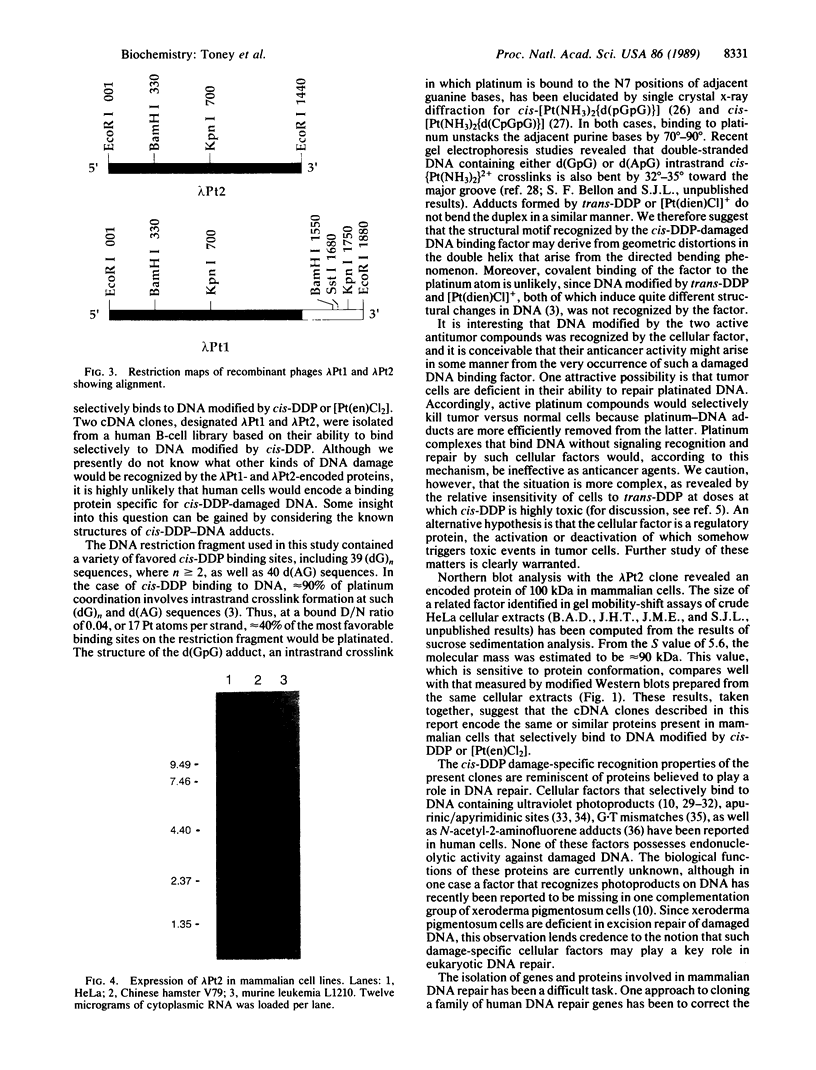
DNA modified by the antitumor drug cis-diamminedichloroplatinum(II) (cis-DDP or cisplatin) was used to identify a factor in mammalian cells that binds to cis-DDP-damaged DNA and hence may play a role in repair. This factor selectively recognizes double-stranded DNA fragments modified by cis-DDP or [Pt(en)Cl2] (en, ethylenediamine). Little or no binding occurs to unmodified double-stranded DNA or to DNA modified with the clinically ineffective compounds trans-DDP and [Pt(dien)Cl]Cl (dien, diethylenetriamine). Low levels of binding to single-stranded DNA modified by cis-DDP are observed. The apparent molecular mass of the factor in a variety of mammalian cells is approximately 100 kDa, as determined by modified Western blotting. Two recombinant phage have been isolated from a human B-cell lambda gt11 library by using a cis-DDP-modified DNA restriction fragment as a probe. The two clones have insert sizes of 1.88 and 1.44 kilobases and are aligned at their 5' ends. The polypeptides encoded by the recombinant phage exhibit DNA binding properties similar to those of the cellular factor identified in crude extracts prepared from mammalian cells. Northern analysis with one of the clones revealed an mRNA of 2.8 kilobases that is conserved in humans and rodents. The methods used here should be applicable in studies of other damage-specific DNA binding proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Solomon M. J., Varshavsky A., Lippard S. J. In vivo effects of cis- and trans-diamminedichloroplatinum(II) on SV40 chromosomes: differential repair, DNA-protein cross-linking, and inhibition of replication. Biochemistry. 1985 Dec 17;24(26):7533–7540. doi: 10.1021/bi00347a005. [DOI] [PubMed] [Google Scholar]
- Deutsch W. A., Linn S. DNA binding activity from cultured human fibrolasts that is specific for partially depurinated DNA and that inserts purines into apurinic sites. Proc Natl Acad Sci U S A. 1979 Jan;76(1):141–144. doi: 10.1073/pnas.76.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Johnson M. S., Husain I., Van Houten B., Thomas D. C., Sancar A. Domainal evolution of a prokaryotic DNA repair protein and its relationship to active-transport proteins. Nature. 1986 Oct 2;323(6087):451–453. doi: 10.1038/323451a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Grossman L. A DNA binding protein from human placenta specific for ultraviolet damaged DNA. Biochemistry. 1976 Jun 1;15(11):2402–2408. doi: 10.1021/bi00656a024. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Lucas J. L., Dannenberg A. A damage-specific DNA binding protein. Large scale purification from human placenta and characterization. J Biol Chem. 1982 Jun 10;257(11):6394–6401. [PubMed] [Google Scholar]
- Feldberg R. S. On the substrate specificity of a damage-specific DNA binding protein from human cells. Nucleic Acids Res. 1980 Mar 11;8(5):1133–1143. doi: 10.1093/nar/8.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Characterization of genes and proteins involved in excision repair of human cells. J Cell Sci Suppl. 1987;6:111–125. doi: 10.1242/jcs.1984.supplement_6.7. [DOI] [PubMed] [Google Scholar]
- Jiricny J., Hughes M., Corman N., Rudkin B. B. A human 200-kDa protein binds selectively to DNA fragments containing G.T mismatches. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8860–8864. doi: 10.1073/pnas.85.23.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U. Comparison of apurinic DNA-binding protein from an ataxia telangiectasia and a HeLa cell line. Evidence for an altered processing of apurinic/apyrimidinic endonuclease. J Biol Chem. 1985 Dec 5;260(28):14918–14924. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Moranelli F., Lieberman M. W. Recognition of chemical carcinogen-modified DNA by a DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3201–3205. doi: 10.1073/pnas.77.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff S. C., Beck D. J., Rupp W. D. Repair of plasmid DNA damaged in vitro with cis- or trans-diamminedichloroplatinum(II) in Escherichia coli. Mutat Res. 1987 Mar;183(2):129–137. doi: 10.1016/0167-8817(87)90055-1. [DOI] [PubMed] [Google Scholar]
- Rice J. A., Crothers D. M., Pinto A. L., Lippard S. J. The major adduct of the antitumor drug cis-diamminedichloroplatinum(II) with DNA bends the duplex by approximately equal to 40 degrees toward the major groove. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4158–4161. doi: 10.1073/pnas.85.12.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Sherman S. E., Gibson D., Wang A. H., Lippard S. J. X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3)2(d(pGpG))]. Science. 1985 Oct 25;230(4724):412–417. doi: 10.1126/science.4048939. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Sonenshein G. E., Siekevitz M., Siebert G. R., Gefter M. L. Control of immunoglobulin secretion in the murine plasmacytoma line MOPC 315. J Exp Med. 1978 Jul 1;148(1):301–312. doi: 10.1084/jem.148.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. S., Kuhnlein U. DNA-binding protein from HeLa cells that binds preferentially to supercoiled DNA damaged by ultraviolet light or N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1982 May 31;697(2):202–212. doi: 10.1016/0167-4781(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]