Abstract
Study Objectives:
Acute myocardial infarction (MI) is followed, within a few hours, by neuronal loss in the central nervous system (CNS), including the limbic system, the hypothalamus, and the brainstem. Sleep before and after MI was investigated in the first experiment. In a parallel experiment, 2 weeks after MI, we quantified brainstem cholinergic neurons known to control paradoxical sleep (PS).
Measurements and Results:
Data were obtained from 28 adult male Sprague-Dawley rats weighing 350-375 g and maintained under a 12-12 light-dark cycle in 2 experiments on 16 and 12 rats, respectively. The 16 animals in the first experiment were implanted with chronic electroencephalographic (EEG) and electromyographic (EMG) electrodes. A week after surgery, these animals were habituated for 2 days to the recording equipment, and baseline sleep was charted for 24 h. The next morning, MI was induced in 8 rats by occluding the left anterior descending coronary artery for 40 min. The remaining 8 rats served as sham-operated controls. Sleep was recorded again 2 weeks after MI. The number of choline acetyltransferase (ChAT)-positive neurons was counted in the second, parallel experiment on 6 MI and 6 sham rats.
Compared to the sham controls, MI rats displayed longer latency to sleep onset, shorter latency to paradoxical sleep (PS), and curtailed PS duration. The number of ChAT-positive neurons in the pedunculopontine tegmentum (PPT) area of MI rats was significantly decreased compared to the sham controls, while the number of laterodorsal tegmentum (LDT) cholinergic neurons was not different.
Conclusion:
Acute MI is accompanied, within 2 weeks, by PS-specific insomnia that can be explained, at least partly, by a specific loss of cholinergic neurons in an area known to control PS.
Citation:
Bah TM; Laplante F; Wann BP; Sullivan R; Rousseau G; Godbout R. Paradoxical sleep insomnia and decreased cholinergic neurons after myocardial infarction in rats. SLEEP 2010;33(12):1703-1710.
Keywords: Paradoxical sleep, myocardial infarction, acetylcholine, depression, rat, model
ACUTE MYOCARDIAL INFARCTION (MI) IS FOLLOWED, WITHIN A FEW MONTHS, BY MAJOR DEPRESSION IN 15% to 30% OF PATIENTS.1 WE RECENTLY replicated this phenomenon in a rat model in which behavioral signs of depression, such as despair and anhedonia, were encountered 2 weeks after MI and reversed by antidepressant treatments.2–4 In humans, depression is also accompanied by sleep disorders, including difficulties initiating and maintaining sleep, less slow wave sleep (SWS) together with facilitation of rapid eye movement (REM) sleep, also identified as paradoxical sleep (PS).5 These sleep disorders have also been documented in rat models of depression, such as learned helplessness,6 chronic mild stress,7 olfactory bulbectomy,8 the Flinders Sensitive Line,9 and the neonatal clomipramine model of endogenous depression.10 The present studies were undertaken to verify if the post-MI rat model of depression3 replicates the sleep findings reported in other animal models of depression and whether brainstem cholinergic neurons, known to be involved in PS control, are also targeted. Our hypotheses were that 2 weeks after MI and compared to sham-operated controls, MI rats would show: (a) increased sleep latency; (b) decreased total sleep time; (c) diminished SWS; (d) reduced latency to PS; and (e) greater total time spent in PS.
METHODS
Experiment 1: Sleep Recording
This experiment was performed to characterize sleep architecture after MI in rats.
Animals
Sixteen adult male Sprague-Dawley rats weighing 350-375 g were acquired through regular trade (Charles Rivers, St-Constant, Quebec, Canada) and housed individually under standard conditions of 12/12-h light/dark cycles with light onset at 08:00, temperature 21-25°C, relative humidity 40%-50%, food and water ad libitum. All procedures described below were conducted according to Canadian Council on Animal Care guidelines and were approved by the animal ethics committee of the Biomedical Research Centre at Hôpital du Sacré-Coeur de Montréal.
Surgical Procedures
Sleep
The animals were first anesthetized with ketamine/xylazine (50 mg/kg i.m. and 5 mg/kg i.m., respectively), then maintained on isoflurane (1%-1.5%) ventilation. Body temperature was controlled by rectal probe connected to a thermostat-equipped heating pad set at 37°C. EEG electrodes were positioned bilaterally with stainless steel screws (diameter = 1.2 mm) in the skull bone over the sensorimotor and frontal cortices; a reference electrode was placed at midline over the cerebellum. Three EMG electrodes, made of fine, flexible, Teflon-coated wire loops (diameter = 0.2 mm, bared 2 mm along the loop), were sown between the levator scapulae dorsalis and acromiotrapezius neck muscles; pairs of EMG electrodes were referenced against each other. All electrodes were attached to a socket (Ginder Scientific, Ottawa, Ontario, Canada) that was fixed to the skull with dental acrylic cement. The animals were returned to their cages after receiving an antibiotic (15,000 IU penicillin G, i.m.) and an analgesic (2 mg/kg of butorphanol, s.c.). The socket was linked to a swivel connector, which was attached to a counter-balancing weight system, enabling the rats to move about freely. Electrophysiological signals were relayed to a Grass Model 78D polygraph, filtered and amplified, before being digitized at 256 Hz and written on disk with a computer-assisted system (Eclipse, Stellate Systems, Montreal, Quebec, Canada). After 5-day postoperative recovery, the rats were connected for 2 days of habituation to the recording equipment under standard housing conditions, and baseline sleep was tracked for 24 h on the seventh day post-surgery. Sleep recordings were initiated after awakening, enforced by brief, gentle handling at light onset (08:00), for 10 to 15 s.
Myocardial infarction
On the morning after baseline sleep recording, the rats were randomized into 1 of 2 groups: those in the experimental (MI) group were subjected to coronary occlusion for 40 min, followed by reperfusion, according to a standard method already published by our group (see below).2–4 The control (sham-operated) group of 8 rats were submitted to the same thoracotomy protocol but without actual coronary artery occlusion. Ketamine and xylazine (50 mg/kg i.m. and 5 mg/kg i.m., respectively) were combined for anesthesia induction, after which the animals were intubated and placed on an artificial respirator, with anesthesia maintained by isoflurane (1.0%-1.5%) ventilation. Then a left thoracotomy was undertaken at the fifth intercostal space, and the left anterior descending coronary artery was occluded for 40 min with a silk thread in 8 rats. Electrocardiogram, temperature, heart rate, and oxygen flow were monitored throughout the surgery. Ischemia was confirmed by ST segment alterations and ventricular subepicardial cyanosis. After 40 min of occlusion (ischemia), the ligature was loosened so that the myocardial tissue could be reperfused. Transient arrhythmias can occur during MI surgery, but no cardiac failure was observed (see below). Reperfusion was confirmed by the disappearance of cyanosis, and the thorax was closed, followed by injections of an antibiotic (15,000 IU penicillin G, i.m.) and an analgesic (2 mg/kg of butorphanol, s.c.), with the animals then being returned to their cages. The analgesic was given every 8 h during the 24 first h post-MI surgery. Two weeks after MI, sleep was recorded again in the same manner as described above.
General condition of animals after surgery
All animals recovered optimally from sleep and MI surgeries. From the beginning of this study to the end, the general condition (quality of hair and grooming, as monitored by animal health technicians of our research centre) and weight gain were similar in control (136.17 ± 23.97 g) and MI rats (122.83 ± 20.17 g). Lung weight after sacrifice served as an indicator of possible heart failure, but no significant differences were apparent between the controls (1.52 ± 0.06 g) and MI rats (1.55 ± 0.07 g). Finally, control and MI rats displayed similar swimming performances in the Morris water maze,3 and run similar distances for 24 h in a running wheel (unpublished data), indicating intact motor performance. It thus can be concluded that there was no sign of pain or heart failure symptoms in MI rats.
Dependent Measures and Data Analysis
Vigilance states
The best 2 EEG and EMG derivations were selected for the measurement of vigilance states with 10-s epochs. The following scoring criteria were implemented, using 10-s epochs: wakefulness was scored on the basis of low-voltage, high-frequency (≥ 6-8 Hz) EEG coupled with high EMG levels. Slower EEG frequencies, vertex sharp waves, and decreased EMG signal were scored as drowsiness. Light SWS (SWS-1) was scored from EEG sleep spindles (5-12 Hz) and more than 20% but less than 50% of EEG delta waves (2-4 Hz) per epoch. Deep SWS (SWS-2) scores were based on the predominance of EEG delta waves and more than 50% of epochs. Finally, PS was scored when EEG activity was low amplitude and stable, with theta activity (5-8 Hz) and general EMG atonia. The following sleep parameters were computed and examined in the data analysis: sleep onset latency was the time elapsed from the beginning of sleep recording at light onset, after forced awakening, to the first occurrence of any sleep stage; the latency to sleep stages was defined as the time elapsed between sleep onset and the first epoch of that stage; total sleep time: total time spent in any of the sleep stages after sleep onset; wake time after sleep onset: number of minutes during the total recording time after sleep onset; sleep efficiency: percentage of total sleep time during recording time after sleep onset; PS periods: uninterrupted bouts of PS lasting ≥ 3 min and terminated by at least 10 consecutive epochs of SWS or wakefulness. All recognizable PS episodes were computed in terms of duration (min) and occurrence (fragments).
Infarct size
Infarct size data were drawn from the animals used for sleep recordings and were analyzed according to a standard method described in our previous work.2–4 Briefly, at the end of the experiment, the rats were sacrificed and their hearts were promptly removed. The left anterior descending coronary artery was occluded at the same site occluded earlier with 4.0 silk suture. Then, the heart was removed and rinsed with saline (0.9%), followed by 0.5% Evans Blue infusion into the aorta to measure the area at risk (AR). The hearts were then frozen at −80°C for 5 min and sliced into 4 coronal sections of 2 mm. Each section was stained at 37°C for 5 min in a 2,3,5-triphenyltetrazolium chloride solution (TTC 1%, pH 7.4) to assess the area of necrosis (I). MI was expressed as a percentage of I of the AR (I/AR). The AR was also expressed as a percentage of left ventricle (LV) area (AR/LV). In terms of appearance, each area was examined according to morphological properties intensified with TTC treatment: necrosed tissue was colored white, healthy tissue was colored red, and Evans Blue identified the area spared from ischemia (colored blue).
Statistical Analyses
The 24-h sleep recording sessions were divided into light and dark periods. The data were evaluated by 1-tailed t-tests for independent groups, with an α level of 0.05. They were first analyzed for homogeneity of variance and for normality, which was the case.
Experiment 2: Quantification of Brainstem Cholinergic Neurons
This experiment was undertaken to examine the relationship between expected group differences in PS and PS control mechanisms located in the brainstem.
Animals
Twelve rats (6 MI and 6 sham) weighing 350-375 g and acquired through regular trade (Charles Rivers, St-Constant, Quebec, Canada) were housed individually under standard conditions (see experiment 1 above). All procedures were conducted according to Canadian Council on Animal Care guidelines and were approved by the animal ethics committee of the Research Centre where the experiments were performed. All surgical procedures for sleep recording and MI were the same as those detailed above (experiment 1). At the end of the sleep recording (i.e., 2 weeks after MI), the rats were sacrificed according to the method explained below.
Perfusion and Histology
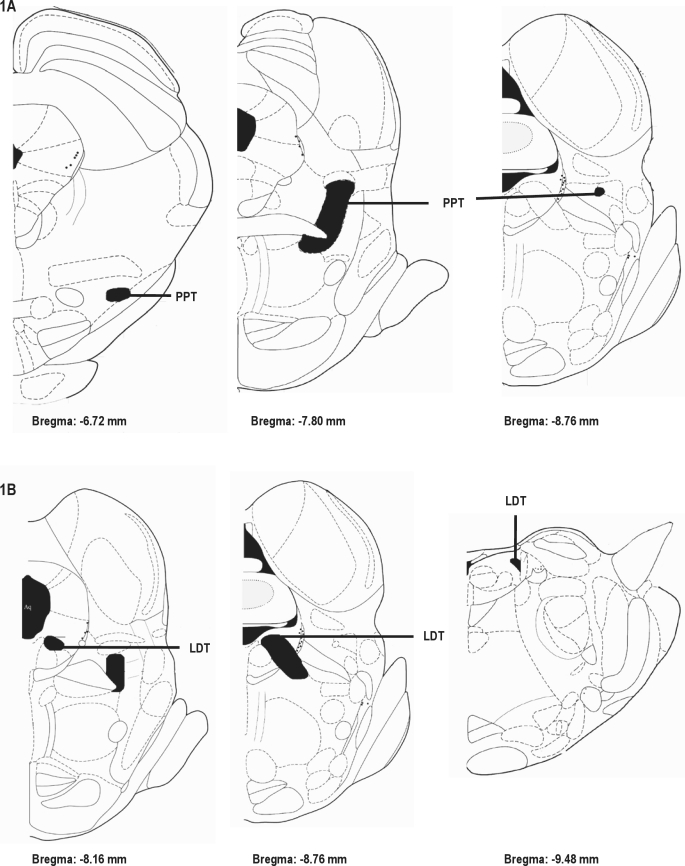
The animals were deeply anesthetized with ketamine/xylazine (50 mg/kg and 5 mg/kg i.m., respectively) and perfused transcardially with 100 mL of 0.9% NaCl, followed by 700 mL of ice-cold 4% paraformaldehyde in 0.1 M sodium tetraborate buffer (pH 9.5). Their brains were post-fixed for 3 h and then cryoprotected in 15% sucrose in KPBS buffer (potassium phosphate 0.02 M; NaCl 0.9%; pH 7.4) overnight at 4°C. Frozen coronal brainstem sections (30 μm thick) were cut in a freezing cryostat at in four 1-in-4 (1-4) series throughout the brainstem, referring to the rat atlas of Paxinos and Watson.11 The beginning and end of the pedunculopontine tegmentum (PPT) were set between Bregma −6.72 and −8.76, respectively; the beginning and end of the laterodorsal tegmentum (LDT) were set between bregma −8.16 and −9.48, respectively (Figure 1Figure 1). Sections were collected in cold cryoprotectant (sodium phosphate 0.05 M; sucrose 15%; ethyl glycol 30%; pH 7.4) and stored at −20°C until histochemical procedures were performed.
Figure 1.
Stereotaxic coordinates based on The Rat Brain in Stereotaxic Coordinates by Paxinos and Watson.11 1A and 1B: Adapted figures respectively showing the beginning and end of the PPT nucleus and LDT nucleus in the brainstem.
Immunohistochemistry
To ascertain the number of choline acetyltransferase (ChAT)-positive neurons, brain sections were first rinsed in KPBS buffer to remove the cryoprotectant, then pretreated for 10 min with 0.3% hydrogen peroxide to reduce endogenous peroxidase activity and for 8 min with 1% sodium borohydrate solution to eliminate free aldehyde, followed by incubation with primary goat anti-ChAT antibody (Chemicon, Temecula, CA) at 1:2,000 in KPBS buffer with 0.3% Triton X-100 and 2% rabbit serum for 48 h at 4°C. The primary antibody was detected with biotinylated rabbit anti-goat immunoglobulin (Vector Laboratories, Burlingame, CA) at 1:200 (48 h) and avidin-DH-biotinylated horseradish peroxidase-H-complex (Vectastain Elite ABC kit, Vector Laboratories). The diaminobenzidine (DAB) reaction product was developed with the DAB substrate kit for peroxidase (Vector Laboratories) according to the manufacturer's instructions.
Dependent Measures and Data Analysis
The PPT and LDT areas were defined by the boundary of the outermost cholinergic neurons in each section. Cells were quantified with a non-stereological method, since MI was not expected to change cell size. Cholinergic neurons were counted from 1-in-4 series of coronal brainstem sections representing 14-16 sections. ChAT-positive stained neurons with a discernible nucleus were counted under 10X magnification to discern any numerical differences between MI and sham-operated rats. The data represent total counts from the left plus the right sides of the brain.
Statistical Analyses
An independent 2-tailed t-test was applied to compare the means of ChAT-positive neurons, with P < 0.05 considered as significant. The data were first analyzed for homogeneity of variance and for normality, which was the case.
RESULTS
Infarct Size
The infarct size data, derived from animals used for sleep recording, corresponded to 46.78% ± 1.34% of the AR in the MI group and > 50% of the LV. No cardiac tissue damage was observed in the sham-operated controls, similar to values reported in our own previous studies as well as those of others.12
Sleep
Before MI, both groups presented similar baseline sleep organization (data not included). The results obtained 2 weeks after MI are summarized in Table 1. Compared to the sham controls, MI rats displayed longer latency to sleep onset (t8 = −2.88, P < 0.01) and shorter latency to PS (t8 = 2.26, P < 0.05). MI rats also manifested significantly less PS time (t8 = 2.17, P < 0.05) and a lower number of PS periods (t8 = 3.66, P < 0.01) during the light period, with significantly fewer PS fragments both during the 24-h recording period (t8 = 2.62, P < 0.05) and in the light period (t8 = 3.52, P < 0.01). There were no significant differences in any sleep parameters analyzed in the dark period except for drowsiness: relative to the sham controls, MI rats showed significantly less drowsiness time (168.70 ± 23.93 vs. 97.75 ± 13.67; t8 = 2.58, P < 0.05).
Table 1.
Sleep organization (mean ± standard error of the mean) in 24-h recording time and light period 2 weeks after MI (n = 8/group).
| SHAM |
MI |
P |
||||
|---|---|---|---|---|---|---|
| Sleep parameters | 24 h | Light | 24 h | Light | 24 h | Light |
| Sleep latency (min) | 10.29 ± 2.52 | 10.29 ± 2.52 | 20.41 ± 2.79 | 20.41 ± 2.79 | 0.01* | 0.01* |
| Total sleep time (min) | 893.60 ± 51.12 | 563.64 ± 18.59 | 816.29 ± 49.07 | 530.47 ± 21.27 | 0.29 | 0.27 |
| Wake time after sleep onset (min) | 515.00 ± 38.45 | 143.87 ± 18.82 | 578.39 ± 53.73 | 156.58 ± 20.46 | 0.35 | 0.65 |
| Sleep efficiency (%) | 62.65 ± 3.54 | 79.66 ± 2.64 | 57.76 ± 3.57 | 76.86 ± 3.05 | 0.34 | 0.50 |
| Drowsy duration (min) | 357.10 ± 47.14 | 193.54 ± 23.92 | 250.52 ± 22.07 | 151.70 ± 13.04 | 0.06 | 0.14 |
| SWS-1 duration (min) | 424.62 ± 43.12 | 286.77 ± 27.65 | 462.45 ± 27.13 | 303.39 ± 13.29 | 0.46 | 0.59 |
| SWS-2 duration (min) | 17.60 ± 7.58 | 13.97 ± 6.40 | 24.47 ± 7.65 | 19.73 ± 7.52 | 0.53 | 0.57 |
| PS latency (min) | 40.62 ± 5.70 | 40.62 ± 5.70 | 22.14 ± 3.95 | 22.14 ± 3.95 | 0.01* | 0.01* |
| PS duration (min) | 103.52 ± 7.78 | 76.08 ± 5.79 | 82.70 ± 8.75 | 58.00 ± 5.97 | 0.09 | 0.04* |
| PS fragments (number) | 83.37 ± 7.08 | 60.87 ± 5.46 | 57.50 ± 6.86 | 38.50 ± 3.22 | 0.02* | 0.003** |
| PS periods (number) | 61.00 ± 3.55 | 43.87 ± 2.28 | 48.62 ± 6.25 | 31.62 ± 2.45 | 0.10 | 0.002** |
Immunohistochemistry
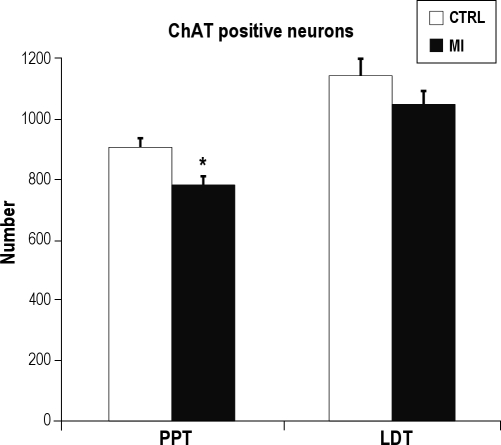
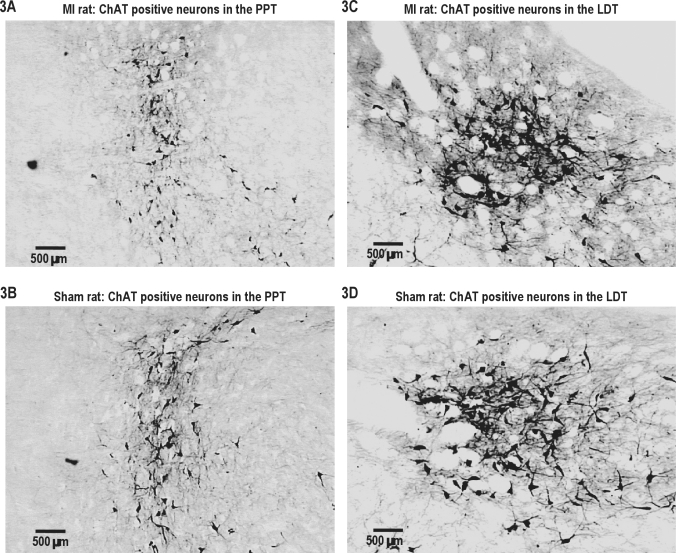
In comparison to the sham controls, MI rats exhibited a significant diminution of ChAT-sensitive neurons in the PPT area (t6 = 2.84, P < 0.01) but not in the LDT area (t6 = 0.84, P > 0.05) (Figures 2 and 3 A to 3D). In both groups, ChAT-sensitive neurons were equally distributed on the right and left sides.
Figure 2.
Number of ChAT-positive neurons in myocardial-infarcted (MI) rats and sham controls (CTRL) in the left + right PPT and LDT areas. There were no differences between the left and right sides of the brainstem. *P < 0.05.
Figure 3.
Examples of ChAT-positive neurons in the left brainstem of a MI and a sham rat. 3A, 3B: left PPT. 3C, 3D: left LDT. There were no differences between the left and right sides of the brainstem.
DISCUSSION
The present paper deals with sleep organization and control mechanisms related to PS/REM sleep after acute MI. The fact that MI is followed by depressed mood and actual major depression in a certain proportion of patients1 suggests that post-MI sleep may be altered either by MI-related mechanisms themselves or by mechanisms associated with the development of depression. In humans, sleep after MI13–15 and sleep in depressed patients16–18 are both characterized by sleep maintenance difficulties, with high amounts of wakefulness and poor sleep efficiency. REM sleep, however, differs between the 2 conditions: post-MI sleep in humans features long REM sleep latency, a short duration of REM sleep, and a low number of REM sleep periods13–15; while depressed patients show overall facilitation of REM sleep.16–18 In the present experiments on rats, we recorded long sleep latency compatible with findings in humans after MI or upon depression while the PS data were mixed: PS latency was facilitated while PS duration was decreased relative to the sham controls.
Laboratory sleep recordings in persons with depression also disclose a reduction of SWS together with diminished latency and increased duration of REM sleep.5 These sleep patterns have been attributed to many possibilities, including faulty mechanisms of SWS control,19 heightened REM sleep pressure,20 and ultradian rhythm disorders.21 Most rat models of depression are compatible with this profile.5,22,23 In our experiments, the finding that insomnia was almost exclusively attributable to loss of PS was the opposite of what was expected, since post-MI rats display clear behavioral signs of depression that can be blocked by antidepressants.3,4 Indeed, the present results could constitute another demonstration that the relationship between PS and depression may sometimes diverge from the usual profile. Indeed, while it is known, for example, that most antidepressant treatments inhibit PS, molecules, such as trimipramine, monoamine oxidase inhibitors, trazodone, and bupropion, do not suppress PS24; whereas nefazodone might even increase it.25 In any case, cholinergic control of PS is achieved through interaction between brainstem cholinergic and cholinoceptive neurons,26 and our data show that a subgroup of pontine cholinergic neurons, namely, the PPT, is decreased 2 weeks after MI compared to the sham controls. Since it has been reported that lesions of PPT cholinergic neurons significantly reduce PS,27,28 the present results may be sufficient to explain the decline of PS seen here in MI rats, even though other mechanisms, such as decreased number of post-synaptic cholinoceptive neurons or increased “REM-off” neuron activity,26 should not be dismissed. It is noteworthy that although the pontine cholinergic/cholinoceptive system has been found to play a role in at least one of the models (the Flinders Sensitive Line),9 ChAT-positive neurons do not seem to contribute to the PS facilitation it displays.29,30 These observations are in accordance with the present data, showing facilitation of REM sleep latency in rats in the face of diminished PPT cholinergic neurons. They further indicate that other elements, selectively and specifically involved in the control of REM sleep onset and shared with the physiopathology of depression, may be important here in maintaining high pressure on PS onset.
The decreased PS time reported here is compatible with what has been observed in patients after MI13–15 and can be conceptually associated with a reduced number of ChAT-sensitive brainstem neurons, as illustrated in the present study. Still, the mechanism(s) by which brainstem cholinergic neurons are lost after MI is unclear. One possibility is that circulating neurotoxic substances released upon post-MI reperfusion might be implicated. It is known that pro-inflammatory cytokines, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) are released after MI.31–33 We have previously observed that pentoxifylline, a nonspecific cytokine synthesis inhibitor, prevents neural apoptosis in the amygdala after MI without effect on infarct size2 and that TNF-α is involved.34 We have also demonstrated that inhibition of the inducible inflammatory enzyme cyclooxygenase-2 (COX-2), during the reperfusion period, attenuates the apoptotic process (decreased number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells and reduced caspase-3 activation) in the amygdala without affecting infarct size.35 Cholino-sensitive cells of the amygdala are thought to be engaged in the control of PS.36,37 Moreover, a relationship between apoptosis and PS loss has been noted: selective PS deprivation,38 but not unselective sleep deprivation, induces apoptosis in the amygdala and PPT/LDT as well as in several other brain regions, including the habenula, locus coeruleus, hippocampus, and areas of the hypothalamus.38,39 The present results thus indicate that pro-inflammatory cytokines could be involved in the decreased post-MI duration of PS reported here, through apoptosis in the amygdala and PPT cholinergic area.
In summary, these data reveal that acute MI is accompanied, within 2 weeks, by PS-specific insomnia that can be at least partly attributed to a particular loss of cholinergic neurons in brainstem areas known to control PS. We conclude that PPT cholinergic neurons are more sensitive than LDT cholinergic neurons to central insults induced by acute MI, a phenomenon that could be explained by the heterogeneity of neuronal cell neurotransmitters released in these 2 nuclei, as well as pro-inflammatory cytokine activity and triggers of cell death.
Study Limitations and Future Studies
Sleep was recorded 2 weeks after MI, but it is possible that sleep patterns were affected at an earlier stage. This possibility needs to be investigated since we have reported the presence of apoptosis in various brain regions as early as 72 h after MI.2,40 If such is the case, then sleep disorders could be considered as early effects of early apoptosis. Moreover, given the reciprocal connectivity of the amygdala with the myocardium and its influence on heart rate control,41 it is crucial to ascertain which nucleus of the amygdala is specifically sensitive to early apoptosis.
Another possible limitation of the present study is that direct insult to the brain, caused by hypoxia during the MI procedure, may have contributed to the results. Although this possibility needs to be considered, many hypoxia-independent functional routes, through which circulating pro-inflammatory cytokines may act on the brain, also deserve to be examined. It is known, for example, that peripheral circulating cytokines have clear effects on the brain by accessing it via circumventricular organs,42 by crossing the blood-brain barrier,43,44 or indirectly, by stimulating peripheral vagal afferents.45,46 Pro-inflammatory cytokines may recruit peripheral immune cells into the brain, which may then express cytokines.47 Finally, it is also apparent that pro-inflammatory cytokines can be produced endogenously within the brain48 through the induction of inducible cyclooxygenase (COX-2) activity in the cerebral microvasculature.49,50
Finally, future research should further explore cholinergic neurons in other areas of the brain, including the hippocampus and the basal forebrain, together with cholinergic receptors and apoptosis markers, possibly by quantified analysis of EEG activity during SWS and PS. The fact that PS was inhibited rather than facilitated should lead to further investigation to evaluate the effects of antidepressants on sleep in post-MI rats.
DISCLOSURE STATEMENT
This study was not industry-supported. The authors declare that they have no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to R.G. and studentships from Fonds de la recherche en santé du Québec (FRSQ) and the J.A. DeSève Foundation to T.M.B. G.R. is a Research Scholar of the FRSQ. We are grateful to Sevan Kaloustian, Élyse Chevrier, Pierre Fortier, Louis Chiocchio, and Caroline Bouchard for their skillful assistance and technical expertise.
REFERENCES
- 1.Guck TP, Kavan MG, Elsasser GN, Barone EJ. Assessment and treatment of depression following myocardial infarction. Am Fam Physician. 2001;64:641–8. [PubMed] [Google Scholar]
- 2.Wann BP, Boucher M, Kaloustian S, Nim S, Godbout R, Rousseau G. Apoptosis detected in the amygdala following myocardial infarction in the rat. Biol Psychiatry. 2006;59:430–3. doi: 10.1016/j.biopsych.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Wann BP, Bah TM, Boucher M, et al. Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: a possible model for human postinfarct major depression. J Psychiatry Neurosci. 2007;32:11–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Wann BP, Bah TM, Kaloustian S, et al. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol. 2009;23:451–9. doi: 10.1177/0269881108089820. [DOI] [PubMed] [Google Scholar]
- 5.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 6.Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–62. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- 7.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 8.Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–47. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–59. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Vogel GW, Neil D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. New York: Elsevier; 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IK, Yoo KY, Han TH, et al. Enhanced cell proliferation and neuroblast differentiation in the rat hippocampal dentate gyrus following myocardial infarction. Neurosci Lett. 2009;450:275–80. doi: 10.1016/j.neulet.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45:348–60. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 14.BaHammam A. Sleep quality of patients with acute myocardial infarction outside the CCU environment: a preliminary study. Med Sci Monit. 2006;12:CR168–72. [PubMed] [Google Scholar]
- 15.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep patterns in patients with acute coronary syndromes. Sleep Med. 2010;11:149–53. doi: 10.1016/j.sleep.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effect of a major stressful event and its resolution. Psychiatry Res. 1991;39:199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- 17.Abad VC, Guilleminault C. Sleep and psychiatry. Dialogues Clin Neurosci. 2005;7:291–303. doi: 10.31887/DCNS.2005.7.4/vabad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendlewicz J. Sleep disturbances: core symptoms of major depressive disorder rather than associated or comorbid disorders. World J Biol Psychiatry. 2009;10:269–75. doi: 10.3109/15622970802503086. [DOI] [PubMed] [Google Scholar]
- 19.Borbely AA. The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry. 1987;20:23–9. doi: 10.1055/s-2007-1017069. [DOI] [PubMed] [Google Scholar]
- 20.Vogel GW, McAbee R, Barker K, Thurmond A. Endogenous depression improvement and REM pressure. Arch Gen Psychiatry. 1977;34:96–7. doi: 10.1001/archpsyc.1977.01770130098010. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz PJ, Rosenthal NE, Kajimura N, et al. Ultradian oscillations in cranial thermoregulation and electroencephalographic slow-wave activity during sleep are abnormal in humans with annual winter depression. Brain Res. 2000;866:152–67. doi: 10.1016/s0006-8993(00)02271-x. [DOI] [PubMed] [Google Scholar]
- 22.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–51. [PubMed] [Google Scholar]
- 23.Jindal RD, Thase RD, Fasiczka AL, et al. Electroencephalographic sleep profiles in single-episode and recurrent unipolar forms of major depression: II. Comparison during remission. Biol Psychiatry. 2002;51:230–6. doi: 10.1016/s0006-3223(01)01226-4. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SJ, Argyropoulos SV. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927–47. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 25.Rush AJ, Armitage R, Gillin JC, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol Psychiatry. 1998;44:3–14. doi: 10.1016/s0006-3223(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 26.Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007;584:735–41. doi: 10.1113/jphysiol.2007.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 29.Greco MA, Magner M, Overstreet D, Shiromani PJ. Expression of cholinergic markers in the pons of Flinders rats. Brain Res Mol Brain Res. 1998;55:232–6. doi: 10.1016/s0169-328x(98)00002-3. [DOI] [PubMed] [Google Scholar]
- 30.Overstreet DH, Russell RW, Crocker AD, Schiller GD. Selective breeding for differences in cholinergic function: pre- and postsynaptic mechanisms involved in sensitivity to the anti-cholinesterase, DFP. Brain Res. 1984;294:327–32. doi: 10.1016/0006-8993(84)91044-8. [DOI] [PubMed] [Google Scholar]
- 31.Deten A, Zimmer HG. Heart function and cytokine expression is similar in mice and rats after myocardial infarction but differences occur in TNFalpha expression. Pflugers Arch. 2002;445:289–96. doi: 10.1007/s00424-002-0930-x. [DOI] [PubMed] [Google Scholar]
- 32.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–71. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 33.Pudil R, Pidrman V, Krejsek J, et al. Cytokines and adhesion molecules in the course of acute myocardial infarction. Clin Chim Acta. 1999;280:127–34. doi: 10.1016/s0009-8981(98)00179-x. [DOI] [PubMed] [Google Scholar]
- 34.Kaloustian S, Bah TM, Mathieu S, et al. Tumor necrosis factor-alpha participates in apoptosis in the limbic system after myocardial infarction. Apoptosis. 2009;14:1308–16. doi: 10.1007/s10495-009-0395-x. [DOI] [PubMed] [Google Scholar]
- 35.Kaloustian S, Wann BP, Bah TM, et al. Celecoxib after the onset of reperfusion reduces apoptosis in the amygdala. Apoptosis. 2007;12:1945–51. doi: 10.1007/s10495-007-0122-4. [DOI] [PubMed] [Google Scholar]
- 36.Sanford LD, Yang L, Tang X, Dong E, Ross RJ, Morrison AR. Cholinergic regulation of the central nucleus of the amygdala in rats: effects of local microinjections of cholinomimetics and cholinergic antagonists on arousal and sleep. Neuroscience. 2006;141:2167–76. doi: 10.1016/j.neuroscience.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 37.Tang X, Yang L, Liu X, Sanford LD. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28:923–30. doi: 10.1093/sleep/28.8.923. [DOI] [PubMed] [Google Scholar]
- 38.Biswas S, Mishra P, Mallick BN. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience. 2006;142:315–31. doi: 10.1016/j.neuroscience.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Hipólide DC, D'Almeida V, Raymond R, Tufik S, Nobrega JN. Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int J Neurosci. 2002;112:155–66. doi: 10.1080/00207450212022. [DOI] [PubMed] [Google Scholar]
- 40.Kaloustian S, Wann BP, Bah TM, et al. Apoptosis time course in the limbic system after myocardial infarction in the rat. Brain Res. 2008;1216:87–91. doi: 10.1016/j.brainres.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection. Neurosci Biobehav Rev. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA. Physiology and pathology of the blood-brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J NeuroVirol. 1999;5:538–55. doi: 10.3109/13550289909021284. [DOI] [PubMed] [Google Scholar]
- 43.Banks WA, Kastin AJ. The interleukins-1α, −1β, and −2 do not acutely disrupt the murine blood-brain barrier. Int J Immunopharmacol. 1992;14:629–36. doi: 10.1016/0192-0561(92)90124-4. [DOI] [PubMed] [Google Scholar]
- 44.Ellison M, Krieg RJ, Povlishock JT. Differential central nervous system responses following single and multiple recombinant IL-2 infusions. J Neuroimmunol. 1990;28:249–60. doi: 10.1016/0165-5728(90)90018-i. [DOI] [PubMed] [Google Scholar]
- 45.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 46.MohanKumar SM, MohanKumar PS, Quadri SK. Effects of bacterial lipopolysaccharade on central monoamines and fever in the rat: involvement of the vagus. Neurosci Lett. 2000;284:159–62. doi: 10.1016/s0304-3940(00)01025-9. [DOI] [PubMed] [Google Scholar]
- 47.Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM, Herkenham M. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brainwide glial activation. Neuroscience. 2002;112:731–49. doi: 10.1016/s0306-4522(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell NJ. Annual review prize lecture cytokines − killers in the brain? J Physiol. 1999;514:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–79. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]