Abstract
Objective
Resolvin E1 (RvE1) is an eicosapentaenoic acid (EPA)-derived specialized pro-resolving mediator generated during resolution of acute inflammation. RvE1 exhibits potent organ-protective actions in vivo and acts on specific cell types including platelets. Here, we investigated the ability of RvE1 to regulate adenosine diphosphate (ADP) activation of platelets via specific receptors because RvE1 reduces platelet aggregation with certain agonists including ADP.
Methods and Results
RvE1 (0.1nM–100nM) incubated with platelets gave reduced ADP-stimulated P-selectin mobilization (IC50 ~1.6×10−12 M) and polymerized actin content compared to control platelets. RvE1 (1–100nM) did not stimulate or block intracellular calcium mobilization. Using a new P2Y12-β-arrestin-coupled cell system, ADP-activated P2Y12 with an EC50 of 5×10−6 M and RvE1 did not directly stimulate P2Y12 or block ADP-P2Y12 signals. In this system, another eicosanoid LTE4 (EC50 1.3×10−11 M) dose dependently activated P2Y12. When recombinant P2Y12-expressing cells were transiently transfected with an RvE1 receptor, human ChemR23 (present on human platelets), addition of RvE1 (0.1nM-10.0nM) blocked ADP signals (IC50 ~1.6×10−11 M) in P2Y12-ChemR23-expressing cells compared to mock transfections.
Conclusions
These results demonstrate that RvE1’s regulatory actions (i.e reducing ADP-stimulated P-selectin mobilization and actin polymerization) are hChemR23-dependent. Moreover, they document specific platelet actions of RvE1 selectively engaged with ADP-activated platelets that illuminate a new cellular mechanism and impact of omega-3 EPA that may contribute to both resolution of vascular inflammation and ADP-dependent platelet activation relevant in pathologic cardiovascular events.
Keywords: Eicosapentaenoic acid (EPA), Resolvin E1, inflammation, platelet, adenosine diphosphate (ADP)
Excessive inflammation is now recognized as a central component in several of the most prevalent human diseases in the developed world, including rheumatic diseases, diabetes, and cardiovascular diseases1–3. Identification of cellular and molecular mechanisms in resolution and inflammation as well as the roles of novel local chemical mediators demonstrate that resolution of acute inflammation is an active process4–6 rather than passive as believed earlier2. It is becoming increasingly apparent that inflammatory diseases, such as periodontal disease7, atherosclerosis8 and Henoch-Schonlein purpura9, arise due to a failure in mounting endogenous resolution programs10. Timely resolution of acute inflammation is actively regulated by a new genus of endogenous mediators collectively called specialized pro-resolving mediators (SPM). This genus includes several families of structurally distinct, potent and local-acting mediators, namely lipoxins, resolvins and protectins, which are biosynthesized from polyunsaturated essential fatty acids, reviewed in11, 12. Results from several clinical studies (recently reviewed in13) demonstrate that diets rich in marine fish omega-3 polyunsaturated fatty acids (PUFA) reduce the risk of coronary events13. The cellular mechanisms responsible for omega-3 fatty acid responses are of increasing general interest14. Along these lines, circulating omega-3 fatty acids (eicosapentaenoic acid, EPA, and docosahexaenoic acid, DHA) rapidly appear in resolving inflammatory exudates carried by edema proteins in vivo, thus emphasizing the importance of circulating levels of omega-3 PUFA and their availability for conversion by exudates into resolvins and protectins15.
Resolvins are enzymatic products of EPA and DHA biosynthesized during the resolution phase of acute inflammation, initially isolated in self-limited murine exudates6, 16. They are agonists that exert both anti-inflammatory (i.e. limit further neutrophil infiltration) and pro-resolving (i.e. stimulate non-phlogistic activation of macrophages) actions11. Specifically, Resolvin E1 (RvE1), a member of the E series of resolvins, is biosynthesized from EPA and was first identified in vivo during the resolution phase of inflammation from exudates6. The levels of RvE1 were monitored in plasma from healthy human volunteers taking fish oil ranged from 0.1 to 0.4 ng/ml.17 This is commensurate with RvE1 bioactions evoked in the pM-nM range.
With isolated human cells, RvE1 is produced by hypoxia-activated human endothelial cells that convert EPA to an 18R-hydroxy-containing intermediate via aspirin-acetylated COX-26. Also, 18R-hydroxy-eicosapentanoic acid is produced by microbial P45018. Once formed, 18R-HEPE is rapidly transformed by activated leukocytes to a 5(6)-epoxide-18R-hydroxy containing intermediate where 5-LOX carries out consecutive steps19. RvE1 complete stereochemistry is established (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) and is a stereoselective agonist that interacts with at least two identified G-protein-coupled receptors (GPCRs), ChemR2317 and BLT120 (reviewed in21). RvE1 possesses potent anti-inflammatory and pro-resolving actions in animal models of disease and acts on a wide range of cell types11 including leukocytes and platelets in whole blood22. Unlike BLT1, which is present on platelets23, we recently identified ChemR23 on megakaryocytes and the surface of human platelets22. RvE1 has stereoselective anti-aggregatory actions with human platelets,22 and at concentrations as low as 1 nM RvE1 blocks adenosine diphosphate (ADP, 10μM)-stimulated platelet aggregation and thromboxane generation22.
The platelet actions of ADP are of particular interest because it is well appreciated as an important local mediator of hemostasis and thrombosis, as well as when aberrantly regulated platelets contribute to the pathogenesis of cardiovascular diseases24, 25. On platelets, ADP specifically binds and activates two G-protein coupled receptors, P2Y1 and P2Y12. Transduction of the ADP signal involves inhibition of adenyl cyclase (via P2Y12) and a concomitant transient increase of intracellular Ca2+ (via P2Y1)25. Further downstream signaling results in robust shape changes, inside-out activation of platelet receptors, such as GPIIbIIIa, and granule secretion25. Current anti-platelet therapies targeted at blocking purinergic receptor signaling, specifically P2Y12 antagonists, are the focal point of platelet therapeutics26 and are among the most widely used pharmacotherapy in cardiovascular diseases27, 28.
Since we found that RvE1 has potent and stereoselective actions in blocking ADP-stimulated platelet aggregation,22 we examined RvE1’s actions in ADP-activated platelets. Here, we report that RvE1 regulates ADP-stimulated P-selectin surface mobilization and actin polymerization, and that RvE1 counterregulates P2Y12 signaling in a receptor-dependant (ChemR23) manner in transfected cells. Together, these findings may have implications for dietary EPA supplementation for the maintenance of vascular homeostasis and in the resolution of acute inflammation.
Materials and Methods
Antibodies and Reagents
Antibodies
Phycoerythrin (PE)-conjugated mouse anti-human CD62P and FITC-CD41 anti-human (BD Biosciences, Rockville, MD), Mouse anti-human ChemR23 (clone 84939) was purchased from R&D (Minneapolis, MN). Goat anti-mouse IgG3 was purchased from Pharmingen (San Jose, CA).
Reagents and cell lines
19-para-fluorophenoxy-RvE1 (RvE1 analog)29, 30 was prepared by total organic synthesis, as in17 (N. Petasis in the Specialized Center for Oral Inflammation and Resolution (CNS P50 DE016191). Prostaglandin E1 and RvE1 were purchased from Cayman (Ann Arbor, MI). Physical and biological properties were matched to published RvE1. The structural integrity and concentration of RvE1 was monitored using UV tandem LC-MS-MS6, 17. ADP was from Sigma Chemical (St. Louis, MO). Permeabilization buffer was purchased from eBioscience (San Diego, CA), Fura-2 fluorescent probe was from Invitrogen (Carlsbad, CA) and FITC-Phalloidin from Sigma (St. Louis, MO). MEG-01 and CHO cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). pcDNA3-hChemR23 CHO cells were prepared as in31. Sensiscript reverse transcription kit and Hotstar Taq polymerase master mix kit were purchased from Qiagen (Valencia, CA). Fugene transfection reagents were from (Roche Diagnostics Corporation, Indianapolis, IN).
Primers
Human ChemR23,5′-ATGAGAATGGAGGATGAAGA-3′and 5′-TCAAAGCATGCCGGTCTCC-3′, human P2Y12, 5′CAAGCCGTCGACAACCTCACCTC-3′ and 5′-TCTCGGCTGCCTGTTGGTCAGAA-3′, human P2Y1, 5′-GTGCTGGTGTGGCTATTGTGGT-3′ and 5′-GGCTTGTATCTCCATTCTGCTTGA-3′, human BLT1, 5′-TATGTCTGCGGAGTCAGCATCTACGC-3′ and 5′-CCTGTAGCCGACGCCCTATGTCCG-3′, GAPDH, 5′-GACCACAGTCCATGACATCACT-3′and 5′-TCCACCACCCTGTTGCTGTAG-3′. All primers were purchased from Invitrogen (Carlsbad, CA).
Platelet P-selectin
Platelet rich plasma (PRP) was isolated from healthy human whole blood donors that had not taken NSAIDs for 14 days prior to donation and anti-coagulated with ACD (1:10), as in22. Briefly, synthetic RvE1 or 19-para-fluorophenoxy-RvE1 (0.001–100 nM) was incubated in PRP for 15 minutes at 37°C. Platelets were then incubated with ADP (10 μM) for 3 minutes (37°C, pH 7.45) with intermittent mixing. Incubations were stopped by adding 6% formalin (15 minutes at 4°C). Platelets were stained with either anti-mouse IgG, PE-mouse anti-human CD62P (1:100 dilution) or FITC-anti-human CD41 (1:100 dilution) for 20 minutes at 4°C. Surface expression was monitored using FACSort (Becton, Dickinson and Company, Franklin Lakes, NJ) and Cellquest software. Platelets were identified by positive CD41 and CD62P staining compared to appropriate IgG control.
ADP-stimulated morphology changes and intracellular calcium mobilization
RvE1 (100 nM) and/or vehicle were added to PRP at 37°C with gentle agitation 15 minutes prior to addition of ADP (10 μM). ADP incubations were carried out for 0, 15, 30 and 60 seconds at 4°C in the presence of vehicle or RvE1. The addition of cold 6% formalin stopped the incubations, and cells were exposed to FITC-phalloidin (1:100) for 1 hour at 4°C. Increases in positive FITC fluorescence compared to the vehicle were quantified (via flow cytometry) as polymerized actin. Intracellular calcium mobilization was assessed by changes in Fura-2 (1μM) fluorescence and analyzed via FlexStationIII (ICCB-Longwood, Harvard Medical Screening Facility).
Human megakaryocyte cell line (MEG-01)
The MEG-01 cell line (ATCC, CRL-2021)32 was cultured in RPMI-1640 supplemented with 10% FBS at 37o C. To assess expression of ChemR23, BLT1, P2Y12 and P2Y1 in MEG-01 cells, PCR was performed. To detect the surface expression of ChemR23 and BLT1, 1×106 cells were stained with anti-human ChemR23, anti-human BLT1, or goat anti-mouse IgG control and analyzed using flow cytometry with CellQuest software. Changes in actin polymerization of MEG-01 (1×105 cells per treatment) were monitored as above. Experiments were carried out using cells that had undergone no more than ten passages.
Actin Polymerization in CHO cells stably expressing human ChemR23
Chinese hamster ovary cells (CHO) stably expressing human ChemR23 or mock (pcDNA3) were prepared as in31 and cultured in Hams F12, FBS 10% and G418 (800 μg/mL). Cells were plated (1×105cells/well) in a 96-well plate and incubated with ADP33 (10 μM) or RvE1 (10 nM) plus ADP (10 μM) for 60 minutes34 at 37°C. FITC-phalloidin was used to monitor changes in actin modification, and fluorescence was acquired using an EnVision plate reader (ICCB-Longwood, Harvard Medical Screening Facility).
P2Y12β-arrestin system
PathHunter CHO-K1 cells over expressing human P2Y12 were cultured as in35 and obtained from DiscoverX (Fremont, CA)36. CHO-P2Y12β-arrestin cells were cultured in HAMs F12 media supplemented with G418 (800 μg/mL), Hygromycin (300 μg/mL), and 10% FBS at 37°C. Cells were incubated (1×105cells/well) in 96-well plates for 24 hours at 37°C. Incubations were carried out in HAMs F12 media (without FBS or antibiotics). ADP (10 nM–100 μM), RvE1 (0.1–100 nM) or RvE1 plus ADP were added for 60 minutes, at 37°C. P2Y12 activation was determined by chemiluminescnce (PathHunter EFC detection kit)36 using an EnVision luminometer (ICCB-Longwood, Harvard Medical Screening Facility). Transient transfection of human ChemR23 or pcDNA3 (as a mock control) were carried out for 48 hours, using FuGENE transfection regents. Flow cytometry was used to validate surface expression of hChemR23.
Statistical analysis
The significance of differences between groups was evaluated using the 2-tailed Student’s t test or ANOVA with Bonferroni post testing. P values of less than 0.05 were taken as statistically significant (GraphPad software).
Results
RvE1 Counters ADP-dependent Platelet Activation
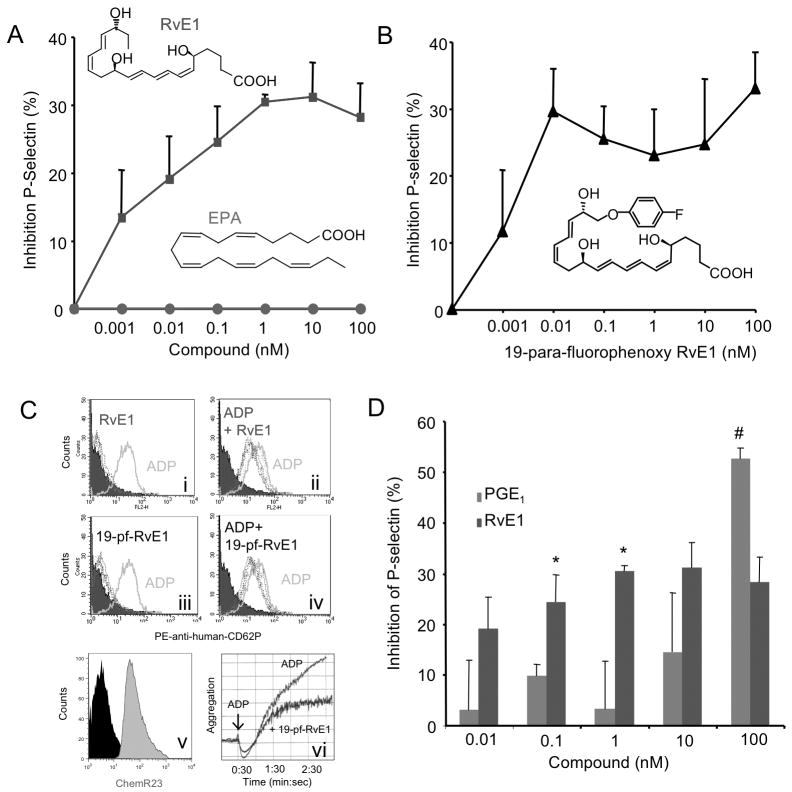
ADP is released from granules within activated platelets during the secondary wave of platelet aggregation that in turn activates neighboring platelets and/or leukocytes25. To determine whether RvE1 regulates ADP-dependent platelet activation, human PRP was incubated with RvE1 (0.001 nM–100nM) for 15 minutes, followed by ADP (10 μM) for 3 minutes, and surface P-selectin was monitored. Appearance of P-selectin on the surface of platelets is a sensitive marker of platelet activation. RvE1 is more potent than its precursor, EPA, and limited ADP-stimulated P-selectin surface mobilization, giving a ~30% reduction at 1nM (Figure 1A). In comparison, an RvE1 stable analog 19-para-fluorophenoxy-RvE129, 30 appeared to be equi-potent (Figure 1B) to RvE1. Representative histograms in Figure 1C demonstrate that neither RvE1 nor its analog 19-para-fluorophenoxy-RvE1 alone (0.001–100nM) increased P-selectin surface mobilization (Figure 1C panel i and C panel ii, respectively). Additionally, we confirmed surface expression of ChemR23 on CD41+ human platelets (Figure 1C, panel v) and found that 19-para-fluorophenoxy-RvE1 reduced ADP-stimulated platelet aggregation (Figure 1C, panel vi). We next compared RvE1’s actions with ADP-stimulated P-selectin mobilization to that of PGE1 (0.01 nM–100 nM), a prostanoid that blocks platelet activation via stimulation of cAMP25. When directly compared, RvE1 (0.1 nM and 1.0 nM) significantly reduced ADP-stimulated P-selectin mobilization as compared on a molar basis to PGE1 (Figure 1D). At low doses RvE1 appeared more potent that PGE1 reduction of P-selectin mobilization while at higher concentration PGE1 gave stronger maximal inhibition.
Figure 1. RvE1 reduces ADP-stimulated P-selectin surface mobilization.
PRP was incubated with either vehicle, (A) RvE1 (0.01–100 nM, blue), EPA (0.01–100 nM, red) (B) 19-para-flurorophenoxy-RvE1 (0.01–100 nM) was incubated for 15 minutes, 37°C, and ADP (10 μM) was added for 3 minutes, 37°C. Platelets were stained with PE-anti-human CD62P (P-selectin) and subjected to flow cytometry. (C) Representative histograms of PE anti-human CD62P surface expression. Vehicle (shaded), ADP (10μM) alone, solid line, RvE1 or 19-para-fluorophenoxy-RvE1 (dashed lines). (Ci) and (Ciii) indicate SPM alone while (Cii) and (Civ) display SPM + ADP. Histograms are representative of n=5–6 separate donors. (Cv) Representative histogram of ChemR23+ platelets. (Cvi) 19-para-fluourophenoxy-RvE1 (10 nM) reduction of ADP-stimulated platelet aggregation, representative of n=3. (D) Direct Comparison between PGE1 (0.01 nM-100 nM) and RvE1 at equi-molar concentrations. Results are mean ± SEM n=3, separate donors, *p<0.05, #p<0.03.
RvE1 Blocks Platelet Actin Polymerization
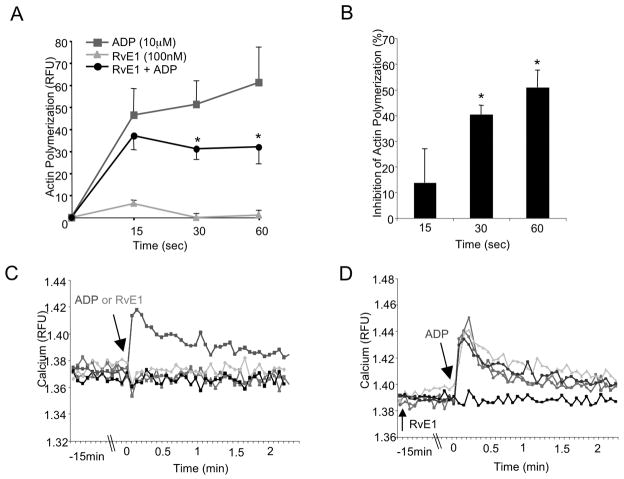
Upon activation, platelets undergo robust shape changes25 . Therefore, it was of interest to assess whether RvE1 modulates the actin cytoskeleton upon ADP-dependent stimulation. As compared to ADP (10 μM), RvE1 (100 nM) alone did not stimulate actin polymerization in PRP (Figure 2A). When administered 15 minutes prior to ADP, RvE1 (100 nM) significantly blocked ADP-stimulated actin polymerization by 40.5% ± 3.6 and 50.0% ± 6.7 at 30 and 60 seconds respectively (Figure 2B). Together, these results indicate that RvE1 dampens platelet responses by blocking potentiation of ADP-stimulated morphology changes.
Figure 2. RvE1 reduces ADP-stimulated actin polymerization: Ca2+ independent.
(A,B) PRP was incubated with either vehicle or RvE1 (100 nM) for 15 minutes, 37°C with gentle mixing then stimulated with ADP (10 μM) for 0, 15, 30, or 60 seconds. Incubation was stopped by the addition of ice-cold formalin (6%) and platelets were permeabilized and stained with FITC-phalloidin (1:100) for 1 hour, 4°C. Changes in platelet morphology were quantified via flow cytometry and Cell Quest software. (B) RvE1 (100nM) percent inhibition of ADP-stimulated actin polymerization. Results are mean±SEM n=3, *p≤ 0.03. (C,D) Fluorescent intracellular calcium indicator, Fura-2. (C) RvE1 (1–100nM) as compared to vehicle and ADP. (D) RvE1 (1–100nM) plus ADP as compared to vehicle. Fluorescence was monitored by an EnVision plate reader and accompanying software. Results are representative of n=3.
RvE1’s actions on human platelets are not a consequence of intracellular calcium mobilization
ADP stimulation via P2Y1 releases intracellular calcium stores, which further enhances platelet activation25. RvE1 itself neither stimulated Ca2+ mobilization (Figure 2C) nor did it block ADP-stimulated intracellular Ca2+ release in human PRP (Figure 2D). To assess whether RvE1 blocks intracellular Ca2+ mobilization from other platelet agonists, the TXA2 receptor agonist U46619 (500 μM) and thrombin (1 U/mL) were each tested (n=3). RvE1 (1–100 nM) did not block either U46619- or thrombin-stimulated Ca2+ mobilization (n=3, data not shown). These results suggest that RvE1’s actions on platelet activation are not a consequence of signaling events via P2Y1 that lead to intracellular Ca2+ mobilization.
RvE1 recombinant human ChemR23 interactions are required to reduce ADP-stimulated actin polymerization
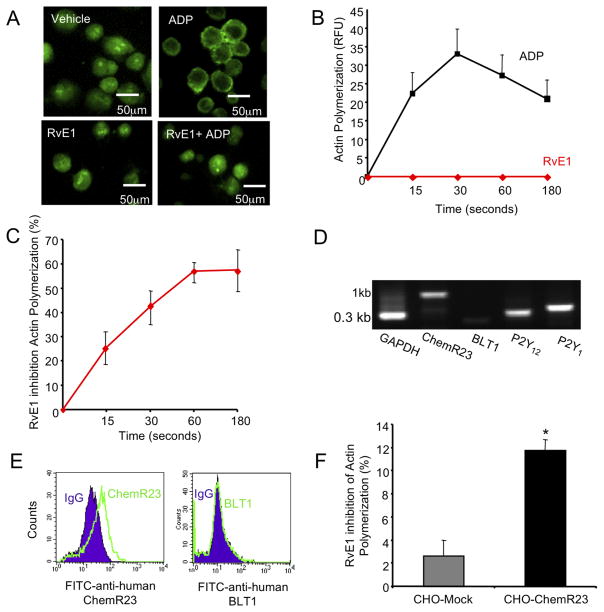
Since an RvE1 receptor ChemR2317 was recently identified on human platelets,22 we tested whether RvE1 actions in regulation of ADP responses were mediated by ChemR23. Upon ADP-stimulation, actin is polymerized as indicated by punctate staining of FITC-phalloidin (Figure 3A,B). ADP stimulated actin polymerization in reaching a maximal response at 30 seconds while RvE1 (100 nM) alone did not (Figure 3B). When given 15 minutes prior to ADP, RvE1 effectively reduced ADP-stimulated actin reaching a maximal inhibition at 60 seconds (Figure 3C). The MEG-01 (human megakaryoblast) cell line was selected because it expresses ChemR23, P2Y12 and P2Y1 but not BLT1 (Figure 3D,E). This was useful because RvE1 can also interact with BLT120 present on human neutrophils. Since CHO cell express P2Y receptors33, we further examined and quantitated ChemR23-dependent counter regulation of ADP activation, we prepared CHO cells that stably over expressed recombinant human ChemR23 (CHO-hChemR23). RvE1 (10 nM) significantly (p<0.01) reduced ADP (10 μM)-stimulated actin polymerization in CHO-hChemR23 expressing cells but not in CHO-mock (Figure 3F).
Figure 3. RvE1 reduces ADP-stimulated actin polymerization in MEG-01.
(A–C) MEG-01 cells (1×105/treatment) were cultured and stimulated with vehicle, ADP (10 μM), RvE1 (100 nM) and ADP plus RvE1 for 0, 15, 30, 60 or 180 seconds, 37°C. Actin polymerization was visualized via fluorescent microscopy (Nikon Eclipse E600 and imported into Spot Diagnostic analysis software) (C) or quantified via flow cytometry (D,E). (A) Images are representative of n=3 and (B,C) values are mean ± SEM of n=3. (D) Representative gel of P2Y12, P2Y1, ChemR23 and BLT1 expression. Note that all products were 0.3kb with the exception of full-length human ChemR23, which was 1kb. (E) MEG-01 surface expression of ChemR23 (left, solid line) or BLT1 are shown as compared to their respective isotype matched controls (shaded). (F) Mock pcDNA3 or hChemR23 was transiently transfected into CHO cells using Lipofectamine 2000 for 48 hours, 37°C. CHO-Mock (grey bar) or CHO-hChemR23 (black bar) cells (1×105) were stimulated with RvE1 (10 nM) 15 minutes prior to ADP (10 μM) stimulation (60 minutes, 37°C). FITC-phalloidin fluorescence was assessed via an EnVision plate reader and software. Values are mean ± SEM of n=3, *p<0.01.
RvE1 Blocks ADP stimulated P2Y12 Activation
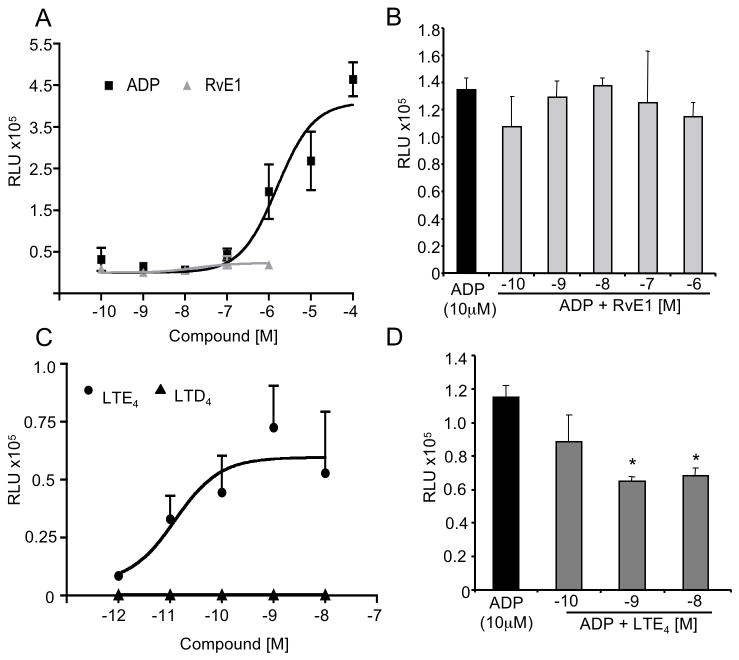
P2Y12, coupled to Gαi, is the ADP receptor on platelets that is primarily responsible for blocking adenyl cyclase and cAMP accumulation and to potentiate P2Y1, (a platelet ADP receptor coupled to Gq and intracellular calcium mobilization) signaling events25. Since RvE1 blocked the potentiation of platelet actin polymerization (Figure 2A,B) without evoking mobilization of intracellular Ca2+ (Figure 2C,D), we set out to investigate P2Y12 signaling. To monitor ligand receptor interactions, we used a new GPCR-β-arrestin-coupled system that consisted of CHO cells stably overexpressing β–arrestin and recombinant human P2Y12 (CHO-P2Y12). ADP (EC50 5×10−6 M) dose-dependently activated CHO-P2Y12β-arrestin cells whereas RvE1 did not (Figure 4A). In addition, RvE1 (10−10 M – 10−6 M) did not block ADP activation of P2Y12 suggesting that RvE1 did not interact with P2Y12 directly (Figure 4B). In light of the recent findings that LTE4 may be a ligand for P2Y12 37, we next sought to investigate LTE4 interaction with P2Y12 using this cell system. We directly compared LTE4 to LTD4 at equi-concentrations (10−12 M – 10−8 M). LTE4 (EC50 1.3×10−11 M) dose dependently activated P2Y12 while LTD4 at the same concentration range was ineffective (Figure 4C). In addition, LTE4 (10−10 M – 10−8 M) blocked ADP activation of P2Y12 in this system (Figure 4D) suggesting that LTE4 directly interacts with P2Y12.
Figure 4. RvE1 counter regulates P2Y12 signaling.
ADP (10−10 M – 10−4 M) added to CHO-P2Y12 (1×105 cells/well), RvE1 (10−10 M – 10−6 M), LTE4 (10−12 M – 10−8 M) or LTD4 (10−12 M – 10−8 M) were added (60 minutes at 37°C). All incubations were stopped with lysis buffer containing emerald substrate reagent for (60 minutes, 21°C). EC50 values were determined by curve fit analysis using GraphPad software (see methods). (A) ADP (squares) and RvE1 (triangles) dose response. Values are mean ± SEM of n=5. (B) RvE1 (10−10 M – 10−7 M), when added simultaneously with ADP. (C) LTE4 (circles) and LTD4 (triangles) dose response. Values are mean ± SEM of n=6. (D) LTE4 (10−9 M – 10−8 M) followed by ADP (10−5 M). Values are mean ± SEM , n=3, *p<0.05.
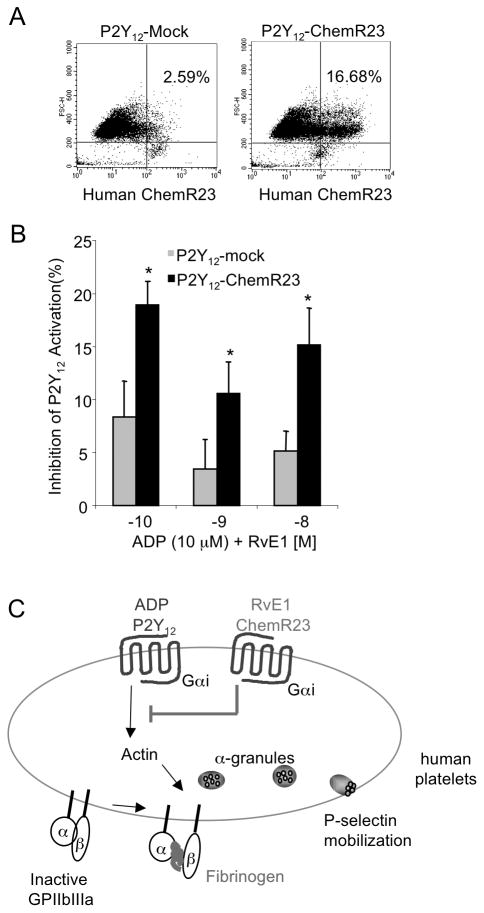
To further elucidate whether RvE1’s counterregulatory action is via ChemR23, we next transiently transfected recombinant human ChemR23 (P2Y12-ChemR23) or pcDNA3 (P2Y12-Mock) as a mock control into the CHO-P2Y12β-arrestin cells. Transfection was confirmed by assessing surface expression of FITC-anti-human-ChemR23 (Figure 5A). RvE1 (10−10 M – 10−8 M), when added 15 minutes prior to ADP, significantly blocked P2Y12 activation in the P2Y12-ChemR23 cells compared to P2Y12-Mock transfected cells. Lower doses of RvE1 (10−11 M) did not block ADP-stimulated P2Y12 activation in P2Y12-ChemR23 cells compared to P2Y12-Mock (data not shown). In contrast, RvE1 (10−10 M – 10−8 M) did not block LTE4 (10−9 M) activation of P2Y12 in P2Y12-ChemR23 or P2Y12-Mock cells (data not shown), suggesting that RvE1 and LTE4 each interact with P2Y12 via different mechanisms.
Figure 5. RvE1 regulates P2Y12 signaling in a ChemR23-dependant manner.
(A,B) CHO-hP2Y12β-arrestin cells were transiently transected with mock-pcDNA3 or hChemR23 using FuGene transfection reagent for 48 hours, 37°C. Cells were plated (1×105 cells/well), and ADP (10−5 M) or ADP plus RvE1 (10−10 M – 10−8 M) were incubated for 60 minutes, 37°C. (A) Human ChemR23 transient transfection was monitored using flow cytometry. Histograms are representative of n=4. (B) Inhibition of P2Y12 by RvE1 was monitored in mock-pcDNA3 (grey bars) or hChemR23 (black bars) Values are mean ± SEM of n=4, * p<0.03, 2-way ANOVA with Bonferroni post testing. (C) Proposed Scheme for RvE1 actions on human platelets (see discussion for details).
Discussion
Results from several clinical studies demonstrate that omega-3 polyunsaturated fatty acids (PUFA) such as EPA and DHA have beneficial actions in diseases where platelets play central pathogenic roles such as CVD and stroke13. In the present manuscript, we report the potent actions of EPA-derived RvE1 on human platelets. EPA is not converted to RvE1 by isolated platelets but is biosynthesized in the vasculature and produced in vivo within plasma/whole blood17 and hence quieces the activation of platelets and leukocytes22. For example, RvE1 regulates ADP- and U46619-stimulated platelet aggregation but not collagen-stimulated platelet aggregation in an agonist specific and stereoselective manner22. Here, we report RvE1 down regulates (i) ADP-stimulated platelet activation by reducing P-selectin mobilization and the potentiation of platelet actin polymerization, (ii) without altering Ca2+ mobilization, (iii) by P2Y12 –ADP signaling via the RvE1 receptor, ChemR23.
P-selectin is an important modulator of coagulation and inflammation because of its role in cell-cell adhesion with platelets, leukocytes and the vascular endothelium1, 25. The present results demonstrate that RvE1 and its metabolically stable analog 19-para-fluorophenoxy-RvE1 reduced ADP-stimulated P-selectin surface mobilization (Figure 1). Additionally we performed aggregation studies and found that 19-para-fluorphenoxy-RvE1 (10 nM) reduced ADP-stimulated platelet aggregation (Figure 1C) comparable to RvE122. RvE1’s biologically inactive isomer, Δ6,14trans-RvE1 did not reduce ADP-stimulated platelet aggregation22. These results underscore that RvE1’s stereoslective actions and ability as a local mediator that may control platelet-platelet and platelet-leukocyte aggregate formation as well as their interactions with activated endothelium. These findings are in agreement with recent results where RvE1 reduces surface expression of selectins and integrins on neutrophils and monocytes in human whole blood and promotes detachment of leukocytes from endothelium in microcirculation22.
Platelets play a pivotal role in circulation through the preservation of vascular integrity and prevention of hemorrhage after injury25. Although the formation of a thrombus (i.e. platelet-platelet and platelet-leukocyte aggregates) is a protective mechanism for wound healing, excessive thrombus formation may lead to pathological events such as acute myocardial infarction or stroke27. The capacity of platelets to form thrombi is dependent on their ability to aggregate, which at the molecular level is in part due to activation of selectins, (i.e. P-selectin) and integrins, such as GPIIbIIIa. Inside-out activation of GPIIbIIIa from downstream ADP signaling occurs. Upon activation, GPIIbIIIa is able to bind its ligand (fibrinogen), stimulating outside-in signaling and thus further activation of the platelet and stabilization of the clot25. At higher concentrations, we found that 100 nM RvE1 reduces fibrinogen binding to platelets38, which points to a potential role for local RvE1 in regulating formation of thrombi and platelet-leukocyte aggregates also observed with RvE1 added to whole blood22. These results are consistent with P-selectin regulation by ADP (Figure 1) where RvE1 appeared to quiesce activation of platelets and subsequent thrombus formation, as well as dampen platelet-leukocyte interactions22. Complete abolition of platelet responses could lead to excessive bleeding, a common side effect of most current anti-platelet therapeutics26. Hence it is noteworthy that RvE1 did not completely block ADP-stimulated platelet activation, underscoring its potential to modulate platelet responses to ADP for the maintenance of vascular homeostasis.
Platelets undergo rapid morphology changes upon activation by a range of agonists including thrombin, thromboxaneA2 and ADP25. Of interest, RvE1 does not regulate either thrombin- or collagen-22 initiated platelet responses suggesting that RvE1’s actions on platelets are highly selective toward regulating ADP’s local vascular roles. When platelets are initially stimulated, the first event is a rearrangement of the cytoskeletal proteins (actin and myosin),and the normally disc-shaped cells change into spheres with filopodia25, 39. Actin is polymerized upon ADP-stimulation25, 39 primarily via engagement of P2Y140. Results with P2Y1-deficient mice demonstrated that P2Y1 accounts for the ability of ADP to induce changes in morphology and the large intra-platelet calcium mobilization that precedes aggregation41, 42. The role of P2Y12 in platelet shape change is to potentiate the actions from P2Y1 signaling25, 43. In the present experiments, we found that RvE1 reduces the potentiation of ADP-stimulated actin polymerization in human platelets (Figure 2). On its own, RvE1 did not stimulate actin polymerization (Figure 2), indicating that RvE1 is not a classic platelet agonist and that its actions appear to be selective in counterregulating the pro-coagulatory agonist, ADP.
P2Y12 signaling completes platelet aggregation initiated by P2Y1 and plays a central role in amplifying aggregation to other known agonists such as thromboxane A2 (TXA2)25, 26. Of note, RvE1 reduced ADP-stimulated thromboxane generation22, which is consistent with RvE1 blocking P2Y12-mediated signaling events. Recently, it was shown that the P2Y1-mediated Ca2+ flux is unchanged in P2Y12−/− mice, demonstrating that P2Y12 does not participate in the Ca2+ response, and that there is no apparent compensatory increase of P2Y1 function in the knockout mice44. Also, P2Y12−/− mice have a diminished aggregation response to ADP44. RvE1’s ability to quiesce ADP-stimulated aggregation22 and actin polymerization content without modulating intracellular Ca2+ mobilization (Figure 2) is consistent with results from platelets congenitally lacking P2Y1245 and from P2Y12 knockout mice44.
P2Y12 is desensitized via G-protein receptor kinases (GRKs) and β-arrestin in human platelets46. When ADP binds to P2Y12, GRKs are activated46 that phosphorylate the c-terminus of P2Y12. Upon phosphorylation, β-arrestin is recruited and physically associates with the activated c-terminal region, which leads to the internalization and desensitization of the GPCR, a process known as homologous receptor desensitization47. P2Y1 signaling in human platelets is desensitized by other mechanisms not associated with β-arrestin46. Thus, we used a recombinant P2Y12-β-arrestin-coupled system to evaluate 1) RvE1’s regulatory actions and 2) members of the leukotriene series of mediators known to be important in slow reacting substances of anaphylaxis (SRS-A) on P2Y12 signaling because LTE4 was identified as a putative ligand37. RvE1 did not directly activate P2Y12 (Figure 4A and B). In contrast, LTE4 directly activated recombinant P2Y12 (Figure 4C,D), a finding consistent with recent reports suggesting leukotriene E4 (LTE4) once considered an inactive SRS-A metabolite48, may be relevant in allergic reactions in humans48, 49.
In addition to nucleotides, eicosanoids are also mediators of thrombosis and vascular homeostasis24. Thromboxane A2 (TxA2) stimulates platelets and prostacyclin counteracts platelet activation50. Of interest, CysLTs were identified at sites of thrombus formation and in atheroma plaque in vivo51. Yet, the actions of CysLTs on platelet function are insufficiently understood. Recently, LTE4 has also been reported to activate mast cells in a P2Y12 dependent fashion49. Therefore, it was of interest in the present experiments with the P2Y12-β-arrestin system that LTE4 is indeed an activating ligand for P2Y12 as well as an effective competitor of ADP activation of this receptor.
Since RvE1 did not directly activate nor block ADP-stimulated recombinant P2Y12 activation (Figure 5A,B), we next sought evidence for whether the expression of recombinant human ChemR23 may mediate RvE1’s counterregulatory actions in ADP-P2Y12 signaling. When recombinant human ChemR23, one of the RvE1 GPCRs, was co-expressed on this cell system, RvE1 significantly blocked P2Y12 activation in the P2Y12-ChemR23 as compared to P2Y12-Mock. In this recombinant system, it appears that RvE1 activation of ChemR23 (RvE1-ChemR23) is a threshold dependent response, likely a phosphorylation event that blocks ADP-P2Y12 signaling to action. These findings suggest that RvE1 counterregulates P2Y12 signaling in a ChemR23-dependent manner (Figure 5B). Also, these results provide the first example that resolvins may interact not only with their cognate receptor, but also with other ligand-activated GPCR-mediated signals.
A classic example of endogenous ligand-GPCR mediated counterregulation in the vasculature is the interaction between prostacyclin (IP) and thromboxane (TPα) receptors24, 50, 52. To limit TXA2 activation, IP can form dimers with TPα53. Thus, it is also feasible that in acute inflammation, agonists of resolution of inflammation and regulators of hemostasis, such as RvE1, act in concert with other receptors to control the magnitude of platelet activation in the local environment.
In summation, ADP is an agonist of cardiovascular events and an improved understanding of the mechanisms by which platelets become inactivated is of immense interest28. RvE1 reduces ADP-stimulated human platelet aggregation22 and in this present report we document that RvE1 counterregulates ADP-stimulated P-selectin surface mobilization and the potentiation of actin polymerization in a Ca2+ independent manner. Together these findings suggest that RvE1 actions are counterregulating P2Y12 signaling in human platelets, illustrated in Figure 5C. In support of this model, results from the GPCR-β-arrestin coupled recombinant system indicate that RvE1’s counter regulation of P2Y12 is RvE1 receptor-(ChemR23) dependent. These findings can provide novel approaches for platelet therapies as well as explain, in part, the actions of dietary EPA via local RvE1 production and platelet interactions that impact cardiovascular diseases and timely return of homeostasis.
Acknowledgments
We thank Dr. Sriram Krishnamoorthy for help with the CHO-P2Y12β-arrestin cells, and Mary H. Small for expert assistance in paper preparation.
Sources of Funding --This work was supported in part by National Institutes of Health(NIH, Bethesda, MD) grant nos. GM38765 and RC2AT005909 (C.N.S.) and DE15566 (T.E.V.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS, NIDDK, or the NIH.
Footnotes
Author Contributions
G.F. carried out experiments, acquired and analyzed data, performed statistical analyses, and contributed to paper preparation; T.E.V.D analyzed and interpreted results and supervised the research, and C.N.S. conceived and designed the research and experiments, interpreted results, supervised the research, and contributed to presenting the manuscript.
Disclosure -- C.N.S. is inventor on patents assigned to Brigham and Women’s Hospital on the resolvins, related compounds, and their analogs and uses (C.N.S. and T.V.D.) that are licensed for clinical development. C.N.S. and T.V.D. retain founder stock in Resolvyx Pharmaceuticals. G.F. declares no competing financial interests.
References
- 1.Majno G, Joris I. Cells, tissues, and disease : Principles of general pathology. New York: Oxford University Press; 2004. [Google Scholar]
- 2.Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and cotran pathologic basis of disease. Philadelphia: Elsevier/Saunders; 2005. [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN. Endogenous chemical mediators in anti-inflammation and pro-resolution. Curr Med Chem Anti Inflamm Anti Allergy Agents. 2002;1:177. [Google Scholar]
- 6.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyke TE, Serhan CN. Resolution of inflammation: A new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 8.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Inverse temporal changes of lipoxin A4 and leukotrienes in children with henoch-schonlein purpura. Prostaglandins Leukot Essent Fatty Acids. 2009;80:177–183. doi: 10.1016/j.plefa.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leon H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: Systematic review. BMJ (Clinical Research Ed ) 2008;337:a2931. doi: 10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:e20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 15.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin e2: Identification and anti-inflammatory actions: Pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 22.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasari VR, Jin J, Kunapuli SP. Distribution of leukotriene B4 receptors in human hematopoietic cells. Immunopharmacology. 2000;48:157–163. doi: 10.1016/s0162-3109(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 24.Marcus AJ. Stratton lecture 1989. Thrombosis and inflammation as multicellular processes: Pathophysiologic significance of transcellular metabolism. Blood. 1990;76:1903–1907. [PubMed] [Google Scholar]
- 25.Michelson AD. Platelets. Amsterdam ; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 26.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL. Platelets in cardiovascular disease. London: Imperial College Press; 2008. [Google Scholar]
- 28.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 29.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 31.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2009 doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogura M, Morishima Y, Ohno R, Kato Y, Hirabayashi N, Nagura H, Saito H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive philadelphia chromosome. Blood. 1985;66:1384–1392. [PubMed] [Google Scholar]
- 33.Marcet B, Chappe V, Delmas P, Verrier B. Pharmacological and signaling properties of endogenous P2Y1 receptors in cystic fibrosis transmembrane conductance regulator-expressing chinese hamster ovary cells. J Pharmacol Exp Ther. 2004;309:533–539. doi: 10.1124/jpet.103.063396. [DOI] [PubMed] [Google Scholar]
- 34.Boccellino M, Biancone L, Cantaluppi V, Ye RD, Camussi G. Effect of platelet-activating factor receptor expression on cho cell motility. J Cell Physiol. 2000;183:254–264. doi: 10.1002/(SICI)1097-4652(200005)183:2<254::AID-JCP12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson KR, Eglen RM. Beta galactosidase complementation: A cell-based luminescent assay platform for drug discovery. Assay and Drug Development Technologies. 2007;5:137–144. doi: 10.1089/adt.2006.052. [DOI] [PubMed] [Google Scholar]
- 37.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 38.Fredman G. PhD Dissertation. Department of Oral Biology, Boston University; Boston: 2009. Resolvin E1 regulation of platelet functions: Novel pathways in resolution. [Google Scholar]
- 39.Paul BZ, Daniel JL, Kunapuli SP. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J Biol Chem. 1999;274:28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- 40.Hechler B, Leon C, Vial C, Vigne P, Frelin C, Cazenave JP, Gachet C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92:152–159. [PubMed] [Google Scholar]
- 41.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 42.Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. The role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351–360. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 44.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992;80:2787–2796. [PubMed] [Google Scholar]
- 46.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and p2y12 receptors for adp desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 47.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Samuelsson B. Leukotrienes: A novel group of compounds including SRS-A. Prog Lipid Res. 1981;20:23–30. doi: 10.1016/0163-7827(81)90010-2. [DOI] [PubMed] [Google Scholar]
- 49.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene e4-induced pulmonary inflammation is mediated by the p2y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1:18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- 51.Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- 52.Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J Biol Chem. 2004;279:53036–53047. doi: 10.1074/jbc.M405002200. [DOI] [PubMed] [Google Scholar]
- 53.Wilson SJ, Dowling JK, Zhao L, Carnish E, Smyth EM. Regulation of thromboxane receptor trafficking through the prostacyclin receptor in vascular smooth muscle cells: Role of receptor heterodimerization. Arterioscler Thromb Vasc Biol. 2007;27:290–296. doi: 10.1161/01.ATV.0000252667.53790.4e. [DOI] [PubMed] [Google Scholar]