Abstract
Objectives. To systematically review and meta-analyse evidence on the effectiveness of the TNF-α inhibitors when used sequentially.
Methods. Systematic review of comparative and single-arm observational studies. Data were synthesized using random-effects meta-analysis. Treatment effects were estimated using four outcome measures from the included studies: European League Against Rheumatism (EULAR) and ACR20 response rates and mean improvement in disease activity score-28 (DAS-20) and HAQ. The effect of other factors was explored via meta-regression and sub-group analyses.
Results. Twenty studies comprising 2705 patients were included in the analysis. All studies were observational and most had no control group. Therefore, our primary analysis considered patient changes from baseline. The mean percentage of ACR20 responders was 60.8% (95% CI 53.8, 67.4), EULAR responders 70.5% (95% CI 63.7, 76.6), mean overall improvement in DAS-28 scores was 1.53 (95% CI 1.25, 1.80) and in HAQ scores was 0.25 (95% CI 0.11, 0.40). Four studies made comparisons with patients who received TNF-α inhibitors for the first time. Response rates associated with sequential TNF-α inhibitor treatment were lower than for first-time use.
Conclusions. Sequential TNF-α inhibitor use is likely to lead to treatment benefit in terms of the signs and symptoms of disease and physical function. There is also some evidence to suggest that the probability of achieving a response is lower, and the average magnitude of response is lower than the first use. Further evidence from randomized controlled trials is required to confirm and further quantify the role specific anti-TNF-α agents have when used sequentially.
Keywords: Rheumatoid arthritis, Systematic review, Meta-analysis, TNF-α inhibitors, Sequential
See page 2235 for the editorial comment on this article (doi:10.1093/rheumatology/keq257)
Introduction
RA is the most common form of inflammatory arthritis with prevalence estimated at 0.8% of the population [1] and incidence between 1.5 per 10 000 for males and 3.6 per 10 000 for females in the UK. [2] Although joint destruction can be painful and disabling, RA is also a systemic disease often affecting extra-articular tissues throughout the body. RA imposes a substantial cost burden both to health services and to the wider economy, particularly due to the fact that many patients are unable to continue working [3].
Biologic treatments are used when conventional DMARDs such as MTX cannot control RA synovitis. The efficacy of TNF-α inhibitors—infliximab, adalimumab and etanercept—the first biologics used in RA, has been established in randomized controlled trials (RCTs) [4–6] and confirmed in meta-analyses [7]. There is also evidence that they may be cost-effective when conventional DMARDs are insufficient [8, 9]. Newer biologics—rituximab, abatacept and tocilizumab—also have proven efficacy [10, 11], though their cost-effectiveness is more controversial.
A substantial minority of RA patients stop taking their first TNF-α inhibitor due to intolerance, lack of response (primary inefficacy) or loss of response (secondary inefficacy). Treatment options in these patients comprise switching to a second TNF-α inhibitor, alternative biologics such as rituximab and reverting to non-biological treatments. Biologics are high-cost treatments. It is, therefore, of crucial importance that policy makers and rheumatologists alike consider the relative effectiveness and cost-effectiveness of switching TNF-α inhibitors compared with these alternative strategies.
There is uncertainty about the relative efficacy of TNF-α inhibitors with first and subsequent use. As there are genetic response predictors for some TNF-α inhibitors in RA [12, 13], there might be a class effect with patients failing to respond to one TNF-α inhibitor failing to respond to other agents in the class. Recent analyses of the cost-effectiveness of sequential TNF-α inhibitor therapy have, therefore, assumed efficacy is equivalent to first use [13, 14]. As a consequence, direct evidence is needed about the effectiveness of TNF-α inhibitor switching to inform clinical and drug reimbursement decision makers.
We have, therefore, estimated the treatment effect of the TNF-α inhibitors, both as a class and as individual agents, when used sequentially. The analysis is based on evidence identified from a systematic review and considers the most widely reported outcome measures in RA: the HAQ and disease activity score (DAS) measures, and the ACR and European League Against Rheumatism (EULAR) response criteria. The analysis also explores reasons for heterogeneity in study results, including reason for switching (intolerance, primary inefficacy or secondary inefficacy) and the TNF-α inhibitor that patients switched from or switched to.
Methods
Systematic review
The aim of the review was to identify all articles published in peer-reviewed journals that reported the clinical effectiveness of either infliximab, adalimumab or etanercept in RA patients that had previously been treated with at least one TNF-α inhibitor.
We conducted comprehensive searches of electronic databases (Cochrane Library, MEDLINE, EMBASE and NHS Database of Reviews of Effectiveness). Reference lists for identified studies were hand searched. A combination of free-text and thesaurus search terms were employed to specify both the relevant population and interventions (see appendix 1 for details, available as supplementary data at Rheumatology Online). Searches were conducted to cover the period from January 2001 to October 2009.
Studies were included if they considered RA patients that had withdrawn from either infliximab and/or etanercept and/or adalimumab (but not all three) and had been switched to a different TNF-α inhibitor. Studies of patients with other conditions such as juvenile arthritis, Crohn’s disease, PsA or other forms of SpA were excluded unless RA patients could be distinguished in the results. Studies reporting switches to anakinra, abatacept or rituximab were not included. At least one of the following outcome measures that reflect the signs, symptoms and impact on physical function of RA had to be reported for a study to be included: ACR, EULAR, HAQ or DAS/DAS-28. We did not consider radiographic outcome measures.
Identified studies were selected for review by one of us (A.J.W.) based on the title and abstract if available. Articles selected were then assessed against the inclusion criteria on the basis of the full study reports. In addition to the criteria given above, several studies were excluded at this stage because they replicated data reported in other studies included in the review.
Data from included studies were extracted independently by two of the authors with any disagreements resolved by consensus. We recorded the TNF-α inhibitor being investigated and the TNF-α inhibitor patients had switched from. The reason for switching was categorized as intolerance or adverse events, primary inefficacy (a failure to achieve a clinical response from the start of treatment), secondary inefficacy (a loss of response over time in patients that had originally achieved primary response) and other. Outcome data were recorded that consisted of number of patients, proportions of responders in case of ACR and EULAR scores and for continuous outcome measures DAS-28 and HAQ, means and standard errors if available. Otherwise s.d.s, medians or inter-quartile ranges were noted. Where studies reported outcomes at multiple time points after switching treatments, data for each time point were extracted. These outcome measures were recorded for whole cohorts described in each of the included studies as well as for sub-groups of patients defined by sequence of the TNF-α inhibitor and by reason for switching. Other patient characteristics extracted from the selected papers included mean age, percentage of females, percentage of patients classified as being RF+, mean disease duration in years, mean number of previous DMARDs, mean duration of previous biologic treatment in months and follow-up time in weeks.
Meta-analysis
Each of the four outcome measures—ACR, EULAR, DAS and HAQ—were considered separately in the analysis, although similar analytic methods were utilized; different measures of effect size were used for the categorical and continuous data. We found that many studies reported only ACR20, not ACR50/70/90, and we, therefore, limit discussion to this outcome measure.
Random-effects meta-analysis models were used from the outset due to the known clinical heterogeneity between studies. Where data on sub-groups only were available, a fixed-effects meta-analysis was carried out to obtain the overall outcome for the whole cohort. For non-comparative studies, the meta-analysis for the binary response data was carried out using the log-odds of being classed as a responder (transformed back to a proportion for interpretation). The effect size for the continuous outcomes was the change from baseline score (improvement in DAS-28 or HAQ scores). Missing data were calculated or imputed for the continuous outcomes where necessary (in particular, for the change from baseline values and the associated s.e.s) using both within-study [15] and across-study imputation methods [16] (see appendix 2 for details, available as supplementary data at Rheumatology Online).
Initially, meta-analyses were conducted treating all TNF-α inhibitors as a class (i.e. assuming equal effectiveness). Variability between the studies was assessed using the I2-statistic, which can take values between 0 and 100% where high values indicate strong heterogeneity. Meta-regression models [17] were then developed with the aim of trying to explain heterogeneity using the study-level demographic covariates of mean age, percentage of females and baseline characteristics that included the percentage of RF+ patients, mean disease duration, mean number of previous DMARDs and the mean duration of previous biologic treatments. Follow-up time was also considered. Sub-group analyses and meta-regressions were then carried out to determine whether there were differences in outcome according to the type and sequence of TNF-α inhibitor received—adalimumab, etanercept or infliximab—or according to the reason for switching to another treatment—primary inefficacy, secondary inefficacy, inefficacy, intolerance or other.
Data estimating the comparative effect of sequential TNF-α inhibitors use vs first-line use were extracted where reported. Meta-analysis was used to pool the results from multiple studies. As for the non-comparative analyses, where the s.e.s of the differences in outcome from baseline were not reported, it was necessary to use imputation methods. Studies that reported data on rituximab controls are not included in the meta-analysis but reported separately.
Publication bias was assessed using funnel plots and Eggers test. All analyses were conducted in Stata v11.0 (StataCorp LP, TX, USA), with a significance level of 5%. No adjustments were made for multiple testing.
Results
Systematic review
Study characteristics
Searches resulted in 453 unique references. A total of 67 studies were identified for review, and of these a total of 20 [18–37] were eligible for inclusion. These provided data on a total of 2705 patients that received sequential TNF-α inhibitor treatment.
Table 1 provides details related to treatments in the included studies, whereas Table 2 lists reported outcome measures indicating also those in comparator groups where available. Fifteen of the studies have no comparator group. Four studies make comparisons with other cohorts of patients taking a first anti-TNF-α and who may or may not include the group that subsequently switched [18, 20, 34, 37]. One of these studies [20] includes data on over 800 switching patients and over 5000 patients receiving their first TNF-α inhibitor. One other study makes comparisons with patients that switched to rituximab [25]. Clearly, such comparisons must be treated with caution owing to the observational nature of these studies. One of the identified studies is an RCT [38] and this is an open-label, pilot study (n = 28). However, this study included patients who responded to the first TNF-α inhibitor and were then randomized. We acknowledge that this study is different and we exclude it from the comparative analysis. Sensitivity analysis showed that excluding this study from the single-arm analysis did not have any impact on the conclusions. Many of the identified studies are relatively small; 14 (61%) have fewer than 50 patients and 4 (17%) have fewer than 20 patients.
Table 1.
Characteristics of included studies
| Study | n | Failed anti-TNF, n | New anti-TNF | Reason for switching, % | Time point, weeks |
|---|---|---|---|---|---|
| Bennett et al. [18] | 26 | INF, ETA, AKA | ADA | PIE (31), SIE (50) | 34a |
| Bingham et al. [19] | 188 | INF | ETA | PIE (15), SIE (85) | 16 |
| Bombardieri et al. [20] | 810 | ETA (168) | ADA | PIE (24), SIE (31), AE (30) | 12 |
| INF (541) | ADA | PIE (30), SIE (19), AE (29) | |||
| ETA and INF (120) | ADA | IE/AE | |||
| Buch et al. [21] | 25 | INF | ETA | PIE | 12 |
| Buch et al. [22] | 7 | INF | ETA | PIE (36), SIE (40), AE (24) | 12 |
| Cohen et al. [23] | 30 | INF (18), ETA (12) | ETA, INF | PIE (76), AE (4) | 12 |
| Di Poi et al. [24] | 18 | INF | ETA | PIE (61), SIE (39) | 42 |
| Finckh et al. [25] | 66 | Unspecified | Unspecified | IE | 24 |
| Haraoui et al. [26] | 22 | INF | ETA | IE/AE | 6, 12 |
| Hjardem et al. [27] | 156 | INF (130), ETA (7), ADA (19) | Unspecified | IE (46), AE (31) | 12 |
| Hyrich et al. [28] | 331 | ETA, INF, ADA | ETA, INF, ADA | IE | 52 |
| Iannone et al. [29] | 37 | INF | ETA | AE (100) | 8, 16, 24 |
| Karlsson et al. [30] | 337 | Unspecified | Unspecified | IE (41), AE (41) | 12 |
| Koike et al. [31] | 411 | INF | ETA | IE/AE | 24 |
| Laas et al. [32] | 26 | INF | ETA | IE (77), AE (23) | 12, 24, 36 |
| Navarro-Sarabia et al. [33] | 83 | Unspecified | Unspecified | IE (58), AE (29) | unclear |
| Nikas et al. [34] | 24 | INF | ADA | IE (38), AE (62) | 48 |
| Van der Bijl et al. [35] | 41 | INF | ADA | PIE (37), SIE (51), AE (12) | 16 |
| van Vollenhoven et al. [36] | 31 | ETA (18), INF (13) | INF, ETA | IE (77), AE (11) | 12 |
| Wick et al. [37] | 36 | INF (27), ETA (9) | ADA | SIE | 12, 24 |
aThis was the mean time of follow-up, not a set time from baseline. INF: infliximab; ETA: etanercept; ADA: adalimumab; AKA: anakinra; IE: inefficacy (primary or secondary); PIE: primary inefficacy; SIE: secondary inefficacy; AE: adverse event.
Table 2.
Reported outcomes in selected studies
| Study | Outcomes reported |
|||
|---|---|---|---|---|
| ACR | EULAR | DAS-28 | HAQ | |
| Bennett et al. [18] | ✓a | ✓a | ✓a | |
| Bingham et al. [19] | ✓ | ✓ | ✓ | ✓ |
| Bombardieri et al. [20] | ✓a | ✓a | ✓a | ✓a |
| Buch et al. [21] | ✓ | |||
| Buch et al. [22] | ✓ | ✓ | ✓ | |
| Cohen et al. [23] | ✓ | ✓ | ||
| Di Poi et al. [24] | ✓ | ✓ | ||
| Finckh et al. [25] | ✓b | |||
| Haraoui et al. [26] | ✓ | ✓ | ||
| Hjardem et al. [27] | ✓ | ✓ | ||
| Hyrich et al. [28] | ✓ | |||
| Iannone et al. [29] | ✓ | |||
| Karlsson et al. [30] | ✓ | ✓ | ||
| Koike et al. [31] | ✓ | |||
| Laas et al. [32] | ✓ | |||
| Navarro-Sarabia et al. [33] | ✓ | ✓ | ✓ | |
| Nikas et al. [34] | ✓a | ✓a | ✓a | |
| Van der Bijl et al. [35] | ✓ | ✓ | ✓ | ✓ |
| Van Vollenhoven et al. [36] | ✓ | |||
| Wick et al. [37] | ✓a | ✓a | ||
Also given are reported data for comparator group that had: anot previously received anti-TNF-α; breceived rituximab.
Follow-up was typically 12–24 weeks from baseline (range 2–96 weeks). In total, 10 studies reported ACR20 response rates [19–22, 26, 30, 34–37], 12 reported EULAR response rates [18–20, 22–24, 27, 30, 31, 33–35], 13 DAS-28 improvement [18–20,22–25,27,32–35,37] and 8 HAQ improvement [19, 20, 27, 31, 33, 35]. Three studies reported all four outcomes of interest [19, 20, 35]. Details are shown in Table 2.
Table 1 also shows that the included studies reported data on patients that switched from their first TNF-α inhibitor for a variety of reasons. Sub-groups of patients were reported according to the reason for discontinuation: primary inefficacy; secondary inefficacy; primary or secondary inefficacy or adverse events.
Characteristics of patients in studies
The mean and range of the reported patient characteristics (weighted by sample size) in the included studies are reported in Table 3. Most studies reported the mean (or median) age and gender of participants. Reporting of other potentially relevant baseline characteristics was less widespread.
Table 3.
Characteristics of patient populations in included studies
| Characteristic | Studies, n | Weighted mean | Range of study means |
|---|---|---|---|
| Mean age, years | 19 | 55.0 | 47.1–58.0 |
| Female, % | 18 | 80.1 | 63.6–92.0 |
| RF+, % | 13 | 71.4 | 44.0–97.0 |
| Mean disease duration, years | 14 | 11.6 | 8.0–16.6 |
| Mean number of previous DMARDs | 10 | 4.6 | 3.4–5.8 |
| Mean previous biologic duration, months | 10 | 14.9 | 9.0–25.2 |
Meta-analysis results
ACR20
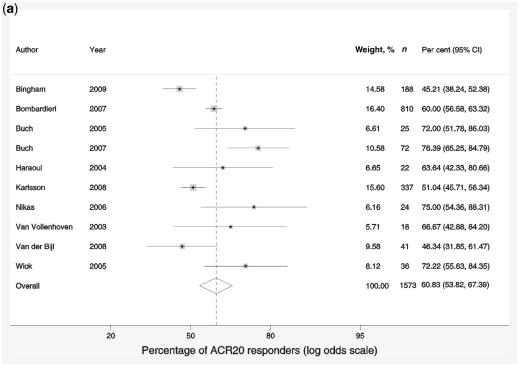
The proportion of ACR20 responders within the studies ranged from 45 to 76%. The pooled response rate was 60.8% (95% CI 53.8, 67.4), suggesting that at least half of patients switching to an alternative TNF-α inhibitor achieved a minimum of an ACR20 response (Fig. 1a). There was a high degree of variability in the estimated proportion due to heterogeneity between the studies (I2 = 77.5), which could not be accounted for by patient demographic or baseline characteristics. There was no significant difference identified in estimates by sub-group based either on the sequence of TNF-α inhibitor or on the reason for switching from one anti-TNF to another.
Fig. 1.
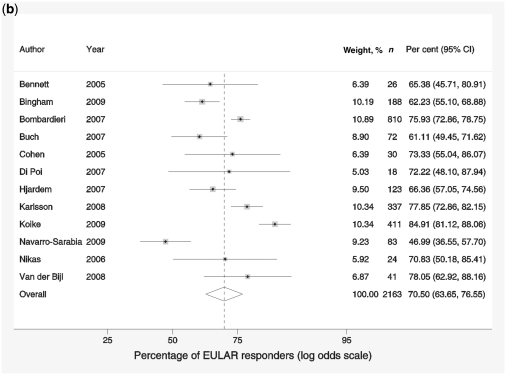
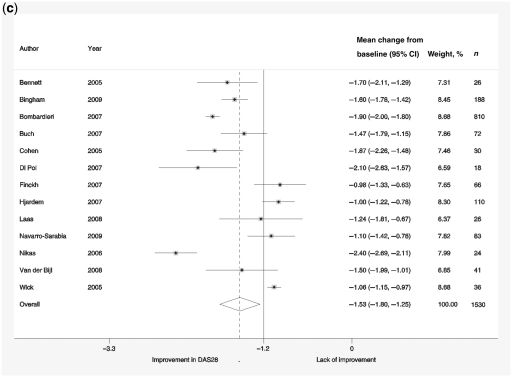
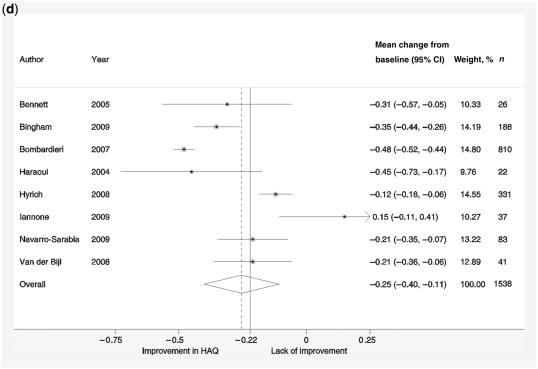
Forest plots showing response rates on the ACR20 scale (a), response rates on EULAR scale (b), response to treatment recorded by change in DAS-28 scores compared with baseline scores (c) and reduction in HAQ scores (d). Weights are from random-effects analysis.
EULAR
The proportion of EULAR responders within the studies ranged from 47 to 85% (Fig. 1b), and the pooled effect size was estimated to be 70.5% (95% CI 63.7, 76.6). There was a high degree of variability in the estimated response rate due to heterogeneity between studies (I2 = 86.3%). No significant effect of baseline characteristics, demographic variables, anti-TNF sequence or reason for switching was found that would explain this heterogeneity.
DAS-28
Change from baseline DAS-28 ranged from −0.98 to −2.4. The overall estimate of the reduction in DAS-28 score (from baseline) was 1.53 (95% CI 1.25, 1.80; Fig. 1c), and the analysis showed a high degree of variability in effect sizes (I2 = 94.8%). The duration of the disease had a significant effect on patients’ DAS-28 responses, giving additional reduction in DAS-28 score by −0.157 (95% CI −0.243, −0.072, P = 0.004) per additional year.
HAQ
HAQ change ranged from increase by 0.15 to decrease by −0.48. The pooled reduction in HAQ score from baseline was 0.25 (95% CI 0.11, 0.40; Fig. 1d). Heterogeneity was also an issue in these studies with I2 = 93.5%. None of the demographic variables, baseline characteristics or sub-groups of reason for switching or anti-TNF sequence had a significant effect on the change from baseline scores.
Comparative meta-analysis
Four studies [18, 20, 34, 37] reported data comparing outcomes of patients receiving sequential TNF-α inhibitors with those receiving the treatment for the first time. Three of these studies [20, 34, 37] reported ACR20 outcomes, three [18, 20, 34] reported EULAR response rates and all four reported improvement in DAS-28. Only two studies reported information on HAQ improvement, [18, 20] and one [18] was excluded due to lack of data.
A random-effects meta-analysis comparing response rates for sequential use with first-time use produced an odds ratio of 0.65 (95% CI 0.56, 0.76) for ACR20 data and 0.60 (95% CI 0.50, 0.71) for EULAR outcomes. Meta-analysis of improvement in DAS-28 gave weighted mean difference of −0.37 (95% CI −0.57, −0.17). Data from one study [20] showed mean difference in HAQ improvement of −0.07 (95% CI −0.11, −0.03). All the above results consistently show superior response in patients receiving anti-TNF-α for the first time. This may, in part, be a selection bias since switchers are by definition, first biologic treatment failures.
One study reported comparative data for patients who switched to second or third TNF-α inhibitor with patients who switched to rituximab after failing at least one TNF-α inhibitor [25]. The estimated difference in DAS-28 improvement between TNF-α inhibitor switchers and rituximab switchers was −0.63 (95% CI −1.14, −0.12). None of the tests for publication bias for any of the analyses was statistically significant.
Discussion
This meta-analysis shows that patients who fail one TNF-α inhibitor due to intolerance, inefficacy or loss of efficacy may still benefit from switching to another drug from this group. However, those studies that compared outcomes for sequential with initial TNF-α inhibitor use showed lower response rates. More evidence is needed, particularly from RCTs, to define the effectiveness of switching. This is happening with new TNF inhibitors; for example, an RCT of switching with golimumab—a recently licensed TNF-α inhibitor—showed 37% of patients achieved an ACR20 response compared with 18% receiving placebo [39]. The only RCT [38] identified within the search for the purpose of this review, reported outcomes of patients who at least partially responded to etanercept and then were randomized to either switch to infliximab or to continue etanercept. The odds ratio for ACR20 response was 3.14 (95% CI 0.94, 10.30) and the difference in DAS-28 improvement was 0.90 (95% CI −0.14, 1.94). Both results favoured switching to infliximab but were not statistically significant.
Our evidence synthesis has several limitations. Most importantly, all the evidence was based on observational studies. Some studies reported comparative data in which controls were patients receiving biologic treatments for the first time. In these studies, a degree of selection bias is inevitable as patients who fail a TNF-α inhibitor will have worse prognoses and are likely to show limited responses to all treatments.
There are a number of other limitations. Many studies did not report s.e.s and these needed to be imputed. There were also inconsistencies in reporting outcomes in some studies that had to be excluded, thus depleting the data available for meta-analysis. Finally, there was marked heterogeneity between studies. This variation was not explained by the study-level covariates used in the sub-group analyses or meta-regressions.
We conclude that there is evidence that RA patients who fail to respond to one TNF inhibitor will show a significant response to a second agent. This view is supported by other analyses of the available literature [5, 10, 40]. However, the relative merits of switching TNF-α inhibitors compared with starting another biologic such as rituximab is controversial; there is some evidence, notably from Finckh et al. [25], that starting rituximab is more effective and this could potentially be more cost-effective. Further RCTs and associated economic evaluations are needed to clarify the role of sequential use of TNF-α inhibitors in RA. However, the value of identifying and synthesizing this evidence should be recognized in the light of the requirements of decision makers, both at the individual patient and drug reimbursement level, at the current time. Bodies such as the National Institute for Health and Clinical Excellence in England and Wales, have a need to issue guidance on sequential use of TNF-α inhibitors despite the shortcomings in the available data.

Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
Funding: This work was partially funded by the Medical Research Council (MRC) grant G0800770. The systematic review element of this work was supported by the NICE Decision Support Unit. Funding to pay the Open Access publication charges for this article was provided by the Department of Health Sciences, University of Leicester.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Symmons D, Turner G, Webb E, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology. 2002;41:793–800. doi: 10.1093/rheumatology/41.7.793. [DOI] [PubMed] [Google Scholar]
- 2.Symmons D, Barrett E, Bankhead C, et al. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol. 1994;33:735–9. doi: 10.1093/rheumatology/33.8.735. [DOI] [PubMed] [Google Scholar]
- 3.Pugner K, Scott D, Holmes J, et al. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29:305–20. doi: 10.1016/s0049-0172(00)80017-7. [DOI] [PubMed] [Google Scholar]
- 4.Klareskog L, van der Heijde D, de Page J, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 5.Keystone E, Kavanaugh A, Sharp J, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy. A randomized, placebo controlled, 52 week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 6.St Clair E, Desiree M, van der Heijde M. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 7.Nixon R, Bansback N, Brennan A. Using mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Stat Med. 2006;26:1237–54. doi: 10.1002/sim.2624. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-F, Jobanputra P, Barton P, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10 doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- 9.Wailoo A, Bansback N, Brennan A, Michaud K, Nixon R, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost effectiveness analysis. Arthritis Rheum. 2009;58:939–46. doi: 10.1002/art.23374. [DOI] [PubMed] [Google Scholar]
- 10.Genovese M, Becker J, Schiff M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Emery P, Greenwald M, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase iii trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 12.Plenge R, Criswell L. Genetic variants that predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis: current challenges and future directions. Curr Opin Rheumatol. 2008;20:145–52. doi: 10.1097/BOR.0b013e3282f5135b. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Batliwalla F, Li W, et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol Med. 2008;14:575–81. doi: 10.2119/2008-00056.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wailoo A, Brennan A, Bansback N, Nixon R, Wolfe F, Michaud K. Modeling the cost effectiveness of etanercept, adalimumab and anakinra compared to infliximab in the treatment of patients with rheumatoid arthritis in the Medicare program, AHRQ; Rockville, MD: Technology Assessment Program; 2006. [Google Scholar]
- 15.Abrams K, Gillies C, Lambert P. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med. 2005;24:3823–44. doi: 10.1002/sim.2423. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd S. Technical Report. University of Leicester; 2008. Evaluating the sequential use of TNF-alpha Inhibitors. [Google Scholar]
- 17.Sutton A, Abrams K, Jones D, Sheldon T, Song F. London: John Wiley; 2000. Methods for meta-analysis in medical research. [Google Scholar]
- 18.Bennett A, Peterson P, Zan A, Grumley J, Panayi G, Kirkham B. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology. 2005;44:1026–31. doi: 10.1093/rheumatology/keh673. [DOI] [PubMed] [Google Scholar]
- 19.Bingham CO, 3rd, Ince A, Haraoui B, Keystone EC, Chon Y, Baumgartner S. Effectiveness and safety of etanercept in subjects with RA who have failed infliximab therapy: 16-Week, open-label, observational study. Curr Med Res Opin. 2009;25:1131–42. doi: 10.1185/03007990902841010. [DOI] [PubMed] [Google Scholar]
- 20.Bombardieri S, McKenna F, Drosos A, et al. Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology. 2007;46:1191–9. doi: 10.1093/rheumatology/kem091. [DOI] [PubMed] [Google Scholar]
- 21.Buch M, Seto Y, Bingham S, et al. c-reactive protein as a predictor of infliximab treatment outcome in patients with rheumatoid arthritis. Defining subtypes of nonresponse and subsequent response to etanercept. Arthritis Rheum. 2005;52:42–8. doi: 10.1002/art.20711. [DOI] [PubMed] [Google Scholar]
- 22.Buch M, Bingham S, Bejarano V, et al. Therapy of patients with rheumatoid arthritis: outcome of infliximab failures switched to etanercept. Arthritis Rheum. 2007;57:448–53. doi: 10.1002/art.22617. [DOI] [PubMed] [Google Scholar]
- 23.Cohen G, Courvoisier N, Cohen J, Zaltni S, Sany J, Combe B. The efficiency of switching from infliximab to etanercept and vice-versa in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:795–800. [PubMed] [Google Scholar]
- 24.Di Poi E, Perin A, Morassi M, et al. Switching to etanercept in patients with rheumatoid arthritis with no response to infliximab. Clin Exp Rheumatol. 2007;25:85–7. [PubMed] [Google Scholar]
- 25.Finckh A, Ciurea A, Brulhart L, et al. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum. 2007;56:1417–23. doi: 10.1002/art.22520. [DOI] [PubMed] [Google Scholar]
- 26.Haraoui B, Keystone E, Thorne J, et al. Clinical outcomes of patients with rheumatoid arthritis after switching from infliximab to etanercept. J Rheumatol. 2004;31:2356–9. [PubMed] [Google Scholar]
- 27.Hjardem E, Østergaard M, Pødenphant J, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumor necrosis factor alpha inhibitor? Ann Rheum Dis. 2007;66:1184–9. doi: 10.1136/ard.2006.054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyrich K, Lunt M, Dixon W, Watson K, Symmons D. Effects of switching between anti-TNF therapies on HAQ response in patients who do not respond to their first anti-TNF drug. Rheumatology. 2008;47:1000–5. doi: 10.1093/rheumatology/ken127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iannone F, Trotter F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249–52. doi: 10.1136/ard.2006.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson J, Kristensen L, Kapetanovic M, et al. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology. 2008;47:507–13. doi: 10.1093/rheumatology/ken034. [DOI] [PubMed] [Google Scholar]
- 31.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 32.Laas K, Peltomaa R, Kautieinen H, Leirisalo-Repo M. Clinical impact of switching from infliximab to etanercept in patients with rheumatoid arthritis. Clin Rheumatol. 2008;27:927–32. doi: 10.1007/s10067-008-0880-6. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-Sarabia F, Ruiz-Montesinos D, Hernandez B, et al. DAS-28-based EULAR response and HAQ improvement in rheumatoid arthritis patients switching between TNF antagonists. BMC Musculoskelet Disord. 2009;10:91. doi: 10.1186/1471-2474-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikas S, Voulgari P, Alamanos Y, et al. Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study. Ann Rheum Dis. 2006;65:257–60. doi: 10.1136/ard.2005.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Bijl A, Breedveld F, Antoni C, et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: Relationship to reasons for failure and anti-infliximab antibody status. Clin Rheumatol. 2008;27:1021–8. doi: 10.1007/s10067-008-0866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vollenhoven R, Harju A, Bran-Newark S, Klareskog L. Treatment with infliximab when etanercept has fialed or visa versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis. 2003;62:1195–8. doi: 10.1136/ard.2003.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wick M, Linblad S, Klareskog L, van Vollenhoven R. Adalimumab (Humira) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade) or etanercept (Enbrel): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol. 2005;34:353–8. doi: 10.1080/03009740510026887. [DOI] [PubMed] [Google Scholar]
- 38.Furst D, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893–9. doi: 10.1136/ard.2006.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wordsworth P, Smolen J, Kay J, et al. Golimumab, a human TNF-alpha monoclonal antibody, in rheumatoid arthritis patients previously treated with anti-TNF-alpha agents (Go-After Study) Rheumatology. 2009;48(Suppl. 1):I18. [Google Scholar]
- 40.Scott D, Steer S. NICE guidelines on anti-tumor necrosis factor therapy for RA. Nat Clin Pract Rheumatol. 2009;5:16–7. doi: 10.1038/ncprheum0964. [DOI] [PubMed] [Google Scholar]