Abstract
Aims
Patients with chronic hepatitis C virus (HCV) are at increased risk for complications of liver disease if they become infected with the hepatitis A (HAV) or hepatitis B (HBV) viruses. All major guidelines for the management of patients with chronic HCV include the recommendation for vaccination against HAV and HBV for those who are not already immune. We examined the rates of testing for HAV, HBV, and HCV, as well as rates of vaccination against HAV and HBV in patients with chronic HCV infection at a methadone maintenance program (MMP).
Design, Participants, and Setting
Retrospective review of a random sample (n=207) of medical records of patients enrolled in an academically affiliated, urban MMP providing on-site primary care services.
Measurements
Serostatus for HAV, HBV, and HCV and rates of vaccination for HAV and HBV in those patients with chronic HCV.
Findings
Almost all patients reviewed were tested for HAV, HBV, and HCV. Of the 111 patients found to be chronically infected with HCV, 53 (48.6%) and 68 (63%) were found to lack immunity to HAV and HBV respectively. Of those lacking immunity, 29 (54.7%) and 2 (2.9%) were then vaccinated for HAV and HBV respectively.
Conclusions
Despite high rates of testing for HAV, HBV, and HCV at an urban methadone maintenance program, approximately half of those with chronic HCV eligible for the HAV vaccine received it and very few of those eligible for HBV vaccine received it.
Introduction
Chronic infection with hepatitis C virus (HCV) is an important cause of morbidity and mortality in the United States, and injection drug users constitute the population at greatest risk of infection1. Participants of methadone maintenance programs have high rates of infection with HCV, with a HCV antibody seroprevalence of 67–96%2, but have poor access to HCV services3. Linking health services to methadone maintenance has been shown to increase access to services for this marginalized population4, 5.
Patients with chronic HCV are at increased risk of complications from acute hepatitis A virus (HAV)6, acute hepatitis B virus (HBV)7, and by chronic co-infection with HBV8 compared to those without underlying liver disease. HAV and HBV are both vaccine-preventable diseases and the vaccines have been shown to be safe and effective in patients infected with chronic HCV9. Due to the increased risk of super-infection and the efficacy of the vaccines, multiple medical organizations and advisory panels including the American Association for the Study of Liver Diseases, the Infectious Diseases Society of America, the American College of Gastroenterology, and the National Institute of Health have recommended that all patients with chronic HCV who are not immune to HAV and HBV be vaccinated against them10, 11.
Based on these guidelines, The Physician Consortium for Performance Improvement, a collaboration between the American Gastroenterological Association Institute and the American Medical Association, recently proposed that vaccination against HAV and HBV, or documentation of immunity to these viruses, be considered two of ten performance measures included in the “Hepatitis C Physician Performance Measurement Set”12. To date, there are few studies examining adherence to the vaccination guidelines, and none that have specifically looked at them within the context of a methadone maintenance program.
We conducted a study of HCV management at an academically affiliated, urban methadone maintenance program in New York City. Among the outcomes we examined were the rates of testing for HCV, as well as testing and subsequent vaccination against HAV and HBV in patients with chronic HCV infection.
Methods
The Mount Sinai Medical Center's Narcotics Rehabilitation Center (NRC) was a methadone maintenance program in New York City that offered on-site primary care to its patients. NRC medical staff included one to two physicians and one physician assistant. While not all NRC patients accessed their primary care at the NRC, all were required to have an annual history and physical exam performed by the NRC medical staff. Routine screening of all NRC patients for HCV, HAV, and HBV as well as vaccination for HAV and HBV was initiated in 2002 and was performed at the time of enrollment in the NRC, or during the annual or interim visits. HAV and HBV vaccines were provided at no cost through a program from the New York City Department of Health and Mental Hygiene and all NRC participants, regardless of insurance status, were eligible for vaccination if clinically indicated. At the time of the study, approximately 650 patients were enrolled in the NRC's methadone maintenance program.
We conducted a retrospective chart review of a random sample of one third of NRC patients (n=217) between December 2005 and January 2006. Data collected included demographics, history of injection drug use, HCV antibody (HCV Ab), HCV viral load (HCV VL), HAV total antibody (HAV Ab), HBV surface antigen (HBsAg), HBV surface antibody (HBsAb), HBV core total antibody (HBcAb), and vaccination against HAV and HBV.
Chronic HCV infection was defined as HCV viremia during at least one point in time. For the purposes of this study, patients were considered to be immune to HAV if they had a positive HAV Ab, and they were considered to be immune to HBV if they had a positive HBsAb or a positive HBcAb with a negative HBsAg13. Vaccination against HAV and HBV were defined as receiving at least one dose of HAV or HBV vaccine respectively, but patients were considered to have received complete vaccine series if they received two and three doses of HAV vaccine and HBV vaccine respectively. As per the Hepatitis C Physician Performance Measurement Set, the performance measure was defined as the “percentage of patients aged 18 years and older with a diagnosis of hepatitis C who have received at least one injection of hepatitis A (or B) vaccination, or who have documented immunity”12. The study was approved by the Institutional Review Board of Mount Sinai Medical Center.
Results
Demographics
Of the 217 charts randomly selected for the review, 10 could not be located at the time of the study. A total of 207 charts were reviewed. The mean age of the patients was 49 +/− 9.2 years. The majority (62.3%) of the patients were male, 52.7% of whom were Hispanic, 30.0% African American, and 13.5% Caucasian. The majority (58.5%) of the patients had a documented history of injection drug use.
HCV
Of all the charts reviewed (n=207), 203 (98.1%) of all patients were tested for HCV, 138 (68.0%) of whom were found to be HCV Ab+. Of these patients, 137 (99.3%) had a HCV VL checked to evaluate for presence of chronic infection. Of all charts reviewed, 111 patients (53.6%) were confirmed to be chronically infected with HCV.
HAV immunity and vaccination
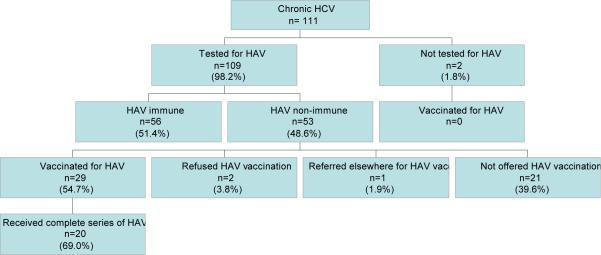
Of those with chronic HCV infection (n=111), 109 (98.2%) were tested for HAV and 53 (48.6%) of them were found to lack HAV immunity. Of those lacking immunity, 29 (54.7%) were vaccinated against HAV. Two additional patients (3.8%) refused HAV vaccination, and one patient was referred elsewhere for HAV vaccination. Among the patients who were not tested for HAV (n=2), none were vaccinated against HAV. Overall, 85 (76.6%) of those with chronic HCV met the performance measure for HAV vaccination by receiving at least one dose of the HAV vaccine or having documented immunity against the virus. Of the 29 patients vaccinated against HAV, 20 (69.0%) received a complete series of the vaccine.
HBV immunity and vaccination
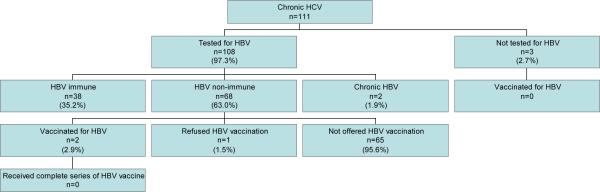
Of those with chronic HCV infection (n=111), 108 (97.3%) were tested for HBV. Two of these patients (1.9%) were found to be chronically infected with HBV and 68 (63.0%) were found to lack HBV immunity. Of those lacking immunity, 2 (2.9%) were vaccinated against HBV, and 1 additional patient refused vaccination. Among the patients who were not tested for HBV (n=3), none were vaccinated against HBV. Overall, 40 (36.7%) of those with chronic HCV who were not co-infected with chronic HBV(n=109) met the performance measure for HBV vaccination by receiving at least one dose of the HBV vaccine or having documented immunity against the virus. None of the patients vaccinated against HBV received a complete series of the vaccine.
Discussion
Our study reveals high rates of HCV, HAV, and HBV testing in a methadone maintenance program. Furthermore, there were high rates of viremic confirmation of chronic HCV in those found to be HCV Ab+. A majority of patients with chronic HCV met the performance measures for HAV vaccination. However, only about half of those with chronic HCV who were not already immune to HAV then received that vaccine. Only one-third of patients met the performance measure for HBV vaccination, but this was primarily due to prior immunity (from previous exposure to HBV or vaccination outside of the NRC) rather than an active on-site vaccination program at the NRC. Almost none of the patients who were not already immune to HBV received that vaccine.
Prior studies of vaccination against HAV and HBV in patients with chronic HCV have reported variable rates of adherence to these guidelines. In a study predating the formal vaccine recommendations, none of the patients in a London clinic with chronic HCV who lacked immunity to HBV were then vaccinated against it14. Similarly, in a study from a Veterans Administration Hospital in New York, only 26.8% of those with chronic HCV found to lack immunity for HAV were then vaccinated15. A more recent study performed within a large regional VA system, however, found that 62% of patients with chronic HCV met the performance measure for both HAV and HBV vaccination13. While the authors found similar rates of immunity to HAV and HBV in those who were tested as we did in our study, 53% and 34% respectively, they described overall higher rates of subsequent vaccination for those who were non-immune: 74% for HAV and 71% for HBV.
It is unclear why rates of vaccination in our study were low in comparison to the high rates of testing, or why there was a discrepancy between vaccination rates against HAV and HBV. Factors that may have contributed include providers' beliefs that vaccines in this population are ineffective, unimportant, or are not cost-effective; more pressing healthcare concerns during visits with providers; or lack of a systematic approach to vaccination. However, considering that all NRC patients, including those who accessed primary care services outside of the NRC, were required to have annual visits with on-site medical providers at the NRC, these visits may represent missed opportunities for vaccination.
Our study has a number of important strengths and weaknesses. As far as we know, this is the first study in the literature to describe adherence to the HAV and HBV vaccine recommendations in those with chronic HCV specifically within the context of a methadone maintenance program. Our results are strengthened by the high rates of testing for HCV, HAV, and HBV, approaching 100% for all three viruses. Furthermore, while the Hepatitis C Physician Performance Measurement Set considers patients that received at least one dose of HAV or HBV vaccine as having fulfilled those respective performance measures, our review also elucidates the number of patients who received complete vaccine series. This is important because according to the Centers for Disease Control and Prevention, while just one dose of HAV vaccine confers immunity in 94–100% of those who receive it, a single dose of HBV vaccine confers immunity in only 30–55% of people16. As a retrospective chart review, however, our study may have missed some important clinical data: patients who lacked immunity to HAV and HBV may have been vaccinated without documentation in the NRC chart if they accessed services outside of the NRC. This limitation may have caused us to underestimate the proportion of eligible patients who were ultimately vaccinated.
Conclusion
We found that despite high rates of testing for HCV, HAV, and HBV at a methadone maintenance program, there were divergent rates of adherence to the recommendation for vaccination against HAV and HBV in those with chronic HCV. While many patients with chronic HCV were found to be immune to HAV and HBV, many of those who lacked immunity did not ultimately receive the vaccines against these viruses. Methadone maintenance programs are uniquely situated to address the current HCV epidemic within this high-risk population that is otherwise subject to multiple barriers to care. Provision of HAV and HBV vaccines for those with chronic HCV is an important aspect of care for these patients and should be integrated into the routine management provided at methadone maintenance programs offering primary care services.
Figure 1.
HAV testing and vaccination
Figure 2.
HBV testing and vaccination
Acknowledgements
(1) For D Fishbein, this project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This research was supported [in part] by the National Institute of Allergy and Infectious Disease.
(2) For D Fishbein, manuscript research previously funded by NIDA K23 DA18623
(3) The authors would like to thank the providers and staff of the Narcotics Rehabilitation Center for their cooperation with this research.
Footnotes
Conflict of Interest: All authors affirm that they have no conflicts of interest to disclose.
References
- 1.Edlin BR. Prevention and treatment of hepatitis C in injection drug users. Hepatology. 2002;36:S210–9. doi: 10.1053/jhep.2002.36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction. 2008;103:905–18. doi: 10.1111/j.1360-0443.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edlin BR, Carden MR. Injection drug users: the overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42:673–6. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286:1715–23. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selwyn PA, Budner NS, Wasserman WC, Arno PS. Utilization of on-site primary care services by HIV-seropositive and seronegative drug users in a methadone maintenance program. Public Health Rep. 1993;108:492–500. [PMC free article] [PubMed] [Google Scholar]
- 6.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–90. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978–80. doi: 10.1111/j.1572-0241.2000.02337.x. [DOI] [PubMed] [Google Scholar]
- 8.Chlabicz S, Grzeszczuk A. Hepatitis B virus vaccine for patients with hepatitis C virus infection. Infection. 2000;28:341–5. doi: 10.1007/s150100070002. [DOI] [PubMed] [Google Scholar]
- 9.Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–37. discussion 237–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed] [Google Scholar]
- 12.Keeffe EB, Williams J. [Accessed February 13, 2010];Hepatitis C physician performance measurement set: Hepatitis C Work Group. 2007 (updated 2009). http://www.amaassn.org/ama1/pub/upload/mm/370/hepatitis-c-ms.pdf. Archived at http://www.webcitation.org/5nWNn4X8X.
- 13.Hernandez B, Hasson NK, Cheung R. Hepatitis C performance measure on hepatitis A and B vaccination: missed opportunities? Am J Gastroenterol. 2009;104:1961–7. doi: 10.1038/ajg.2009.252. [DOI] [PubMed] [Google Scholar]
- 14.Wong V, Wreghitt TG, Alexander GJ. Prospective study of hepatitis B vaccination in patients with chronic hepatitis C. BMJ. 1996;312:1336–7. doi: 10.1136/bmj.312.7042.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim M, Khaykis I, Park J, Bini EJ. Susceptibility to hepatitis A in patients with chronic liver disease due to hepatitis C virus infection: missed opportunities for vaccination. Hepatology. 2005;42:688–95. doi: 10.1002/hep.20830. [DOI] [PubMed] [Google Scholar]
- 16.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55:1–94. [PubMed] [Google Scholar]