Abstract
Background & Aims
The colonic migrating motor complex (CMMC) is a motor pattern that regulates the movement of fecal matter, through a rhythmic sequence of electrical activity and/or contractions, along the large bowel. CMMCs have largely been studied in empty preparations; we investigated whether local reflexes generated by a fecal pellet modify the CMMC to initiate propulsive activity.
Methods
Recordings of CMMCs were made from the isolated murine large bowel, with or without a fecal pellet. Transducers were placed along the colon to record muscle tension and propulsive force on the pellet and microelectrodes were used to record electrical activity from circular muscle cells anal and oral of a pellet and in colons without the mucosa.
Results
Spontaneous CMMCs propagated in both an oral or anal direction. When a pellet was inserted, CMMCs increased in frequency and propagated anally, exerting propulsive force on the pellet. The amplitude of slow waves increased during the CMMC. Localized mucosal stimulation/circumferential stretch evoked a CMMC, regardless of stimulus strength. The serotonin (5-hydroxytryptamine-3) antagonist ondansetron reduced the amplitude of the CMMC, the propulsive force on the pellet, and the response to mucosal stroking, but increased the apparent conduction velocity of the CMMC. Removing the mucosa abolished spontaneous CMMCs, which still could be evoked by electrical stimulation.
Conclusions
The fecal pellet activates local mucosal reflexes, which release serotonin (5-hydroxytryptamine) from enterochromaffin cells, and stretch reflexes that determine the site of origin and propagation of the CMMC, facilitating propulsion.
Abnormal colonic motor patterns have been implicated in the pathogenesis of severe constipation and diarrhea.1–5 One such major motor pattern involved in regulating the movement of fecal matter is the colonic migrating motor complex (CMMC).3–8 The CMMC consists of a rhythmically occurring sequence of electrical activity and/or contractions that can occur as either a stationary event or as a propagating wave of contractions that conduct in either an oral or anal direction over varying lengths of the large bowel. CMMCs are generated by the enteric nervous system because they are blocked by tetrodotoxin and hexamethonium, and reduced by atropine.3–7 Although it has been proposed that discrete neural “pattern generators” within the myenteric plexus and distributed along the colon generate the CMMC,8 our current studies suggest that serotonin (5-hydroxytryptamine [5-HT]) release from the mucosa is required for triggering the neural circuitry underlying the CMMC.
A study of the literature suggests that, similar to heart rate,9 the frequency of the CMMC may be related inversely to body mass, occurring with higher frequency in small mammals such as the mouse,4–7 compared with the colon of larger mammals,3,10–14 including human beings.1–3,13 Although the CMMC occurs during both the fasted and fed state,3,11 they have been observed to be of a higher frequency in the fed state.11,15 In human beings, CMMCs appear to be the events referred to as propagating (pressure) sequences.13 Propagating sequences propagate mainly, but not exclusively, in the anal direction in control subjects, whereas in patients with obstructed defecation there is a significant increase in orally propagating sequences.13
Serotonin (5-HT) plays an essential role in gastrointestinal motility.16,17 5-HT3–receptor antagonists, which reduce intestinal transit, are used to treat multiple symptoms in patients with diarrhea-predominant irritable bowel syndrome. However, because of rare, but potentially serious, side effects, their use currently is restricted to patients who are refractory to conventional therapy because they can predispose some patients to develop ischemic colitis.16–18 5-HT3–receptor antagonists such as ondansetron and alosetron have been shown to reduce the amplitude of spontaneous CMMCs in the isolated empty murine large bowel.19,20 In the isolated colon 5-HT3 antagonists can act only on enteric mechanisms. 5-HT is known to be secreted by enterochromaffin (EC) cells and acts on 5-HT3 receptors located on intrinsic primary afferent neurons or in neurons in descending nerve pathways.16,17
We have examined whether CMMCs are essential for fecal pellet propulsion in the isolated murine large bowel. Importantly, we found that there appears to be a synergistic relationship between the CMMC and the presence of a fecal pellet that generates local intrinsic reflexes to regulate the generation and direction of propagation of the CMMC.
Materials and Methods
C57BL/6 mice (20–90 days old) of either sex were killed humanely by inhalation of anesthetic (Nembutal) and cervical dislocation, in accordance with the animal ethics committee of the University of Nevada School of Medicine, and the whole colon was removed.6,7,20
Preparations
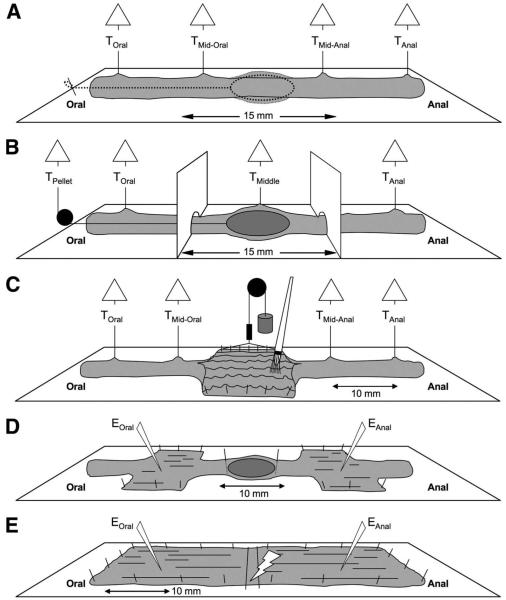
Several preparations were used in this study to monitor circular muscle (CM) tension, propulsive force on a pellet, and electrical activity during spontaneous or evoked CMMCs, as well as in preparations devoid of the mucosa. The preparations were designed to do the following: (1) monitor spontaneous CMMC activity with or without a fixed pellet (Figure 1A); (2) confine drugs using a partitioned chamber with a fixed pellet to sites around the pellet (Figure 1B); (3) evoke intrinsic reflexes by mucosal stimulation applied with a brush or circumferential stretch (Figure 1C); (4) record intracellular electrical activity from CM cells oral and anal to a fixed pellet (Figure 1D); and (5) record electrical activity in preparations without the mucosa (Figure 1E).
Figure 1.
Experimental setups. (A) Effect of a fecal pellet on the CMMC. An epoxy-coated fecal pellet attached to a silk thread was inserted into an isolated segment of colon and was fixed in position with a pin when it reached the middle of the colon. Isometric tension transducers monitored CM tension before and after insertion of the pellet. (B) Partitioned bath. An epoxy-coated fecal pellet was held in position between the 2 partitions by attaching a thread via a pulley to a tension transducer. (C) Mucosal and stretch reflex. A section of the colon was cut open and pinned with the mucosa uppermost, allowing mechanical stimulation with a brush. Circumferential stretch was applied to one edge of the opened region by attaching it to a pulley system to which weights could be added. Tension transducers monitored contractile activity. (D) Microelectrode recordings made on either side of a fecal pellet. Two 10-mm sections of colon were cut open, on either side of a fecal pellet, and pinned circumferentially, with the serosa uppermost, so that electrical activity could be recorded from the underlying CM. (E) Microelectrode recordings from preparations without the mucosa. The mucosa was dissected away from the colon, which was pinned circumferentially. Microelectrode recordings were made 10-mm anal of transmural stimulating electrodes (tms).
The isolated large bowel was attached to the floor of the organ bath by pinning the mesentery. An epoxycoated fecal pellet, with a thread glued to one end, was inserted into the oral end, allowed to propagate down to the middle of the colon and held by a thread (Figure 1A) or a force transducer (Figure 1B), or by pinning in front and behind a pellet (Figure 1D).
Two greased Perspex partitions were slotted into position (15-mm apart) around the pellet (Figure 1B). The partitions effectively divided the colon, for pharmacologic purposes, into an oral segment, a middle (stimulation) segment, and an anal segment.21
Each chamber or preparation was perfused continuously with Krebs solution at 37.0°C ± 0.5°C.20,21
Recording the Mechanical Activity of the Circular Muscle
Suture silk was used to connect force transducers (model TST125C; Biopac Systems Inc., Santa Barbara, CA) along the colon (Figure 1A–C). Resting tension was initially set at 8 mN, and monitored using an MP100 interface and recorded on a PC running Acqknowledge software 3.2.6 (Biopac Systems, Inc.).20
Evoking Neural Reflexes
The colon was opened along a 10-mm length in the middle, the oral end, or the anal end of the preparation and pinned with the mucosa uppermost (Figure 1C). Pressure or brush strokes were applied to the mucosa with an artist’s brush. A rake-holder system then was attached to the opposite edge so that it could be stretched circumferentially using weights (2–20 g) added to a pulley system (Figure 1C).23,24 The stretch was monitored by viewing the preparation with a video camera positioned over the stimulation site (see later). When no stretch was applied the opened segment of colon had a width of 4.20 ± 0.01 mm. After the addition of weights the average width increased as follows: 2 g: 5.42 ± 0.01 mm; 5 g: 6.05 ± 0.03 mm; 10 g: 6.40 ± 0.01 mm; 15 g: 6.70 ± 0.03 mm; and 20 g: 6.90 ± 0.03 mm (n = 4). The average circumference of a fecal pellet was 6.4 ± 0.01 mm, which corresponded to the width applied by a 10-g weight.
Intracellular Recording From Whole-Colon Preparations With and Without Mucosa
Dual microelectrode impalements were made into CM cells as described previously.7,22–25 Electrical recordings were made from the CM on either side of a fixed pellet by advancing the electrode through the serosal surface of the open segment (Figure 1D).
In other experiments, the mucosa was dissected away from the whole colon that was pinned circumferentially with the serosa uppermost. Platinum transmural stimulating wires (diameter, 0.2 mm), which were connected to a Grass SD stimulator (MA), were placed above and below the preparation (1-mm apart and 10-mm oral of the recording site).
Video Recording of Colonic Movements and Spatiotemporal Map Analysis
The propagation of a fecal pellet along the colon was monitored with a video camera (WV-BP330; Panasonic CCTV, Powermac G4 Apple). Video frames were converted into spatiotemporal diameter or D maps, from which the velocity could be calculated.22,25
Analysis of Data and Statistical Methods
The frequency, duration, and amplitude of contractile complexes were measured using Acqknowledge 3.2.6 (Biopac Systems, Inc.) and tests for statistical significance were made using Sigma Plot 5.0 (Jandel Scientific, San Rafael, CA).20 The propagation velocity was determined by calculating the delay between the 50% amplitude points of CMMCs.20
Statistical comparisons of data were performed using the Student (paired or unpaired) t tests or analysis of variance, and a minimum level of significance was reached at a P value of less than .05. In the Results section, n refers to the number of animals from which tissue was taken. All data are presented as means ± standard error of the mean.
Drugs and Solutions
Hexamethonium bromide and ondansetron were purchased from Sigma (St. Louis, MO). Alosetron was a gift from Glaxo Pharmaceuticals (NC, USA). The Krebs solution was (in mmol/L) 120.35 NaCl, 5.9 KCl, 15.5 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, and 11.5 glucose, gassed continuously with a mixture of 3% CO2–97% O2 (vol/vol), to give a final pH of 7.3–7.4.
Results
Spontaneous Activity Without a Fecal Pellet
Tension recordings of spontaneous CMMCs were made along the isolated large bowel (Figures 1A and 2A). At the oral recording site in the proximal colon these consisted of irregular low-frequency (0.28 ± 0.08 c/min; n = 8), low-amplitude (12.0 ± 1.3 mN), and long-duration (150.00 ± 14.6 s) CMMCs that consisted of a series of phasic contractions that usually failed to propagate farther down the proximal colon (Figure 2A*). These CMMCs were interspersed with higher-amplitude (18.03 ± 1.27 mN), shorter-duration (61.63 ± 7.68 s) CMMCs, which occurred at a frequency of 0.31 ± 0.03 c/min) that propagated in either an oral or anal direction (Figure 2A#). Both types of spontaneous CMMCs were blocked by the nicotinic antagonist hexamethonium (100 μmol/L; n = 5), suggesting they required fast synaptic transmission to be generated.
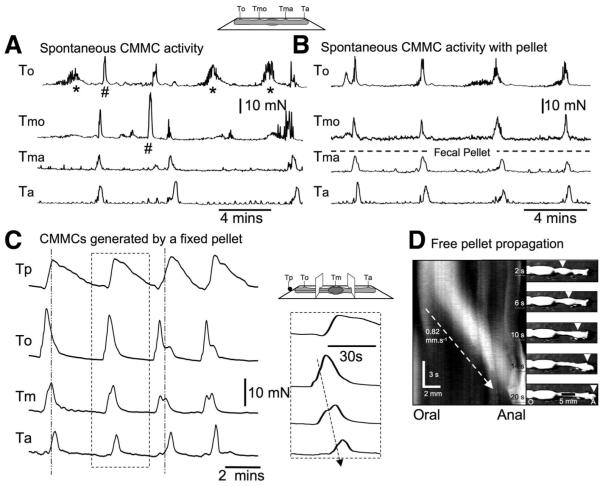
Figure 2.
Effect of a fecal pellet on spontaneous CMMCs. (A) CMMC activity in an empty colon. *Nonpropagated burst activity. #Propagated CMMCs. (B) When an artificial pellet was introduced into the middle of the colon, CMMC activity became more organized and propagated from the oral tension transducer (to) to the anal tension transducer (ta). (C) CMMCs propagated in an oral to anal direction (see inset) and were associated with considerable force on the pellet (Tp top trace). The peak tension on the pellet coincided with the peak of the CMMC in the middle chamber. (D) The conduction velocity of a freely moving fecal pellet down the colon was measured from the spatiotemporal map.
Activity Induced by a Fecal Pellet
When an epoxy-coated fecal pellet was allowed to propagate down the colon and then held in position when it reached the middle of the colon, regular CMMCs were generated that were of shorter duration but higher amplitude, an example of which is shown in Figure 2B. When the pellet was present, the overall frequency of CMMCs was faster (control: 0.17 ± 0.01 c/min; with pellet: 0.31 ± 0.03; P < .01; n = 8). The CMMCs appeared to originate oral to the pellet and propagate down the colon in an oral to anal direction (Figure 2B and C).
We also measured the propulsive tension on the fixed pellet held in the middle of the large bowel, as well as tension of the CM along the colon (Figure 1B). In these experiments, CMMCs appeared to originate oral to the pellet and propagate anally. The peak propulsive tension on the pellet, which often outlasted the CMMC, occurred when the CMMC was detected just anal to the pellet (Figure 2C). The apparent conduction velocity of the CMMC over the fixed pellet was 0.80 ± 0.10 mm/s (n = 6), which was similar to the velocity of a pellet that was allowed to propagate freely down the colon (0.82 ± 0.1 mm/s; n = 6; Figure 2C and D). When the 3-chambered partitioned bath was used, hexamethonium (100 μmol/L; n = 5) applied to the middle chamber containing the pellet completely blocked the CMMC in both the middle and anal chambers and disrupted the CMMC in the oral chamber (Figure 3A). Therefore, long cholinergic nerve pathways are involved in the coordinating and propagation of the CMMC.
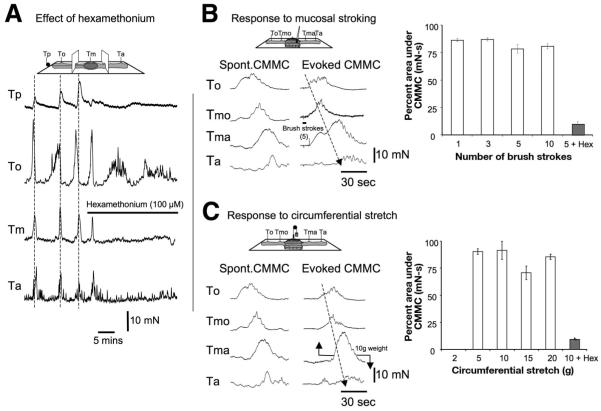
Figure 3.
Effect of nicotinic antagonist and local reflexes. (A) Partitioned bath: Hexamethonium (100 μmol/L) added to the middle chamber blocked the CMMCs in the middle and anal chambers and the tension on the pellet, and disrupted the CMMC in the oral chamber. (B) Comparison of spontaneous and evoked CMMCs. Graphs show responses to stroking the mucosa compared with a spontaneous CMMC. Each series of brush strokes elicited a maximal CMMC response. The CMMC responses were measured at the most oral transducer and compared with CMMCs (100%) that occurred spontaneously. (C) CMMCs could be evoked readily by applying between 5 and 20 g of circumferential stretch on the colon. Hexamethonium (100 μmol/L) significantly reduced the responses to mucosal stroking and circumferential stretch.
Pellet Propulsion and Intrinsic Reflexes
We attempted to determine whether a pellet controls the initiation of the CMMC by activating local reflexes.
Stroking the mucosa or applying pressure with the brush (1–10 strokes or pressure) or circumferential stretch (5–20 g) in the middle, or at either end, always generated a CMMC that was similar if not identical to spontaneous CMMCs. Evoked CMMCs were usually more synchronized and propagated smoothly along the entire length of the colon (Figure 3B and C). There was no response to circumferential stretch to weights less than 5 g that corresponds to a width of less than 6.05 mm (unstretched flap, 4.20 mm), suggesting stretch responses had a threshold for activation that was slightly less than the circumference of a fecal pellet (6.40 mm). The level of circumferential stretch at 10 g (6.40 mm) was the same as the circumference of a fecal pellet. Unlike intrinsic nervous reflexes in the guinea pig and rat large bowel, a graded response to stimulation was never observed regardless of the number of brush strokes or the level of circumferential stretch (Figure 3B and C).21–26 The evoked CMMC responses to either set of stimuli were blocked completely by hexamethonium (100 μmol/L; n = 5; Figure 3B and C).
Effect of 5-HT3 Antagonist on CMMCs Generated by a Pellet
When ondansetron (5 μmol/L) was added to the middle chamber containing the fixed fecal pellet it significantly reduced the CMMC amplitude and the propulsive tension on the pellet by approximately 50%, suggesting that circumferential stretch also may be important in initiating the CMMC (Figure 4A and C). Ondansetron inthe middle chamber also increased the apparent conduction velocity between CMMCs (control: 0.71 ± 0.04 mm/s; ondansetron: 3.03 ± 0.29 mm/s; P < .01; n = 8; Figure 4B).
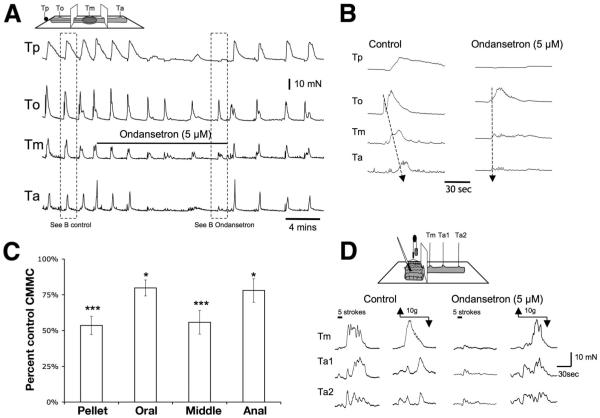
Figure 4.
Effect of 5-HT3 antagonist on CMMCs evoked by a pellet and to reflex stimulation. (A) Ondansetron (5 μmol/L) added to the middle chamber containing a fixed pellet significantly reduced the CMMC amplitude and the propulsive tension on the pellet. (B) Ondansetron increased the apparent conduction velocity between the CMMCs. (C) Summary of the inhibitory effect of ondansetron on CMMCs when applied to the middle chamber. *P < .05; ***P < .001. (D) Control CMMC evoked by stroking the mucosa with a brush (5 strokes) and to circumferential stretch (10 g) applied oral to the recording sites. After addition of ondansetron (5 μmol/L) in the oral stimulation chamber the mucosal response, but not the stretch response, was reduced.
Effect of 5-HT3 Antagonists on Local Reflexes
Either ondansetron (5 μmol/L) or alosetron (5 μmol/L) applied specifically to the stimulation chamber significantly reduced the CMMC response to stroking the mucosa, but did not significantly affect the CMMC response to circumferential stretch (Figure 4D; Table 1).
Table 1.
| Control | Ondansetron (5 μmol/L) | n | P value | |
|---|---|---|---|---|
| CMMCs evoked by local reflexes | ||||
| Mucosal brush strokes, area under the curve mN-s | 343.85 ± 35.61 | 65.85 ± 20.26 | 4 | <.01 |
| Circumferential stretch, area under the curve mN-s | 374.85 ± 46.2 | 343.72 ± 20.26 | 4 | >.05 |
| Alosetron, 5 μmol/L |
||||
| Mucosal brush strokes, area under the curve mN-s | 288.7 ± 33.6 | 45.7 ± 20.3 | 4 | <.01 |
| Circumferential stretch, area under the curve mN-s | 333.4 ± 46.2 | 326.7 ± 33.6 | 4 | >.05 |
| Ondansetron, 1–3 μmol/L |
||||
| CMMCs evoked by a fixed pellet | ||||
| Resting membrane potential, mV | 62.47 ± 2.61 | 60.98 ± 3.63 | 5 | >.05 |
| Interval between CMMCs, s | 225.22 ± 39.54 | Blocked | 4 | <.01 |
| Duration of CMMCs, s | 50.39 ± 2.46 | Blocked | 4 | <.01 |
| Amplitude of hyperpolarization, mV | 12.18 ± 0.50 | Blocked | 4 | <.01 |
| Amplitude of depolarization, mV | 15.35 ± 1.41 | Blocked | 4 | <.01 |
| Number of action potentials on depolarization | 2.60 ± 0.27 | Blocked | 4 | <.01 |
| Ondansetron, 1–3 μmol/L |
||||
| Spontaneous junction potentials | ||||
| Interval between IJPs, s | 2.10 ± 0.15 | 12.05 ± 0.65 | 5 | <.05 |
| Amplitude of IJPs, mV | 10.05 ± 0.57 | 4.79 ± 0.57 | 5 | <.05 |
| Duration of IJPs, mV | 0.65 ± 0.03 | 0.58 ± 0.05 | 5 | <.05 |
| Duration of EJPs, s | 0.60 ± 0.05 | 1.23 ± 0.11 | 5 | <.05 |
| Amplitude of EJPs, mV | 8.49 ± 0.59 | 9.34 ± 0.25 | 5 | >.05 |
| Atropine, 1 μmol/L |
Ondansetron, 1 μmol/L |
|||
| CMMCs evoked by TNS (no mucosa) | ||||
| Amplitude of depolarization, mV | 16.42 ± 1.55 | 17.19 ± 1.55 | 5 | >.01 |
| Duration of depolarization, s | 44.81 ± 5.66 | 49.7 ± 4.34 | 5 | >.01 |
| Amplitude of hyperpolarization, mV | 13.44 ± 2.05 | 3.74 ± 0.36 | 5 | <.01 |
| Duration of hyperpolarization, s | 44.81 ± 5.66 | 21.85 ± 2.48 | 5 | <.01 |
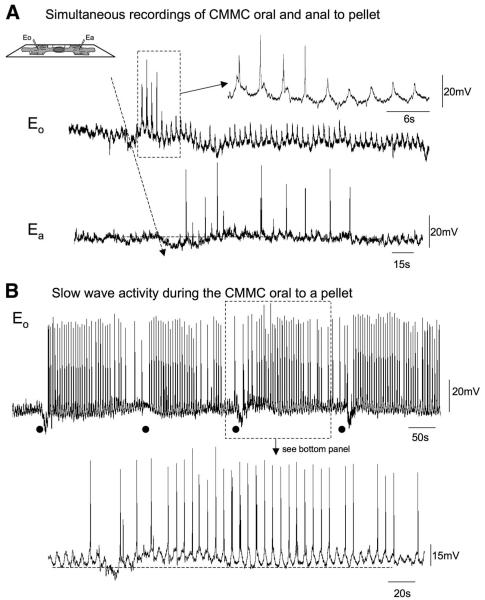
CMMCs Recorded With Intracellular Microelectrodes
Simultaneous intracellular microelectrode recordings were made from CM on the oral and anal side of a fixed pellet (Figure 1D) in unparalyzed muscle preparations, which had a resting membrane potential (between complexes) of 62.47 ± 2.61 mV (n = 5). Regular CMMCs were observed that consisted of a hyperpolarization followed by a prolonged depolarization, upon which were superimposed excitatory junction potentials (EJPs) (Table 1).7 Several action potentials (2.60 ± 0.27) were observed on the depolarizing phase of the CMMC. It was clear that the CMMC originated oral of a fixed pellet and propagated anally (Figure 5A). Between CMMCs, spontaneous inhibitory junction potentials (IJPs) occurred at a frequency of 28.57 cpm (n = 5; Table 1).
Figure 5.
Electrical activity recorded oral and anal to a fixed pellet. (A) The top trace (eO) illustrates slow-wave activity in the CM oral to the pellet, and the bottom trace (ea) shows the electrical activity recorded from a CM muscle cell anal to the pellet. (B) Slow-wave activity during the CMMC oral of a fecal pellet. Note that before the CMMC occurs there is a reduction in the excitability of the cell, whereas when the CMMC occurs there is an increase in the amplitude of slow waves, which elicit action potentials. Black dots indicate the start of the CMMC. The expanded trace shows the characteristic depolarization during the CMMC.
Furthermore, when the electrode was advanced deeper into the tissue towards the submucosal border electrical slow waves were recorded (Figure 5A and B). The slow waves became more periodic and increased in amplitude and elicited action potentials during the CMMC (Figures 5B and 6A). During this period, slow waves within the deep CM had a frequency of 17.14 c/min, an amplitude of 20.39 ± 1.46 mV (maximum amplitude, 35 mV), and a duration of 3.50 ± 0.16 s (n = 4). The slow waves were distinguished easily from EJPs (duration, 0.60 ± 0.05 s; amplitude, 8.49 ± 0.59 mV; P < .01) because slow waves had a significantly longer duration and unlike the EJPs were not blocked by atropine (1 μmol/L; n = 3).
Figure 6.
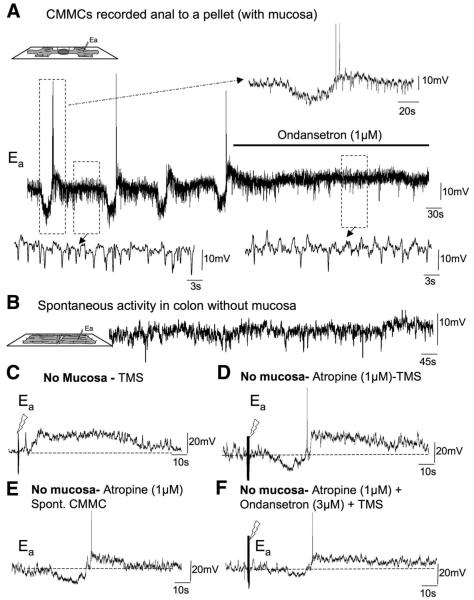
Effect of 5-HT3 antagonist on electrical activity. (A) Spontaneous CMMCs were observed in a CM cell 10-mm anal to a fixed pellet. Between and during the CMMC, spontaneous fast IJPs occurred. Ondansetron (1 μmol/L) blocked the CMMCs but did not affect the resting membrane potential. The spontaneous IJPs (see expanded left-hand trace) were reduced in frequency and EJPs were revealed (see expanded right-hand trace). (B) When the mucosa was removed from the colon only spontaneous IJPs were observed. (C) TMS (0.4 ms, 30 V, 10–20 Hz for 1 s) applied oral to the recording electrode evoked a CMMC in preparations in which the mucosa was removed. (D) Atropine revealed the hyperpolarization before the depolarizing phase of the evoked CMMC. (E) On occasion, after several periods of stimulation, a spontaneous CMMC was observed. (F) The addition of ondansetron significantly reduced the hyperpolarizing component of the CMMC while leaving the depolarizing phase relatively unaffected.
Effect of 5-HT3 Antagonist
When intracellular microelectrode recordings were made from unparalyzed CM just anal to a fixed fecal pellet (Figure 1D) we found that ondansetron (1–3 μmol/L; n = 4) abolished the CMMCs without a significant change in the resting membrane potential (Figure 6A; Table 1). Ondansetron significantly reduced the amplitude, duration, and frequency of spontaneous IJPs and revealed more ongoing EJPs (Figure 6A; Table 1).
Effect of Removing the Mucosa
The effects of ondansetron on the CMMC suggest that 5-HT release from EC cells in the mucosa16,17 may be important for generating the CMMC. To test this hypothesis we removed the mucosa from the colon (Figure 1E). When impalements were made from CM cells in preparations without the mucosa the resting membrane potential (63.08 ± 3.52 mV; P > .01; n = 4) was similar to that in colons with an intact mucosa. In these preparations without the mucosa, CMMCs were not present but ongoing fast IJPs still occurred at a frequency (21.43 cpm) similar to those in preparations with the mucosa intact (Figure 6B; Table 1).
Transmural nerve stimulation (TNS) (pulses of 10–20 Hz for 1 s, 0.4 ms, 30 V) evoked a CMMC, consisting of either a depolarization, or a hyperpolarization followed by a depolarization (Figure 6C; Table 1). The addition of atropine (1 μmol/L) significantly increased the amplitude and duration of the preceding hyperpolarizion response to TNS, whereas the depolarizing response was similar to control (Figure 6D; Table 1). After several TNSs, a spontaneous CMMC occasionally was observed (Figure 6E).
In the presence of atropine, the addition of ondansetron (1 μmol/L) caused a significant reduction in the TNS-evoked hyperpolarizing response, but did not significantly affect the depolarizing response (Figure 6F;Table 1). This suggests that ondansetron was reducing fast synaptic transmission via 5-HT3 receptors27 to inhibitory motor neurons. In addition, the spontaneous IJPs were reduced in frequency by ondansetron (3.16 c/min).
Discussion
Our study suggests that there is a synergistic relationship between the spontaneous CMMCs and local reflexes generated by a fecal pellet in the murine large bowel. The propagation of a fecal pellet down the colon is dependent on the CMMC, which normally occurs spontaneously in an empty colon. However, the presence of a fecal pellet determines the site of origin of a colonic CMMC and its apparent propagation down the colon by activating both mucosal reflexes and reflexes activated by circumferential stretch. The neural circuitry primarily generates a CMMC in response to input from local reflexes, rather than peristaltic reflexes. CMMCs initiated by mucosal reflexes likely involve 5-HT release from EC cells in the mucosa, which activate 5-HT3 receptors on intrinsic sensory neurons.16,17 The neural circuitry for generating the CMMC is in the myenteric plexus, but 5-HT release from the mucosa is essential for triggering the CMMC.
Propagation of a Pellet
Previously it has been assumed that the CMMC drives pellet propulsion.4–7 We found that a freely moving pellet propagates down the colon at a velocity that is identical to the apparent conduction velocity of a CMMC generated by a fixed pellet, suggesting the 2 events may be equivalent and that the CMMC drives pellet propulsion. This strongly is supported by the observation that when a pellet is fixed in the colon, the CMMC nearly always propagates in an oral to anal direction and is associated with considerable tension on a pellet. The tension on the pellet reached a maximum after the passage of the CMMC over the pellet.
Slow Waves and the CMMC
Slow waves in the large bowel originate in interstitial cells of Cajal at the submucosal border.28,29 The reason slow waves have not been reported during the murine CMMC is that they are sensitive to L-type channel blockers,29 which are used routinely to prevent muscle contraction.7 Slow waves near the submucosal border became more periodic and elicited action potentials during the CMMC.
Local Intrinsic Reflexes
The fact that the presence of a fecal pellet organizes the CMMC suggests that the pellet itself exerts a dominant effect on the neural circuitry responsible for generating CMMCs. We assumed that the pellet would either stimulate the mucosa or stretch the bowel to activate local peristaltic reflexes.24 In the guinea pig and rat colon these reflexes are graded according to the stimulus strength.21,24,26 Therefore, we were surprised to find that mucosal stimulation and circumferential stretch applied to the murine large bowel elicited a maximal CMMC response above a threshold regardless of the strength of stimulation.
Role of 5-HT3 Receptors
We found that ondansetron (and alosetron) substantially reduced the responses to mechanical stimulation of the mucosa but not the responses to circumferential stretch, as well as significantly reducing the propulsive tension on the fixed pellet and the amplitude of the CMMC over the pellet when added to the middle chamber. Presumably, the incomplete blockade of the CMMCs by odansetron in the middle chamber may be owing to the reflex response to circumferential stretch elicited by the pellet. It could be argued that the remaining response in the middle chamber was owing to other 5-HT receptors,30–32 however, this seems unlikely because ondansetron and alosetron almost completely blocked the response to mucosal stimulation. In addition, ondansetron also completely blocked CMMCs recorded with intracellular microelectrodes without changing resting membrane potential. These results suggest that 5-HT released from EC cells in the mucosa is important for initiating mucosal reflexes generated by stroking the mucosa or from mucosal compression by the pellet. Previously, it has been shown that 5-HT can activate the endings of myenteric intrinsic primary afferent neurons (or AH neurons) within the mucosa of both the guinea pig large and small intestine,33,34 largely by stimulating 5-HT3 receptors.34 In contrast, the mucosal endings of submucosal AH neurons are activated by 5-HT1P receptors.35
However, ondansetron added to the chamber containing the fecal pellet also increased the apparent conduction velocity of the CMMC, suggesting that it also may be blocking 5-HT3 receptors mediating fast synaptic transmission27 in descending inhibitory nervous pathways (Figure 7). Likely, 5-HT3 receptors are on inhibitory motor neurons because ondansetron reduced the number of spontaneous IJPs and the hyperpolarization evoked by TNS. The activation of descending inhibitory nerve pathways is essential for the apparent propagation of the CMMC.7
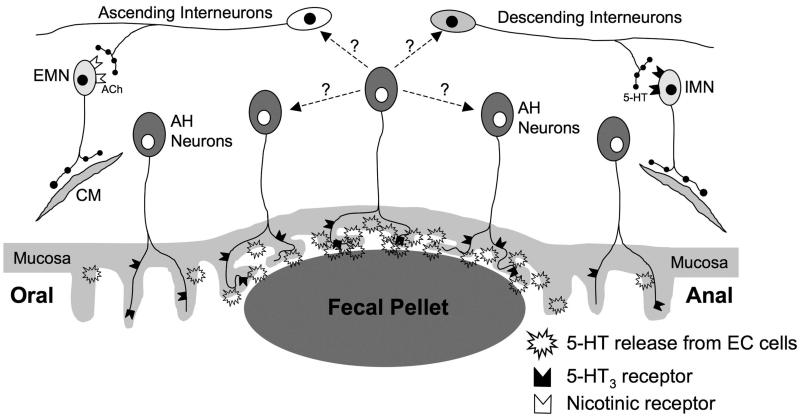
Figure 7.
Possible elements of intrinsic neural circuit underlying the CMMC. The presence of a pellet increases 5-HT release from EC cells in the mucosa, which activates sensory neurons via 5-HT3 receptors. Activation of intrinsic sensory neurons leads to the generation of the CMMC as well as the activation of ascending excitatory cholinergic nerve pathways, which activate excitatory motor neurons (emn) via nicotinic receptors and descending serotonergic nerve pathways that excite inhibitory motor neurons (imn) via 5-HT3 receptors. The interneurons also may be activated by circumferential stretch.
Mucosa Necessary for Triggering the CMMC
Removing the mucosa appeared to abolish spontaneous CMMCs, suggesting that the mucosa is normally critical for their generation. However, transmural nerve stimulation could elicit a CMMC-like response. Also, in atropine, an occasional spontaneous CMMC was observed after several periods of TNS, suggesting that the neural circuitry underlying the CMMC was within the myenteric plexus, but stimuli arising from the mucosa were necessary for triggering the CMMC.
The trigger for the CMMC appears to be spontaneous or evoked (ie, a fecal pellet) release of 5-HT from EC cells to stimulate AH neurons, whereas reflexes activated by circumferential stretch may involve mechanosensitive interneurons.23,36 A CMMC can be initiated from any part of the colon, however, fecal pellets focus the initiation site and propagation direction (oral to anal) of the CMMC. Presumably, the pressure exerted by a pellet on the mucosa increases 5-HT release that would increase the probability that a critical number of myenteric AH neurons are activated for the generation of the CMMC. In fact, the migrating motor complex in the small intestine has been modeled using recurrent connections between AH neurons.37 The concept of discrete “pattern generators” distributed along the colon8 is unlikely because each ganglia contains AH neurons that are likely to integrate different levels of sensory information from the mucosa to form the CMMC, probably through the activation of slow excitatory postsynaptic potentials by release of CGRP.30,36 The effects of hexamethonium suggest that the enteric neural pathways along the whole length of the colon are involved in propagating a CMMC. The apparent propagation of the CMMC involves activation of descending inhibitory nerve pathways, and the sudden coordination of many ascending and descending interneurons (Figure 7).7
In summary, there is a synergistic relationship between fecal matter and the CMMC. A fecal pellet influences the site of generation and propagation of the CMMC by activating local reflexes that enhance activity in intrinsic sensory neurons. Furthermore, the interactions between many fecal pellets and the CMMC needs further study because these are likely to be more complex.22,25
Acknowledgments
The authors disclose the following: This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK45713).
Abbreviations used in this paper
- CM
circular muscle
- CMMC
colonic migrating motor complex
- EC
enterochromaffin
- EJP
excitatory junction potential
- 5-HT
5-hydroxytryptamine (serotonin)
- IJP
inhibitory junction potential
- TNS
transmural nerve stimulation
Footnotes
D.J.H. and E.J.D. contributed equally to this work and were responsible for the tension and electrophysiologic recordings, respectively.
References
- 1.Bueno L, Fiormonti J, Flexinos J, et al. Colonic myoelectrical activity in diarrhea and constipation. Hepatogastroenterology. 1980;27:381–389. [PubMed] [Google Scholar]
- 2.Snape WJ, Jr, Carlson GM, Matarazzo SA, et al. Evidence that abnormal myoelectrical activity produces colonic motor dysfunction in the irritable bowel syndrome. Gastroenterology. 1977;72:383–387. [PubMed] [Google Scholar]
- 3.Sarna SK. Physiology and pathophysiology of colonic motor activity (1) Dig Dis Sci. 1991;36:827–862. doi: 10.1007/BF01311244. [DOI] [PubMed] [Google Scholar]
- 4.Brann L, Wood JD. Motility of the large intestine of piebald-lethal mice. Am J Dig Dis. 1976;21:633–640. doi: 10.1007/BF01071956. [DOI] [PubMed] [Google Scholar]
- 5.Spencer NJ, Bayguinov P, Hennig GW, et al. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol. 2007;292:G546–G555. doi: 10.1152/ajpgi.00352.2006. [DOI] [PubMed] [Google Scholar]
- 6.Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarisations and rapid oscillations in circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer NJ, Hennig GW, Dickson E, et al. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol. 2005;564:829–847. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149–158. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- 9.West GB, Brown JH, Enquist BJ. The origin of universal scaling laws in biology. In: Brown JH, West GB, editors. Scaling in biology; Santa Fe Institute Studies in the Sciences of Complexity Proceedings; Oxford University Press. 2000.pp. 87–112. [Google Scholar]
- 10.Roger T, Ruckebusch Y. Pharmacological modulation of postprandial colonic motor activity in the pony. J Vet Pharmacol Ther. 1987;10:273–282. doi: 10.1111/j.1365-2885.1987.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 11.Cherbut C, Ruckebusch Y. The effect of indigestible particles on digestive transit time and colonic motility in dogs and pigs. Br J Nutr. 1985;53:549–557. doi: 10.1079/bjn19850064. [DOI] [PubMed] [Google Scholar]
- 12.Ferré JP, Ruckebusch Y. Myoelectrical activity and propulsion in the large intestine of fed and fasted rats. J Physiol. 1985;362:93–106. doi: 10.1113/jphysiol.1985.sp015665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinning PG, Szczesniak MM, Cook IJ. Twenty-four hour spatiotemporal mapping of colonic propagating sequences provides pathophysiological insight into constipation. Neurogastroenterol Motil. 2008;20:1017–1021. doi: 10.1111/j.1365-2982.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott SM, Pilot MA, Barnett TG, et al. Prolonged ambulatory canine colonic motility. Am J Physiol. 268:G650–G662. doi: 10.1152/ajpgi.1995.268.4.G650. 199. [DOI] [PubMed] [Google Scholar]
- 15.Powell AK, Fida R, Bywater RA. Motility in the isolated mouse colon: migrating motor complexes, myoelectric complexes and pressure waves. Neurogastroenterol Motil. 2003;15:257–266. doi: 10.1046/j.1365-2982.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 16.Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil. 2007;19(Suppl 2):19–24. doi: 10.1111/j.1365-2982.2007.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Giorgio R, Barbara G, Furness JB, et al. Novel therapeutic targets for enteric nervous system disorders. Trends Pharmacol Sci. 2007;28:473–481. doi: 10.1016/j.tips.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Manag. 2008;4:41–48. doi: 10.2147/tcrm.s140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fida R, Bywater RA, Lyster DJ, et al. Chronotropic action of 5-hydroxytryptamine (5-HT) on colonic migrating motor complexes (CMMCs) in the isolated mouse colon. J Auton Nerv Syst. 2000;80:52–63. doi: 10.1016/s0165-1838(00)00074-6. [DOI] [PubMed] [Google Scholar]
- 20.Bush TG, Spencer NJ, Watters N, et al. Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am J Physiol. 2001;281:G974–G983. doi: 10.1152/ajpgi.2001.281.4.G974. [DOI] [PubMed] [Google Scholar]
- 21.Smith TK, McCarron S. Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. J Physiol. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson EJ, Spencer NJ, Hennig GW, et al. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 23.Spencer NJ, Dickson EJ, Hennig GW, et al. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J Physiol. 2006;576:519–531. doi: 10.1113/jphysiol.2006.109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson EJ, Hennig GW, Heredia DJ, et al. Intrinsic neural reflexes in response to colonic elongation. J Physiol. 2008;86:4225–4240. doi: 10.1113/jphysiol.2008.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grider JR, Makhlouf GM. Regulation of the peristaltic reflex by peptides of the myenteric plexus. Arch Int Pharmacodyn Ther. 1990;303:232–251. [PubMed] [Google Scholar]
- 27.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 28.Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987;252:C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda S, Takano H, Takaki M, et al. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol. 2002;542:887–897. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther. 2003;307:460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 1996;271:G849–G857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- 32.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- 33.Smith TK. Myenteric AH neurons are sensory neurons in the guinea-pig proximal colon: an electrophysiological analysis in intact preparations. Gastroenterology. 1994;862:A-216. [Google Scholar]
- 34.Bertrand PP, Kunze WA, Furness JB, et al. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 35.Gershon MD, Kirchgessner AL. Identification, characterization and projections of intrinsic primary afferent neurones of the submucosal plexus: activity-induced expression of c-fos immunoreactivity. J Auton Nerv Syst. 1991;33:185–187. [Google Scholar]
- 36.Smith TK, Spencer NJ, Hennig GW, et al. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomas EA, Sjövall H, Bornstein JC. Computational model of the migrating motor complex of the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G564–G572. doi: 10.1152/ajpgi.00369.2003. [DOI] [PubMed] [Google Scholar]