Abstract
Recent studies indicate that secondary bile acids promote colon cancer cell proliferation but their role in maintaining cell survival has not been explored. We found that deoxycholyltaurine (DCT) markedly attenuated both unstimulated and TNF-α-stimulated programmed cell death in colon cancer cells by a phosphatidylinositol 3-kinase (PI3K)-dependent mechanism. To examine the role of bile acids and PI3K signaling in maintaining colon cancer cell survival, we explored the role of signaling downstream of bile acid-induced activation of the epidermal growth factor receptor (EGFR) in regulating both apoptosis and proliferation of HT-29 and H508 human colon cancer cells. DCT caused dose- and time-dependent Akt (Ser473) phosphorylation, a commonly used marker of activated PI3K/Akt signaling. Both EGFR kinase and PI3K inhibitors attenuated DCT-induced Akt phosphorylation and Akt activation, as demonstrated by reduced phosphorylation of a GSK-3-paramyosin substrate. Transfection of HT-29 cells with kinase-dead EGFR (K721M) reduced DCT-induced Akt phosphorylation. In HT-29 cells, EGFR and PI3K inhibitors as well as transfection with dominant negative AKT attenuated DCT-induced cell proliferation. DCT-induced PI3K/Akt activation resulted in downstream phosphorylation of GSK-3 (Ser21/9) and BAD (Ser136), and nuclear translocation (activation) of NF-κB, thereby confirming that DCT-induced activation of PI3K/Akt signaling regulates both proproliferative and prosurvival signals. Collectively, these results indicate that DCT-induced activation of post-EGFR PI3K/Akt signaling stimulates both colon cancer cell survival and proliferation.
Colon cancer results from a sequential progression of normal colon epithelial cells to benign adenomas that display increasing dysplasia before developing into locally invasive and metastatic cancer. These phenotypic changes are associated with the accumulation of somatic genetic alterations that alter regulation of cell proliferation, apoptosis, and DNA repair (Kinzler and Vogelstein, 1996). Genetic and epigenetic mechanisms underlying this progression include mutation and/or methylation of oncogenes, tumor suppressors, and mismatch repair genes. After tumor initiation, chronic exposure to environmental factors, including dietary components and fecal bile acids,1 plays an important role in colon cancer cell proliferation, thereby promoting tumor growth and spread (Glinghammar and Rafter, 1999).
Alterations in fecal bile acid concentration, particularly secondary bile acids (lithocholic and deoxycholic acids), have long been implicated by epidemiological and animal studies to play an important role in the pathogenesis of intestinal tumors [reviewed in Glinghammar and Rafter (1999)]. For instance, in mice with an APC gene mutation, removal of colon grafts from the fecal stream prevents adenoma formation (Gould and Dove, 1996). In rodents treated with carcinogens, increasing bile acid concentration in the lumen of the colon augments colon cancer formation (Narisawa et al., 1974; Reddy et al., 1976; Nagengast et al., 1995). In contrast, in rats fed ursodeoxycholic acid, a treatment that lowers the proportion of fecal deoxycholic acid, a reduced number of colon tumors is observed (Earnest et al., 1994; Narisawa et al., 1998). In humans, a similar reduction in colorectal dysplasia and cancer is observed with ursodeoxycholic acid treatment (Pardi et al., 2003; Alberts et al., 2005). Hence, compelling data support an important role for bile acids in colonic neoplasia.
In general, following cancer induction, colon cancer cell survival and promotion depends on a favorable interplay of proproliferative and prosurvival signals. Nonetheless, molecular mechanisms whereby bile acids alter colon cancer cell signaling are only now being elucidated. Observations by Martinez et al. (1998) in colon cancer cells focused on stimulation of apoptosis by deoxycholic acid, not on cell survival or proliferation. Likewise, Schlottman et al. (2000) reported that deoxycholic and chenodeoxycholic acids cause concentration- and time-dependent apoptosis in SW480 and HT-29 colon cancer cells.
Jean-Louis et al. (2006) provided evidence that deoxycholic acid-induced ligand-independent epidermal growth factor receptor (EGFR) signaling and apoptosis are mediated by changes in plasma membrane structure, primarily redistribution of membrane cholesterol. In contrast, Merchant et al. (2005) reported that in HCA-7 and HCT-8 colon cancer cells deoxycholic acid induces ligand (amphiregulin)-dependent activation of EGFR. However, these actions were observed with bile acid concentrations known to induce apoptosis (Martinez et al., 1998; Cheng et al., 2002) and bile acid-induced EGFR activation was not shown to alter either cell proliferation or survival (Merchant et al., 2005).
In HT-29 and Caco-2 colon cancer cells, Milovic et al. (2002) reported that low concentrations (up to 20 µM) of deoxycholic acid stimulate cell proliferation, whereas higher concentrations (>100 µM) stimulate apoptosis. Likewise, Pai et al. (2004) showed that micromolar concentrations of deoxycholic acid stimulate both β-catenin activation and colon cancer cell proliferation. Upstream signaling resulting in these actions was not elucidated. Overall, these findings indicate that the ability of unconjugated bile acids to induce either apoptosis or cell proliferation is concentration-dependent.
Work from our laboratory indicates that, in contrast to the actions of their unconjugated parent molecules, both glycine- and taurine-conjugates of lithocholic and deoxycholic acids stimulate colon cancer cell proliferation at concentrations that do not stimulate apoptosis (Cheng et al., 2002, 2007; Cheng and Raufman, 2005). As observed previously with cholinergic agonists (Cheng et al., 2003), this proliferative action of conjugated secondary bile acids requires ligand-dependent transactivation of EGFR and activation of post-receptor signaling (Cheng and Raufman, 2005; Cheng et al., 2007). Bile acids stimulate EGFR-dependent activation of p44/42 mitogen-activated protein kinase (MAPK) signaling and of p90 ribosomal S kinase (p90RSK), a downstream nuclear response protein (Cheng and Raufman, 2005). Blocking activation of either EGFR or MAPK signaling greatly attenuates the proliferative actions of bile acids (Cheng and Raufman, 2005; Cheng et al., 2007).
In colon cancer, EGFR activation is central to proliferative signaling by many agents (Roberts et al., 2002). In addition to p44/42 (ERK1/2) MAPK signaling, post-EGFR signaling can be mediated by other pathways (Wennstrom and Downward, 1999; Roberts et al., 2002). In particular, our attention was drawn to recent work indicating that in approximately 40% of colorectal cancers, mutations are detected in genes that regulate phosphatidylinositol-3′-OH kinase (PI3K)/Akt signaling (Parsons et al., 2005). In this pathway, ligand binding to EGFR stimulates activation of PI3K and production of phosphatidylinositol 3,4,5-trisphosphosphate (IP3) that recruits Akt to the cell membrane. Akt, a serine/threonine protein kinase that regulates both apoptosis and proliferation, is activated by site-specific phosphorylation at Ser473 and Thr308. Altered regulation of PI3K/Akt signaling is common in many cancers (Datta et al., 1999; Parsons et al., 2005; Song et al., 2005). Relevant to the current work, conjugated bile acids were reported to activate PI3K signaling in liver cells by both EGFR-independent and -dependent mechanisms (Rust et al., 2000; Dent et al., 2005). Moreover, in rat hepatocytes selected bile acids (ursodeoxycholic and tauroursodeoxycholic acids) attenuate deoxycholic acid-induced programmed cell death (Castro et al., 2007).
The foregoing observations suggested to us that activation of PI3K/Akt signaling in colon cancer cells may provide an additional EGFR-dependent mechanism whereby conjugated secondary bile acids stimulate proproliferative and/or prosurvival signaling. In the proximal colon, as observed previously with ursodeoxycholic acid treatment (Pardi et al., 2003; Alberts et al., 2005), naturally occurring DCT in the aqueous phase of human fecal water may protect neoplastic cells from apoptosis. Using two well-characterized human colon cancer cell lines, we sought to determine the role of PI3K/Akt signaling in mediating the actions of bile acids on cell survival and proliferation. Herein, we demonstrate that DCT rescues colon cancer cells from both unstimulated and stimulated apoptosis. Moreover, we show that DCT-induced EGFR-dependent activation of PI3K/Akt signaling results in phosphorylation of GSK, BAD, and other key downstream targets. These findings in two human colon cancer cell lines provide novel evidence that fecal bile acids can contribute to cancer progression and resistance to therapy by rescuing cancer cells from apoptosis. Some of these data were published previously in abstract form (Guo and Raufman, 2006).
Experimental Procedures
Chemicals and supplies
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture medium, RPMI 1640 and McCoy’s 5A Medium, was purchased from Invitrogen (Carlsbad, CA) and Quality Biological (Gaithersburg, MD). Fetal bovine serum (FBS) was purchased from Biowhittaker (Walkersville, MD). Biochemicals were obtained from Sigma-Aldrich (St. Louis, MO). Deoxycholyltaurine (DCT), obtained from Sigma-Aldrich, was maintained as a 0.1 M stock solution in deionized water. EGFR kinase inhibitors (AG1478 and PD168393), PI3K inhibitors (LY294002 and wortmannin) and MEK inhibitor (PD98059) were obtained from Calbiochem (San Diego, CA) and stock solutions were maintained in Me2SO (DMSO). TNF-α was obtained from Chemicon (Temecula, CA). Antibodies against p-Akt, total Akt, p-GSK-3, p-BAD, BAD, EGFR, β-actin, and histone H2A were purchased from Cell Signaling Technology (Danvers, MA), antibodies against NF-κB p65 purchased from BD Biosciences (San Jose, CA) and antibodies against PARP p85 from Promega (Madison, WI).
Cell lines
Cell lines were purchased from the American Type Culture Collection (ATCC). H508 human colon cancer cells were maintained in RPMI 1640 and HT-29 cells in McCoy’s 5A Medium supplemented with 10% FBS. Adherent cells were passaged weekly at subconfluence after trypsinization. Cultures were maintained in an incubator at 37°C in an atmosphere of 5% CO2 and 95% air.
Cell proliferation assay
Cell proliferation was determined using the sulforhodamine B (SRB) colorimetric assay, characterized and validated by Skehan et al. (1990). HT-29 cells, H508 cells, and transfected HT-29 cells were seeded on 96-well plates (Corning Glass Works) at approximately 10% confluence and allowed to attach for 24 h. The growth medium was removed and fresh medium without FBS and containing the indicated concentration of test agent was added. Cells were incubated for the described periods of time at 37°C in an atmosphere of 5% CO2 and 95% air. After incubation, cells were treated for 30 min with 0.4% (w/v) SRB dissolved in 1% acetic acid. Protein-bound dye was extracted with unbuffered 10 mM Tris base. Absorbance was measured at 560 nm using a computer-interfaced, 96-well microtiter plate reader (Spectramax Plus384, Molecular Devices, Sunnyvale, CA).
Plasmids
pRc/CMV constructs encoding the human wild-type EGFR (WT-EGFR) and K721M “kinase dead” EGFR, kindly provided by Dr. John G. Koland (University of Iowa College of Medicine, Iowa City, IA), was previously characterized by Cheng and Koland (1998). Full-length cDNAs of AKT (WT-AKT) and dominant negative AKT mutant (DNM-AKT) resulting in substitution of methionine (ATG) for lysine (AAG) at residue 179 in the expressed protein were obtained from Dr. Jian-Ying Wang (University of Maryland School of Medicine, Baltimore, MD), was characterized previously by Zhang et al. (2004). These cDNAs were inserted into the Klenow-blunted NheI and PmeI sites of expression vector pUSEamp(+) (Upstate Biotechnology, Charlottesville, VA) with the cytomegalovirus (CMV) promoter. HT-29 cells were transfected with wild-type EGFR, dominant negative EGFR, wild-type AKT and dominant negative AKT using the LipofectAMINE kit as recommended by the manufacturer (Invitrogen). After a 5-h incubation, the transfection medium was replaced by standard growth medium containing 10% FBS for 19 h.
Western blot analysis
Cells were serum-starved for 24 h, washed with medium without FBS, and incubated for 1 h at 37°C before adding test agents. After incubation with test agents, cell samples were washed with cold PBS and lysed with lysis buffer [150mM NaCl, 10 mM Tris/HCl, 1% (w/v) deoxycholic acid, 1% (v) Nonidet-40, 0.1% (w/v) SDS, 4 mM EDTA, 1 mM Na3VO4, 20 (µg/ml leupeptin, 20 (µg/ml aprotinin, 250 (µg/ml nitrophenylphosphate, and 1 mM phenylmethylsulfonyl fluoride, pH 8], and incubated on ice for 30 min. The lysis solution was centrifuged at 14,000 rpm for 20 min at 4°C. The protein concentration of the supernatant was measured by the Bradford method. Protein (40 µg) in the supernatant was boiled with electrophoresis sample buffer for 5 min and subjected to electrophoresis on 10% acrylamide gels (Invitrogen) according to the procedure of Laemmli (1970). Resolved proteins were transferred electrophoretically to nitrocellulose membranes (Micron Separations, Westborough, MA). Membranes were incubated overnight at 4°C in 5% BSA/Tris-buffered saline-Tween-20 buffer containing specific antibodies against p-Akt, total Akt, p-GSK, p-BAD, BAD, PARP p85, EGFR, β-actin, histone H2A, or NF-κB p65. Membranes were subsequently washed with 1 × Tris-buffered saline-Tween-20 and incubated with secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. Finally, immunocomplexes on membranes were reacted for 1 min with chemiluminescence reagent (NEL-100 PerkinElmer Life Sciences, Shelton, CT).
Immunofluorescence microscopy
H508 cells were subcultured in 4-well Lab-TekII chamber slides (Nalge Nucn International, Rochester, NY; 5 × 104 cells/well), incubated for 24 h at 37°C, and serum-starved for an additional 24 h. After incubation with PBS for 30 min, cells were incubated with test agents for another 5 min at 37°C. After washing with PBS, PBS/2M NaCl, cells were kept on ice, fixed with cold MeOH for 10 min, treated with 0.1% Triton X-100 for 10 min, and blocked for 30 min with PBS/5% serum derived from the same animal species as the secondary antibody. Cells were then incubated at 4°C overnight with the primary antibodies. After incubation, cells were washed in PBS, incubated with secondary Alexa Fluor555-conjugated antibodies at room temperature for 30 min and washed. Cell nuclei were visualized by Hoechst staining (Sigma-Aldrich). Stained cells were analyzed using immunofluorescence microscopy (Nikon Eclipse 80i).
Measurement of Akt kinase activity
Akt kinase activity was determined using a non-radioactive Akt kinase kit according to the manufacturer’s instructions (Cell Signaling Technology). Briefly, cells were lysed with lysis buffer (20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1 mM PMSF), and immunoprecipitated with anti-Akt antibody. Pellets were washed twice with 500 µl lysis buffer and twice with 500 µl of kinase buffer (25 mM Tris (pH 7.5), 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, 10 mM MgCl2). Exogenous glycogen synthase kinase-3 (GSK-3), a downstream target of phosphorylated Akt, served as the substrate. The kinase reaction was conducted in 40 µl kinase buffer containing 200 µM ATP and 1 µg of GSK-3 fusion protein at 37°C for 30 min and the reaction was stopped by adding 20 µl of 3 × SDS sample buffer. Akt kinase activity was determined by Western blotting using anti-phospho-GSK-3α/β Ser21/9 antibody. To confirm equal loading of samples, membranes were washed twice with 0.4% Tween-20 in PBS for 5 min, stained with India Ink (100 µl Higgins India Ink added to 100 ml 0.4% Tween-20 in PBS) for 3 h and washed three times with PBS for 2 h (all at room temperature).
Preparation of nuclear fractions
Cells plated in duplicate on 100-mm Petri plates (9 ×106 cells/ plate) were maintained in a humidified environment (5% CO2) at 37°C for 24 h followed by serum deprivation overnight. Cells were treated with 100 µM DCT for 2 h, with and without inhibitors. When inhibitors were used, cells were pre-incubated with the agent for 30 min. Control cells were exposed to diluent alone. Cytoplasmic and nuclear fractions were separated according to manufacturer’s instructions (NE-PERR kit, Pierce Biotechnology, Rockford, IL). Briefly, ice-cold CER I was added to the cell pellet and fully resuspended by vortexing. Tubes were then incubated on ice for 10 min followed by addition of ice-cold CER II, vortexed and incubated on ice for 1 min. Samples were centrifuged for 5 min in a microcentrifuge (~16,000g). Supernatants (cytoplasmic extracts) were immediately transferred to a clean pre-chilled tube. Insoluble (pellet) fractions were resuspended in ice-cold NER. Samples were repeatedly vortexed for 15 sec every 10 min (at 4°C) for a total of at least 40 min and microcentrifuged (~ 16,000g). Supernatant fractions (nuclear extracts) were immediately transferred to clean pre-chilled tubes and stored at −80°C until use.
DAPI staining for apoptosis
Cells were grown on sterile glass cover slips in 6-well plates for 18–24 h in medium containing FBS, followed by incubation for an additional 12–18 h in medium without FBS. Cells were then treated with test agents for the times indicated, fixed with 10% formalin for 10–15 min, and washed twice with 1 × PBS. Fixed cells were treated with 0.1% Triton X-100 in PBS for 5 min, and washed twice with PBS, stained in the dark for 30 min with DAPI solution (Sigma-Aldrich; 0.5 µg/ml and 0.1% BSA in PBS). Stained cells were washed with PBS, placed on a glass slide with 15 µl Mowoil (Sigma-Aldrich) and examined under a UV fluorescence microscope (Nikon Eclipse 80i) by an investigator masked to treatment group. Three hundred to 400 cells were examined, the number of cells with fragmented nuclei was determined, and the percentage of apoptotic cells calculated.
Poly(ADP-ribose) polymerase (PARP) degradation
TNF-α-induced apoptosis was determined by proteolytic cleavage of 116 kDa PARP into an 85-kDa peptide product (Haridas et al., 1998). Briefly, H508 and HT-29 cells were grown to near confluence (106 cells/well) in 6-well plates. Cells were pre-treated with or without 100 µM DCT, with and without a PI3K inhibitor (overnight for H508 cells and 24 h for HT-29 cells) and then stimulated with 100 ng/ml TNF-α for 6 and 24 h (H508 and HT-29 cells, respectively) at 37°C. After treatment, cell extracts were prepared by incubating cells for 30 min on ice in 0.02 ml lysis buffer containing 20 mM HEPES (pH 7.4), 2 mM EDTA, 250 mM NaCl, 0.1% NP-40, 2 µg/ml leupeptin, 2 µg/ml aprotinin, 1 mM PMSF, 0.5 µg/ml benzamidine, and 1 mM dithiothreitol. Lysates were centrifuged and supernatants collected. Cell extracts (40 µg) were resolved in 10% SDS–PAGE, electro-transferred onto nitrocellulose membranes, blotted with rabbit anti-PARP antibody specific for the p85 cleaved fragment of PARP that does not recognize intact 116 kDa PARP. Binding was detected by chemiluminescence (ECL; Pierce Biotechnology).
Statistical analysis
Qualitative data were repeated at least three times to ensure reproducibility. Quantitative results are expressed as mean ± SD from at least three separate experiments. Student’s t-test was used to determine the significance of the difference between means (SigmaPlot, Systat Software, Inc., San Jose, CA).
Results
DCT-induced attenuation of unstimulated and TNF-α-stimulated apoptosis in HT-29 colon cancer cells is regulated by PI3K
Our previous work focused on stimulation of colon cancer cell proliferation by bile acids (Cheng and Raufman, 2005; Cheng et al., 2007). To test the hypothesis that conjugated secondary bile acids can also prolong cell survival by inhibiting programmed cell death, we used DAPI staining to detect nuclear fragmentation (Fig. 1A) and examined the effects of adding DCT to HT-29 human colon cancer cells (Fig. 1B). For these experiments, we used 100 µM DCT based on dose–response curves showing stimulation of both p44/42 MAPK signaling and cell proliferation at this bile acid concentration (Cheng and Raufman, 2005; Cheng et al., 2007). The results presented in Figure 1B reveal a low basal rate of apoptosis (~ 1% of HT-29 cells exhibited nuclear fragmentation after 7-h incubation at 37°C). Resistance to apoptosis is common in malignant cells (Green and Evan, 2002) and resistance of HT-29 cells, in particular, to apoptosis was reported by others (Glinghammar et al., 2002).
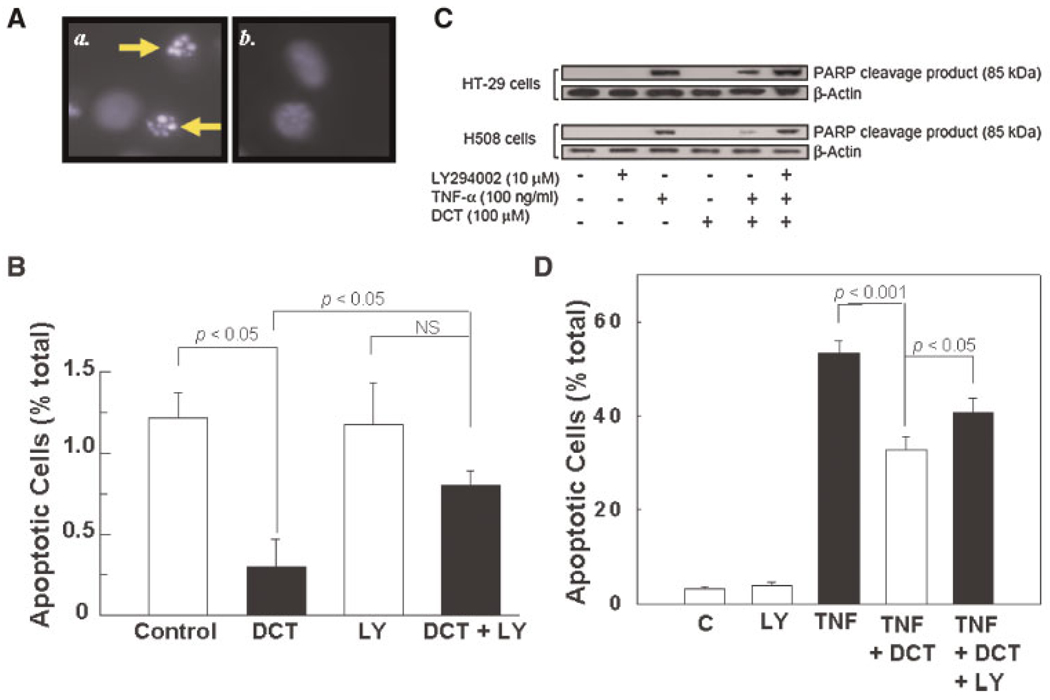
Fig. 1.
Actions of DCT on unstimulated and TNF-α-stimulated apoptosis in HT-29 colon cancer cells. A: Photomicrograph of DAPI-stained HT-29 cells. Apoptosis was determined by using DAPI staining to identify fragmented cell nuclei as described in Experimental Procedures Section. Nuclear DNA is colored blue after staining with DAPI. Cells with fragmented nuclei are indicated by the arrows in a. For comparison, b. shows non-apoptotic HT-29 cells. B: To determine the effect of the bile acid on unstimulated apoptosis, HT-29 cells were pre-incubated alone or with 100 µM DCT for 2 h at 37°C. Cells were then incubated with or without 30 µM LY 294002 for an additional 5 h. As a control, Me2SO (DMSO; 0.1%, vol/vol), the vehicle for LY294002, was added to all samples. This concentration of Me2SO alone does not alter apoptosis (not shown). Results are expressed as mean ± SD of at least three separate experiments. NS indicates comparisons that are not significant at the 0.05 level. C: DCT attenuates TNF-α-stimulated PARP degradation in HT-29 and H508 colon cancer cells by a PI3K-dependent mechanism. Following treatments described in Methods Section, cell extracts were examined for the 85-kDa cleavage product of PARP degradation using a specific monoclonal antibody that does not recognize 116-kDa PARP. The quantity of protein added was verified by immunoblotting with antibody for β-actin. Results shown are representative of three separate experiments. D: Microscopic examination of TNF-α-treated HT-29 cells, alone or with DCT and LY294002, for changes consistent with apoptosis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Strikingly, incubation of HT-29 colon cancer cells with 100 µM DCT caused a reproducible 75% reduction in the percentage of apoptotic cells (Fig. 1B). Moreover, adding a PI3K inhibitor, 30 µM LY294002, attenuated the prosurvival actions of DCT (Fig. 1B). The data shown in Figure 1B reveal that DCT rescues colon cancer cells from unstimulated apoptosis. Nonetheless, the level of apoptosis in these cancer cells is low; approximately 1%. Hence, even the ability of the bile acid to reduce this level of apoptosis by 75% (Fig. 1B) is unlikely to have a major impact on cell number compared to the ability of the bile acid to stimulate robust cell proliferation (Cheng and Raufman, 2005; Cheng et al., 2007). To determine whether activation of PI3K/Akt signaling might play a more important role under conditions of cell stress, we examined the ability of DCT to rescue cells from stimulated apoptosis.
Many agents, including hydrogen peroxide, staurosporine, deoxycholic acid, and tumor necrosis factor-α (TNF-α) are used to stimulate programmed cell death. For the following experiments, we chose TNF-α for this purpose based upon its previous association with PI3K/Akt-dependent, NF-κB-mediated effects on apoptosis, its use for this purpose by many investigators (Manna and Aggarwal, 2000; Marasa et al., 2006; Radeff-Huang et al., 2007), and, in our cell lines, a more robust induction of apoptosis compared to other agents tested. To increase sensitivity for detection of programmed cell death, we also used a biochemical assay that measures degradation of PARP downstream of caspase-2, -3, and -9 activation (Manna and Aggarwal, 2000; Glinghammar et al., 2002). This assay provides an early marker for the detection of apoptosis, since cleavage of PARP occurs prior to DNA fragmentation, measured by DAPI or TUNEL assays. In this assay, apoptosis is represented by cleavage of 116-kDa PARP into an 85-kDa peptide product.
Preliminary time-course experiments indicated that maximal TNF-α-induced apoptosis was observed at shorter time intervals in H508 compared to HT-29 cells (not shown). Delayed apoptosis, requiring long-term measurement, has been observed previously in HT-29 compared to other colon cancer cell lines (Glinghammar et al., 2002). Hence, H508 cells were incubated with TNF-α for 6 h whereas HT-29 cells were incubated for 24 h before assaying for PARP degradation. As shown in Figure 1C, under the conditions used in this assay, neither LY294002 nor DCT alone altered detection of the 85-kDa PARP cleavage product. In contrast, treatment of both HT-29 and H508 cells with TNF-α caused a robust induction of programmed cell death as evidenced by the appearance of an intense band for the 85-kDa cleavage product (Fig. 1C). As observed with unstimulated apoptosis (Fig. 1B), pre-treatment with DCT markedly attenuated TNF-α-induced apoptosis. The intensity of the 85-kDa band was reduced by ~70% in the presence of DCT. Moreover, as observed with unstimulated cells, the anti-apoptotic actions of DCT were effectively reduced in the presence of LY294002 (85-kDa PARP intensity was the same as with TNF-α alone; Fig. 1C). As shown in Figure 1D, microscopic examination of TNF-α-treated HT-29 cells for nuclear features of apoptosis confirmed the results of PARP assay (Fig. 1C). With TNF-α alone, approximately 50% of cells showed features consistent with apoptosis (Fig. 1D). Adding DCT reduced TNF-α-stimulated apoptosis by ~50%, an effect that was attenuated by LY294002. In summary, DCT rescues TNF-α-treated cells from apoptosis and this effect is attenuated by adding a PI3K inhibitor (Fig. 1D). To exclude a role for ERK signaling in mediating the anti-apoptotic actions of DCT, we used PD98059, an effective inhibitor of MEK, the upstream kinase that activates ERK1/2 (Cheng and Raufman, 2005). PD98059 (25 µM) did not alter unstimulated apoptosis and did not alter the reversal of DCT-induced anti-apoptosis observed with LY294002 (not shown).
Collectively, these data provide evidence that DCT reduces the sensitivity of colon cancer cells to both unstimulated (Fig. 1B) and stimulated (Fig. 1C,D) programmed cell death by a PI3K–dependent mechanism. These initial findings were consistent with a regulatory role for PI3K/Akt signaling in DCT-induced attenuation of apoptosis. Nonetheless, although LY294002 is the most widely used inhibitor of PI3K activity, this agent is not specific for this function (Gharbi et al., 2007). Hence, in proceeding to parse the role of post-EGFR signaling in mediating prosurvival effects of DCT and to confirm the role of PI3K/Akt we used two human colon cancer cell lines and several complementary approaches.
DCT stimulates EGFR kinase- and PI3K–dependent Akt phosphorylation in colon cancer cells
The serine-threonine kinase Akt [also known as protein kinase B (PKB)], an important downstream target of PI3K activation, plays a pivotal role in regulating apoptosis and cell cycle progression (Parsons et al., 2005). To determine whether DCT induces Akt activation, we examined Akt1 (PKBα) Ser473 phosphorylation. As shown in Figure 2A, in both HT-29 and H508 colon cancer cells, DCT caused a dose- and time-dependent increase in Akt Ser473 phosphorylation. For both cell lines, we observed reproducible biphasic time-courses for Akt Ser473 phosphorylation. Phosphorylation in the initial rapid phase was maximal by 5 min and a second phase of phosphorylation was observed at 45–60 min (Fig. 2A). A biphasic time-course may result from changes in protein phosphatase activity that was not explored in the present work. Increased Akt Ser473 phosphorylation was observed with concentrations of DCT (10–300 µM) that were previously shown to stimulate colon cancer cell proliferation (Cheng and Raufman, 2005; Cheng et al., 2007) and which overlap those present in the cecum of the human colon (Hamilton et al., 2007). Nonetheless, for optimal detection of changes in protein phosphorylation, in the following experiments we used maximal concentrations of DCT (i.e., 200–300 µM).
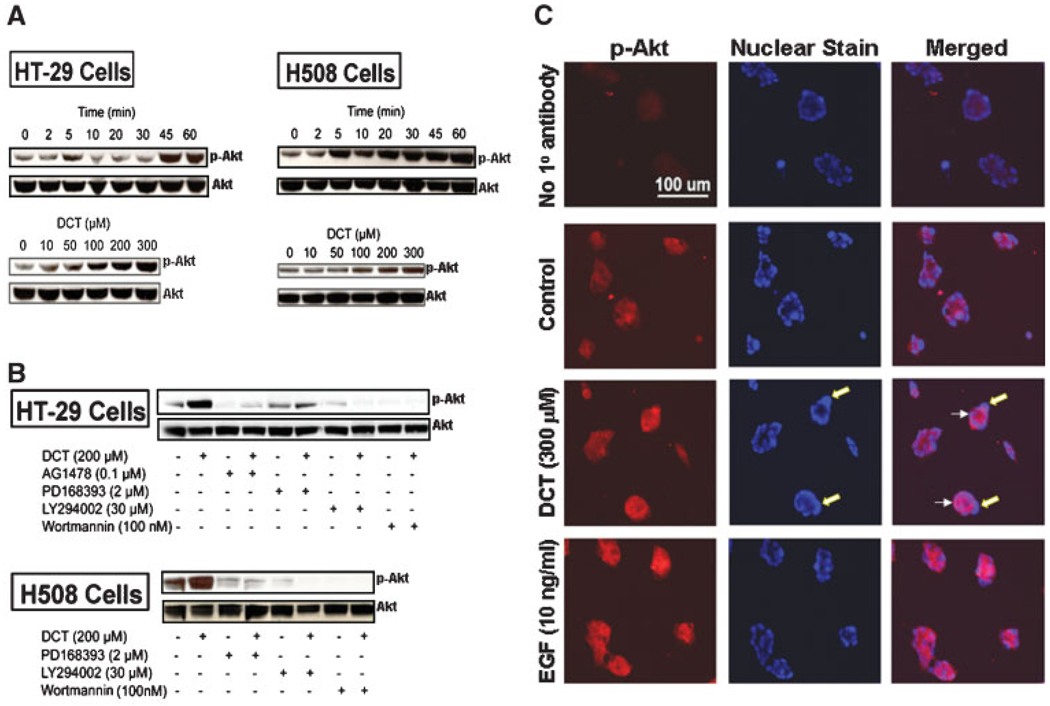
Fig. 2.
DCT-induced Akt (Ser473) phosphorylation in colon cancer cells. A: To determine the time-course for bile acid-induced phosphorylation of Akt, HT-29, and H508 cells were incubated with 300 µM DCT for up to 60 min and cell extracts were immunoblotted at the times indicated to determine changes in phospho-Akt Ser473 (p-Akt). To determine the dose–response for bile acid-induced activation of Akt, cells were incubated for 5 min with increasing concentrations of DCT and cell extracts were immunoblotted for phospho-Akt Ser473. B: HT-29 and H508 cells were pre-incubated with or without inhibitors of EGFR kinase and PI3K for 30 min and then incubated for 5 min with or without DCT. Cell extracts were immunoblotted to determine changes in phospho-Akt Ser473. In these experiments, the quantity of protein added was verified by immunoblotting with antibody specific for total Akt. C: Immunofluorescence staining of H508 cells for phospho-Akt Ser473 in cells incubated for 5 min with water, 300 µM DCT, or 10 ng/ml EGF. After treatment, cells were immunostained as described in Methods Section. The first row shows results of control staining in the absence of primary (phospho-Akt) antibody. The first column shows staining with addition of secondary antibody (Alexa Fluor 555). The middle column shows Hoechst nuclear staining. The third column showing merged images of the signals obtained with the secondary antibody and Hoechst nuclear staining demonstrates cytoplasmic localization of the phospho-Akt Ser473 signal. Large arrows (middle part) indicate staining of nuclei; small arrows (right part) indicate accumulation of phosphorylated Akt in the cytoplasm of DCT-treated cells. Results are representative of three separate experiments.
We used selective inhibitors to determine whether DCT-induced Akt Ser473 phosphorylation was EGFR kinase- and/or PI3K–dependent. As shown in Figure 2B, both EGFR activation inhibitors (AG1478 and PD168393) and PI3K inhibitors (LY294002 and wortmannin) markedly inhibit DCT-induced Akt Ser473 phosphorylation. Although wortmannin may block other signaling molecules, the low concentrations used in our experiments, 50–100 nM, more specifically inhibit PI3K (Fruman et al., 1998; Davies et al., 2000). Collectively, these findings indicate that DCT-induced Akt Ser473 phosphorylation is both EGFR kinase- and PI3K–dependent.
In H508 cells, we used immunofluorescence microscopy to compare DCT-to EGF-induced Akt Ser473 phosphorylation. As shown in Figure 2C, in untreated cells there was negligible immunofluorescence staining for phosphorylated Akt Ser473. Treatment with either DCT or EGF resulted in accumulation in the cytoplasm of phosphorylated Akt. Counterstaining to highlight cell nuclei and merging of the nuclear and phospho-Akt stains confirmed that in these multi-nucleated malignant cells, the accumulation of phosphorylated Akt is localized to the cytoplasm (Fig. 2C, right column, arrows). Moreover, these findings confirm the Western blot data by demonstrating DCT-induced Akt Ser473 phosphorylation.
Additional evidence that DCT-induced Akt activation is EGFR kinase- and PI3K–dependent
Structurally, Akt is composed of an N-terminal pleckstrin homology (PH) domain, a C-terminal regulatory domain and a central kinase domain. Akt possesses two regulatory phosphorylation sites, Ser473 in the C-terminal regulatory domain and Thr308 in the activation loop within the kinase domain (Song et al., 2005). Growth factor-induced Akt activation requires both PI3K activation and Akt (PH domain-dependent) translocation to the plasma membrane; this is followed by Akt Ser473 and Thr308 phosphorylation (Song et al., 2005). Full activation of Akt requires phosphorylation of both Ser473 and Thr308 (Alessi et al., 1996). Although we observed consistent, reproducible DCT-induced Akt Ser473 phosphorylation (Fig. 2), despite using two commercially available antibodies, we were unable to demonstrate in HT-29 and H508 cells that either EGF or DCT caused Akt Thr308 phosphorylation (data not shown).
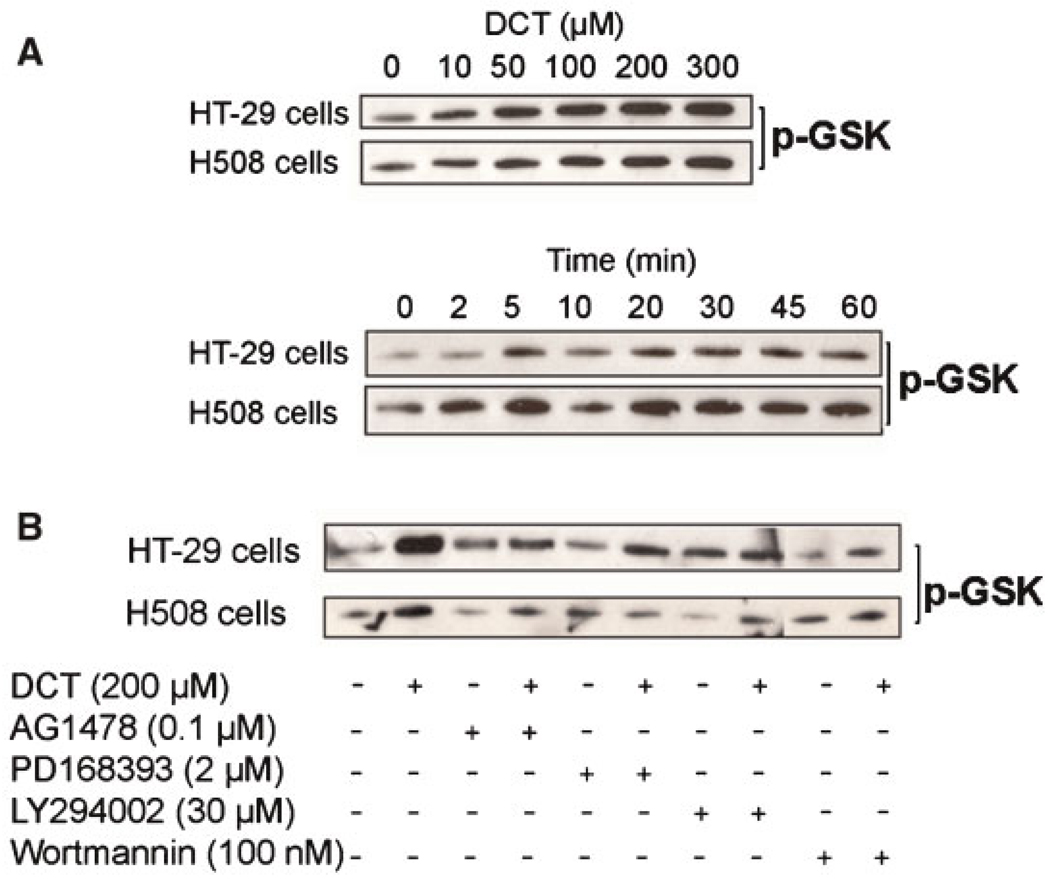
To confirm that treatment with DCT caused Akt activation, we performed in vitro Akt kinase assays using glycogen synthase kinase (GSK)-3α/β, the first identified physiological Akt target (Cross et al., 1995), as substrate (Diehl et al., 1998). After treatment with or without DCT, total Akt was immunoprecipitated from HT-29 and H508 cell extracts and the in vitro kinase assay was performed using a GSK-3-paramyosin fusion protein substrate that was probed with phospho-GSK-3α/β Ser21/9 antibody. As shown in Figure 3A, in both colon cancer cell lines DCT caused dose-dependent increases in GSK phosphorylation that were detectable with 10 µM and maximal with 300 µM DCT, the highest concentration examined. Both the dose–response and time-course for Akt activation (Fig. 3A) were similar to those for Akt Ser473 phosphorylation (Fig. 2A). To confirm that Akt-mediated GSK phosphorylation was both EGFR kinase- and PI3K–dependent, we examined the actions of inhibitors. As shown in Figure 3B, for both HT-29 and H508 cells, DCT-induced GSK phosphorylation was attenuated by adding EGFR inhibitors (AG1478 and PD168393) and PI3K inhibitors (LY294002 and wortmannin). The results presented in Figure 3 confirm that in colon cancer cells, DCT causes EGFR kinase- and PI3K–dependent Akt activation. In both HT-29 and H508 cells, phosphorylation of Akt Ser473 correlates with Akt activity measured by the in vitro kinase assay.
Fig. 3.
Akt kinase activity in colon cancer cells treated with DCT alone or with DCT plus inhibitors of EGFR and PI3K. A: Dose–response and time-course for the actions of DCT on GSK substrate phosphorylation. B: Effect of adding EGFR kinase inhibitors (AG1478 and PD168398) and PI3K inhibitors (LY294002 and wortmannin). Serum-starved cells were pre-incubated with inhibitors or Me2SO (DMSO; vehicle) for 30 min followed by 5-min incubation with or without DCT. Total Akt was immunoprecipitated from HT-29 and H508 cell extracts and the in vitro kinase assay was performed using a GSK-3-paramyosin fusion protein substrate probed with phospho-GSK-3α/β Ser21/9 antibody. India Ink staining was used to determine sample loading (not shown, see Experimental Procedures Section). Results shown are representative of at least three separate experiments.
Transfection of HT-29 colon cancer cells with kinase-dead EGFR attenuates DCT-induced Akt activation
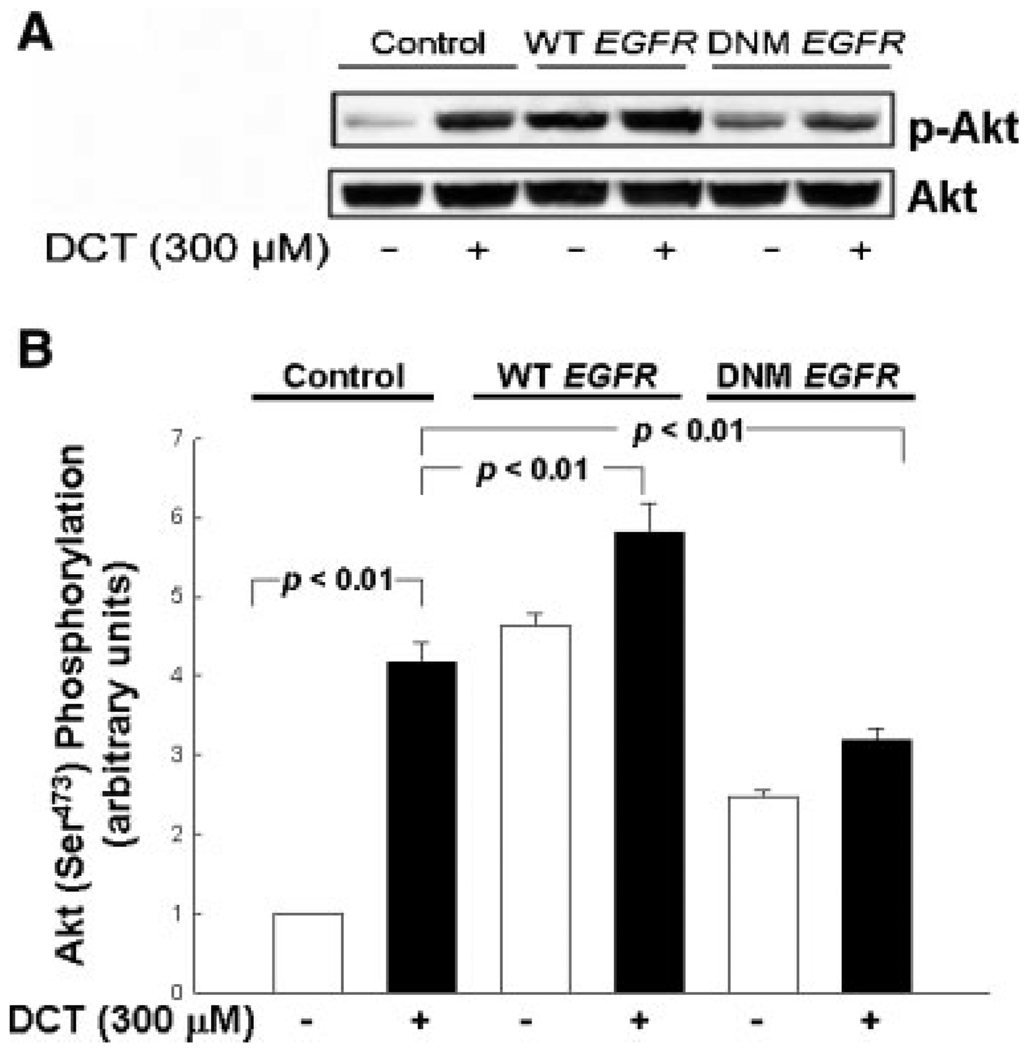
To confirm the role of EGFR kinase in mediating the signaling effects of bile acids, we examined the effects of transfecting colon cancer cells with EGFR plasmids containing a K721M mutation. This modification attenuates the kinase activity of EGFR (so-called kinase-dead mutation) by abolishing the phosphor-transfer reaction (Cheng and Koland, 1998). For comparison, to induce over-expression of the receptor, we transfected cells with plasmids containing wild-type EGFR. As a consequence of low transfection efficiency in H508 cells (not shown) these experiments were performed only in HT-29 cells. As shown in Figure 4A, transfection with a plasmid containing wild-type EGFR increased both basal and DCT-induced Akt Ser473 phosphorylation. In contrast, transfection with a plasmid containing kinase-dead EGFR markedly reduced DCT-induced Akt Ser473 phosphorylation. Whereas in non-transfected HT-29 cells DCT treatment caused a 3.8-fold increase in Akt Ser473 phosphorylation, in cells transfected with dominant negative EGFR, DCT caused only a negligible (20%) increase in Akt phosphorylation (Fig. 4B). Transfection of cells with these plasmids did not alter total cellular Akt (Fig. 4A). In concert with results presented in Figure 3B, the data presented in Figure 4 confirm that EGFR kinase activity is necessary to mediate DCT-induced activation of Akt.
Fig. 4.
Transfection of colon cancer cells with dominant negative EGFR attenuates DCT-induced Akt phosphorylation. A: Control HT-29 cells and HT-29 cells transfected with plasmids containing wild-type (WT) and Lys721 to Met (DNM) EGFR (Cheng and Koland, 1998) were incubated for 5 min with DCT and cell extracts were immunoblotted to determine changes in phospho-Akt Ser473 (p-Akt). The quantity of protein added was verified by immunoblotting with antibodies for total Akt. Three separate experiments were performed that showed similar results. B: Graphic representation of densitometry data from immunoblots prepared as described in A. Densitometry units are arbitrary based on the value for the water control in non-transfected HT-29 cells (first lane) defined as 1. Results are expressed as mean ± SD of at least three separate experiments.
Transfection of HT-29 colon cancer cells with kinase-dead AKT inhibits DCT-induced cell proliferation
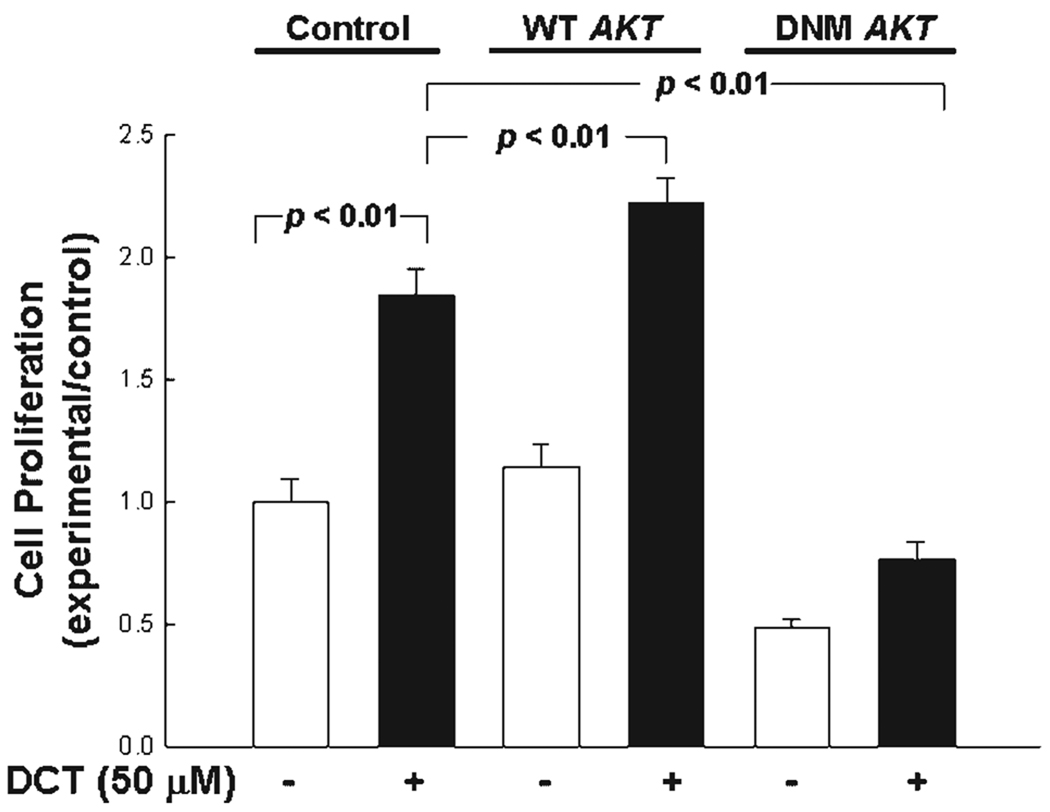
To define further the role of Akt activation in bile acid-induced colon cancer cell proliferation, we examined the actions of PI3K inhibitors and transfection of mutant AKT in HT-29 cells. Both LY294002 (50 µM) and wortmannin (50 nM) attenuated DCT (50 µM)-induced cell proliferation (~26% reduction in cell proliferation; P < 0.01; not shown). HT-29 cells transfected with plasmids containing wild-type and dominant negative mutant AKT (L179M mutation; Zhang et al., 2004) were treated with DCT. As shown in Figure 5, cell transfection to increase expression of wild-type Akt caused a small but significant increase in DCT-induced colon cancer cell proliferation. Transfection with the dominant negative mutant reduced both basal and DCT-induced cell proliferation. Thus, employing two different methods of blocking the actions of Akt (either by using inhibitors to prevent activation of PI3K upstream of Akt or by over expression of kinase-dead AKT) we observed attenuated DCT-induced cell proliferation. These observations indicate that in HT-29 cells Akt plays a role in mediating the proliferative actions of bile acids. Although PI3K inhibitors also attenuated DCT-induced H508 cell proliferation (~35% reduction in cell proliferation, P < 0.05; not shown) the aforementioned low efficiency of transfection in H508 cells prevented us from confirming the role of Akt expression using kinase-dead AKT.
Fig. 5.
Transfection of colon cancer cells with kinase-dead Akt attenuates DCT-induced cell proliferation. Control HT-29 cells and HT-29 cells transfected with plasmids containing wild-type (WT) and mutant AKT (DNM; Zhang et al., 2004) were incubated with 50 µM DCT for 5 days at 37°C. Cell proliferation was determined by the sulforhodamine blue (SRB) colorimetric assay (Skehan et al., 1990). Results are expressed as mean ± SD of at least three separate experiments.
DCT-induced PI3K/Akt signaling modulates activation of downstream prosurvival and proproliferative molecules
In colon cancer cells, proliferative effects of PI3K/Akt activation are mediated by a variety of downstream signaling molecules (Bellacosa et al., 2005; Song et al., 2005; Woodgett, 2005). It did not seem necessary, or reasonable, to examine broadly the effects of the bile acid on these molecules. Nonetheless, to verify the importance of PI3K/Akt signaling in colon cancer cells, we selected for examination several molecules that are key mediators of either programmed cell death or proliferation. In many of these experiments, to reduce the possibility of missing delayed effects of downstream signaling by PI3K/Akt activation (Janes et al., 2006), we extended time-course studies to 16 h.
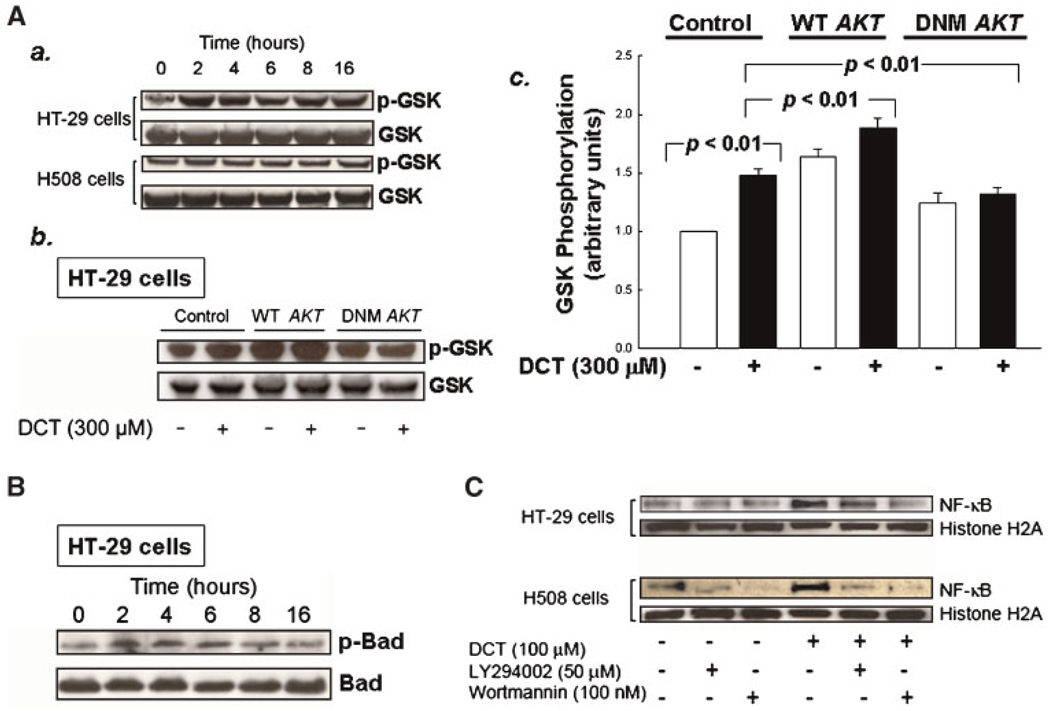
As described above, GSK-3α/β phosphorylation and inactivation by Akt results in proproliferative signaling (Diehl et al., 1998; Bellacosa et al., 2005; Li et al., 2005). The Akt kinase assay using exogenous GSK-3 as substrate demonstrated the ability of DCT to activate Akt via PI3K signaling (Fig. 3). To determine whether colon cancer cell GSK-3 is a potential mediator of downstream DCT-induced PI3K/Akt effects on cell survival, we used immunoblotting to determine whether DCT treatment resulted in phosphorylation of endogenous GSK-3 (Ser21/9). As shown in Figure 6A (part a), in both HT-29 and H508 cells, we observed DCT-induced, time-dependent phosphorylation of endogenous GSK. The increase in GSK phosphorylation after stimulation with the bile acid was more robust in HT-29 compared to H508 cells. Hence, we used transfection of HT-29 cells with WT and dominant negative AKT mutants to explore further the actions of Akt in phosphorylation of endogenous GSK. As shown in Figure 6A (parts b,c), when HT-29 cells were transfected with wild-type and dominant negative AKT, we observed augmentation and attenuation, respectively, of DCT-induced phosphorylation of endogenous GSK. Whereas in non-transfected HT-29 cells DCT treatment caused an approximately 1.5-fold increase in GSK phosphorylation, in cells transfected with dominant negative AKT, DCT did not increase GSK phosphorylation. Collectively, these findings confirm that DCT-induced PI3K/Akt activation results in endogenous GSK phosphorylation.
Fig. 6.
Actions of DCT on phosphorylation of GSK and BAD, and on nuclear translocation of NF-κB. A, a: HT-29 and H508 cells were incubated with DCT(300 µM) at 37°C for up to 16 h and cell extracts were immunoblotted at the times indicated to determine changes in phospho-GSK Ser21/9 (p-GSK). The quantity of protein added was verified by immunoblotting with antibodies for total GSK. b: Control HT-29 cells and HT-29 cells transfected with plasmids containing wild-type (WT) and mutant (DNM) AKT (Zhang et al., 2004) were incubated with DCT for 2 h at 37°C and cell extracts were immunoblotted to determine changes in phospho-GSK. The quantity of protein added was verified by immunoblotting with antibody for total GSK. c: Graphic representation of densitometry data from immunoblots prepared as described in b. Densitometry units are arbitrary based on the value for the control lane (untransfected HT-29 cells treated with water) defined as I. Results are expressed as mean ± SD of at least three separate experiments. B: HT-29 cells were incubated with 300 µM DCT for up to 16 h at 37°C and cell extracts were immunoblotted at the times indicated to determine changes in phospho-BAD Ser136 (p-BAD). The quantity of protein added was verified by immunoblotting with antibody for total BAD. C: HT-29 and H508 cells were pre-incubated with the indicated concentrations of PI3K inhibitors for 30 min and then incubated with or without DCT (100 µM) for 2 h at 37°C. Nuclear extracts were immunoblotted for NF-κB as described in Methods Section. The quantity of protein added was verified by immunoblotting using antibody for a nuclear protein, histone H2A. Results shown are representative of three separate experiments.
BAD was the first member of the Bcl-2 protein family identified as a target of Akt (Zha et al., 1996). Unphosphorylated BAD interacts with and inactivates prosurvival Bcl-2 family members (e.g., Bcl-XL) thereby promoting apoptosis. Akt-induced BAD Ser136 phosphorylation causes dissociation of BAD from Bcl-XL and its association instead with cytoplasmic 14-3-3 proteins, thereby providing a survival signal (Zha et al., 1996; Song et al., 2005). BAD contains several phosphorylation sites, including Ser112, a target of p90RSK, and Ser136 which appears to be site-specific for phosphorylation by Akt (Zha et al., 1996; Song et al., 2005). Epinephrine-stimulated activation of β2-adrenergic receptors was recently shown to protect prostate cells from apoptosis by downstream phosphorylation and inactivation of BAD (Sastry et al., 2007). To determine whether DCT-induced modulation of PI3K/Akt signaling in colon cancer cells alters BAD activity, we examined DCT-induced phosphorylation of BAD Ser136. As shown in Figure 6B, incubation of HT-29 cells with DCT resulted in robust, prolonged phosphorylation of BAD Ser136 that persisted for at least 6 h. DCT-induced BAD phosphorylation was also detected by immunoblotting in H508 cells, but the intensity of the bands was less than that observed in HT-29 cells (not shown).
We considered that NF-κB activation, a critical regulator of both proproliferative and prosurvival signaling (Jobin and Sartor, 2000; Karin, 2006), might play a role downstream of DCT-induced Akt signaling. Because the signaling actions of NF-κB require cytoplasm to nuclear translocation, we determined whether DCT stimulated nuclear translocation of this transcription factor and whether PI3K inhibitors blocked this action. In both cell lines, as shown in Figure 6C, 2-h incubation with DCT stimulated the appearance of a robust nuclear signal for p65 NF-κB (RelA). This signal was attenuated by adding two PI3K inhibitors. In HT-29 cells inhibition was more striking with LY294002 than with wortmannin (Fig. 6C). These findings provide evidence in colon cancer cells that DCT-induced NF-κB activation is regulated by a PI3K–mediated mechanism.
Collectively, the observations depicted in Figure 6 provide evidence to support the hypothesis that DCT-induced activation of PI3K/Akt signaling alters the function of several downstream mediators of colon cancer cell survival and proliferation. In the present study and previous work (Cheng et al., 2002, 2007; Cheng and Raufman, 2005) we demonstrated proproliferative effects of conjugated bile acids. Moreover, the data shown in Figure 6B,C suggest that conjugated bile acids also inhibit programmed cell death by multiple PI3K/Akt-mediated mechanisms, including phosphorylation of BAD and NF-κB activation. NF-κB is of particular interest since the primary role of this molecule is considered to be activation of anti-apoptotic genes (Karin and Lin, 2002; Karin, 2006).
Discussion
Alterations in the composition and concentration of luminal bile acids are associated with an increased risk of colon cancer; however, the molecular mechanisms whereby bile acids promote colon cancer cell proliferation are not well understood. Increasing evidence indicates that EGFR is a major regulator of bile acid actions on colon cancer cells (Cheng and Raufman, 2005; Merchant et al., 2005; Jean-Louis et al., 2006; Cheng et al., 2007). In the present study we show that in addition to EGFR-dependent activation of ERK signaling, a largely proliferative action (Cheng and Raufman, 2005; Cheng et al., 2007), exposure of colon cancer cells to DCT also results in EGFR-dependent sustained activation of PI3K/Akt signaling, a prosurvival action. Multiple independent but complementary approaches, including the use of EGFR and PI3K inhibitors, and transfection with plasmids containing both kinase-dead EGFR and mutant AKT, indicate that bile acid-induced PI3K/Akt signaling downstream of EGFR activation is a key regulator of colon cancer cell survival. Specifically, this conclusion is based on the following functional and biochemical evidence: (i) DCT-induced attenuation of unstimulated and TNF-α-induced apoptosis is reduced by PI3K inhibition; (ii) DCT-induced phosphorylation of Akt and in vitro phosphorylation of a GSK-paramyosin substrate are reduced in the presence of EGFR and PI3K inhibitors; (iii) DCT-induced Akt phosphorylation is augmented in cells transfected with WT EGFR and reduced in cells transfected with dominant negative EGFR; and (iv) DCT-induced phosphorylation and/or activation of signaling molecules downstream of EGFR/PI3K/Akt signaling (i.e., GSK, BAD, and NF-κB) is attenuated by PI3K inhibitors or transfection with kinase-dead AKT.
Consistent with our current findings, others reported that activation of Akt family proteins provides prosurvival signals that protect cancer cells from programmed cell death (Yao and Cooper, 1995; Wendel et al., 2004). Among the many reported targets of activated Akt are components of molecular cascades resulting in apoptosis, including inducible nitric oxide synthase, forkhead transcription factors, telomerases, NF-κB, and others (Datta et al., 1999). A major consequence of PI3K/Akt activation and phosphorylation of these target molecules is suppression of apoptosis (Yao and Cooper, 1995; Datta et al., 1999; Rust et al., 2000). To confirm in colon cancer cells that bile acid-induced PI3K/Akt activation affected key mechanisms for initiation of apoptosis, we examined BAD phosphorylation and NF-κB activation. Primarily in HT-29 cells, we observed DCT-induced sustained phosphorylation of BAD (greater than 6 h duration). Further, as observed in rat hepatoma cells with hydrophobic bile acids (chenodeoxycholic acid conjugates; Rust et al., 2000), we demonstrate that DCT, a more hydrophilic bile acid, also stimulates PI3K/Akt-dependent NF-κB activation, evidenced by nuclear translocation of NF-κB that is attenuated in the presence of PI3K inhibitors (Fig. 6C). Activation of NF-κB provides an additional mechanism for the initiation of PI3K/Akt-dependent prosurvival signaling (Karin and Lin, 2002; Karin, 2006). NF-κB activates several anti-apoptotic genes, including cellular inhibitors of apoptosis (c-IAPs), caspase-8-c-FLIP (FLICE inhibitory protein), A1 (Bfl1), TNFR-associated factor 1 (TRAF1), and TRAF2 (Karin and Lin, 2002).
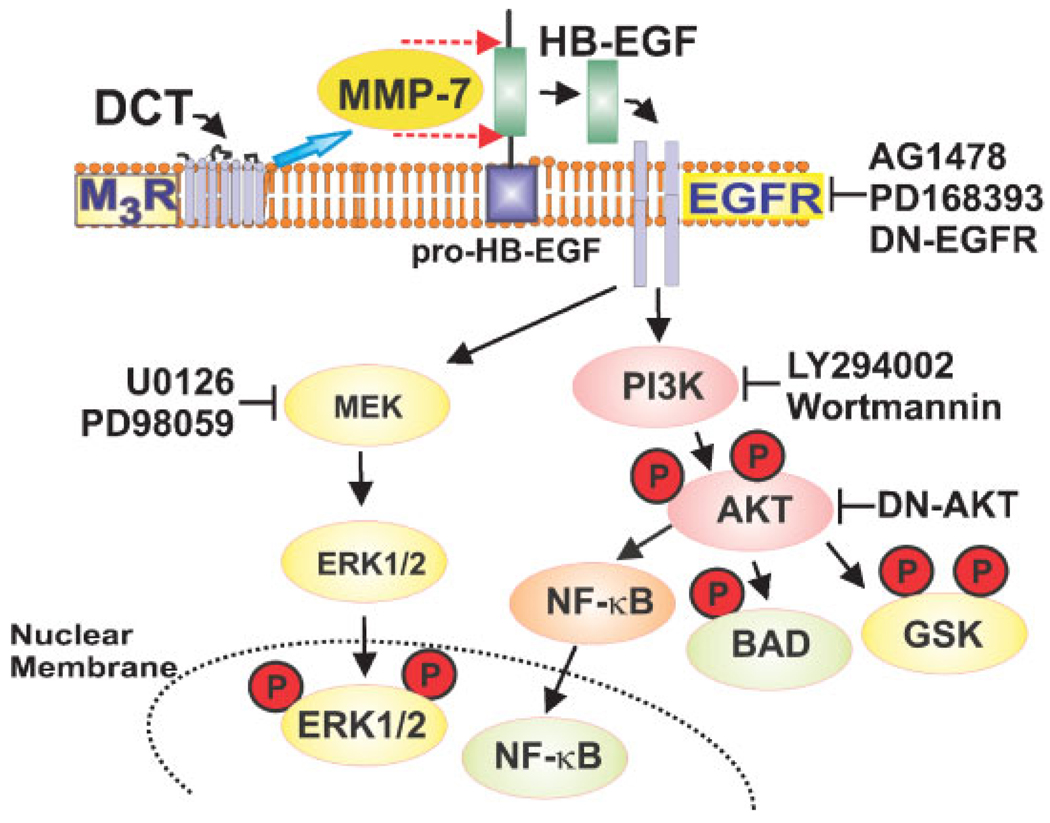
Conjugated bile acids initiate colon cancer cell proliferation by interacting with M3 muscarinic receptors, thereby activating matrix metalloproteinase-7 and releasing HB-EGF, an EGFR ligand that mediates transactivation of EGFR (Cheng and Raufman, 2005; Cheng et al., 2007). Post-EGFR proproliferative signaling is primarily by the ERK1/2 cascade (Fig. 7; Cheng and Raufman, 2005; Cheng et al., 2007). The findings described herein indicate that DCT-induced post-EGFR signaling through the PI3K/Akt cascade activates both prosurvival and proliferative signaling (Fig. 7). We provide novel evidence that DCT-induced activation of PI3K/Akt signaling in colon cancer cells causes phosphorylation of GSK (Figs. 3 and 6A). Bile acid-induced GSK phosphorylation and cell proliferation were both attenuated in HT-29 cells transfected with dominant negative AKT. Inactivation of GSK by phosphorylation is known to promote nuclear translocation of β-catenin, a proliferative transcription factor and reduces degradation of cyclin D1, a critical cell cycle regulatory protein (Diehl et al., 1998). Moreover, direct Akt-mediated site-specific phosphorylation of β-catenin by a GSK-independent mechanism can also stimulate β-catenin nuclear translocation (Fang et al., 2007). Hence, bile acid-induced activation of Akt can alter downstream proliferative signaling by multiple mechanisms. Regardless of mechanism, our findings strongly support a regulatory role for Akt in bile acid-induced cell proliferation; blocking PI3K/Akt activity with inhibitors or by transfection with kinase-dead AKT reduces colon cancer cell proliferation.
Fig. 7.
Intracellular signaling cascades activated by interaction of DCT with plasma membrane receptors. Evidence for these interactions derives from the present work and data presented in (Cheng and Raufman, 2005; Cheng et al., 2007). Stimulation of M3 muscarinic receptors (M3R) leads to activation of matrix metalloproteinase-7 (MMP-7), release of HB-EGF and activation of EGFR [blocked by AG1478, PD168393, and dominant negative (DN)-EGFR] (Cheng and Raufman, 2005; Cheng et al., 2007). Molecules indicated in yellow, activated primarily by post-EGFR ERK signaling (blocked by MEK inhibitors U0126 and PD98059), stimulate cell proliferation. In contrast, molecules indicated in green, activated primarily by post-EGFR PI3K/Akt signaling (blocked by LY294002, wortmannin and dominant negative-AKT), are largely anti-apoptotic (prosurvival). Key phosphorylations are indicated by a circled P. Both activated NF-κB and phosphorylated ERK undergo nuclear translocation where these molecules alter transcription of cell cycle and other critical genes that regulate cell proliferation and survival.
In hematopoietic and neuronal cells inactivation of GSK by Akt-mediated phosphorylation also regulates apoptosis (Maurer et al., 2006; Shaw and Cantley, 2006). Moreover, downregulation of Akt is associated with up-regulation of ERK signaling (Irie et al., 2005). Hence, categorizing PI3K/Akt target molecules as stimulating either cell proliferation or survival is somewhat arbitrary. Both GSK and NF-κB have downstream actions that fall into both of these categories (Karin, 2006; Maurer et al., 2006). In colon cancer cells, our findings indicate clearly that bile acids stimulate PI3K/Akt-dependent GSK phosphorylation and NF-κB nuclear translocation. Regardless of whether the actions of these PI3K/Akt target molecules are categorized as stimulating proliferation, survival, or some balance of the two, the end result is tumor progression. This is evidenced by our complementary experimental approaches showing that inhibition of PI3K/Akt signaling attenuates both DCT induced cell survival and proliferation.
Overall, our findings provide substantial novel information to fill important gaps in knowledge regarding bile acid actions in colon cancer. We show that DCT promotes survival of colon cancer cell lines derived from human tissue, that DCT attenuates both unstimulated and TNF-α-induced apoptosis, and by parsing upstream signaling that mediates this reduction in programmed cell death, we identified the role of EGFR and post-receptor activation of PI3K/Akt signaling that results in both activation and inhibition of key downstream regulatory molecules.
Similar actions of DCT on two human colon cancer cell lines suggest that our findings are germane to colon cancer in vivo. In humans, the composition and concentration of bile acids in the colon depend on multiple factors, including diet, intestinal bacteria, and the integrity and health of the distal small intestinal epithelial lining that plays a pivotal role in enterohepatic circulation of bile acids. For example, dietary meat, which increases taurine conjugation of bile acids, is identified as a risk factor for colon cancer (Glinghammar and Rafter, 1999). Nonetheless, the in vivo spectrum of human intestinal bile acids is difficult to measure, particularly in the proximal colon where cancer is most commonly associated with alterations in bile acids (Glinghammar and Rafter, 1999). Evidence now exists that low micromolar concentrations of conjugated dihydroxy bile acids, like DCT, are achieved in the proximal colon of healthy persons without colon cancer (Hamilton et al., 2007). DCT concentrations as low as 10 µM, which are achieved in the proximal human colon where H508 and HT-29 cells originate (Hamilton et al., 2007), induce activation of PI3K/Akt signaling (Figs. 2 and 3). Cecal concentrations of conjugated bile acids may increase in the presence of ileal mucosal disease or resection. Moreover, fecal levels of conjugated secondary bile acids may be increased in persons with colon cancer; this remains to be determined. The role of higher concentrations of hepatic bile acids in modulating growth of colon cancer implants in the liver, a common site of colon cancer metastasis, remains to be explored.
Our results may have important implications regarding the progression of colon cancer. The present study demonstrating that sustained activation of EGFR-mediated PI3K/Akt signaling plays a role in regulating bile acid-induced proliferation and survival of human colon cancer cells identifies a novel additional mechanism whereby luminal bile acids promote colon cancer (Glinghammar and Rafter, 1999). Moreover, DCT-induced rescue of colon cancer cells from stress-induced apoptosis may increase tumor resistance to radiation or chemotherapy. Further unraveling these mechanisms will enhance our understanding of the tumor-promoting effects of luminal bile acids and provide clues to facilitate targeting treatments to prevent or retard colon cancer growth.
Acknowledgments
We thank Dr. John Koland, Department of Pharmacology, University of Iowa College of Medicine, and Dr. Jian-Ying Wang, Departments of Surgery and Pathology, University of Maryland School of Medicine, for providing plasmids containing wild-type and dominant negative EGFR and AKT, respectively. This study was supported by the National Cancer Institute (CA107345 to JPR) and Office of Research and Development, Medical Research Service, Department of Veterans Affairs Merit Award to JPR.
Contract grant sponsor: National Cancer Institute;
Contract grant number: CA107345.
Contract grant sponsor: Office of Research and Development, Medical Research Service, Department of Veterans Affairs Merit Award.
Footnotes
The term bile acid refers to the protonated form and bile salt the ionized form. In this report as is common in the literature, these terms are used interchangeably. Bile acid nomenclature conforms to recommendations by Hofmann et al. (1992).
Literature Cited
- Alberts DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, Fales L, Koonce K, Parish D, Clouser M, Roe D, Lance P. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–853. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Castro RE, Amaral JD, Sola S, Kren BT, Steer C, Rodrigues CM. Differential regulation of cyclin D1 and cell death by bile acids in primary rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G327–G334. doi: 10.1152/ajpgi.00093.2007. [DOI] [PubMed] [Google Scholar]
- Cheng K, Koland JG. Nucleotide-binding properties of kinase-deficient epidermal-growth-factor-receptor mutants. Biochem J. 1998;330:353–359. doi: 10.1042/bj3300353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Cheng K, Chen Y, Zimniak P, Raufman J, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta. 2002;1588:48–55. doi: 10.1016/s0925-4439(02)00115-1. [DOI] [PubMed] [Google Scholar]
- Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–6750. [PubMed] [Google Scholar]
- Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol. 2007;73:1001–1012. doi: 10.1016/j.bcp.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S, Hylemon PB. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 2005;42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, Roy H, Khare S, Brasitus TA. Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res. 1994;54:5071–5074. [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinghammar B, Rafter J. Carcinogenesis in the colon: Interaction between luminal factors and genetic factors. Eur J Cancer Prev. 1999;8:S87–S94. [PubMed] [Google Scholar]
- Glinghammar B, Inoue H, Rafter JJ. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis. 2002;23:839–845. doi: 10.1093/carcin/23.5.839. [DOI] [PubMed] [Google Scholar]
- Gould KA, Dove WF. Action of Min and Mom1 on neoplasia in ectopic intestinal grafts. Cell Growth Differ. 1996;7:1361–1368. [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Guo C, Raufman J-P. Bile acids activate epidermal growth factor receptor (EGFR) kinase-dependent phosphatidylinositol 3kinase (PI3K)/Akt proliferative signaling in colon cancer cells. Gastroenterology. 2006;130:A119. [Google Scholar]
- Hamilton JP, Xie G, Raufman JP, Hogan S, Griffin TL, Packard CA, Chatfield DA, Hagey LR, Steinbach JH, Hofmann AF. Human cecal bile acids: Concentration and spectrum. Am J Physiol Gastrointest Liver Physiol. 2007;293:G256–G263. doi: 10.1152/ajpgi.00027.2007. [DOI] [PubMed] [Google Scholar]
- Haridas V, Darnay BG, Natarajan K, Heller R, Aggarwal BB. Overexpression of the p80 TNF receptor leads to TNF-dependent apoptosis, nuclear factor-kappa B activation, and c-Jun kinase activation. J Immunol. 1998;160:3152–3162. [PubMed] [Google Scholar]
- Hofmann AF, Sjovall J, Kurz G, Radominska A, Schteingart CD, Tint GS, Valacevic ZR, Setchell DR. A proposed nomenclature for bile acids. J Lipid Res. 1992;33:599–604. [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelialmesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281:14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- Jobin C, Sartor RB. The I kappa B/NF-kappa B system: A key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kinzler DW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J, Mizukami Y, Zhang X, Jo WS, Chung DC. Oncogenic K-ras stimulates Wnt signaling in colon cancer through inhibition of GSK-3beta. Gastroenterology. 2005;128:1907–1918. doi: 10.1053/j.gastro.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Manna SK, Aggarwal BB. All-trans-retinoic acid upregulates TNF receptors and potentiates TNF-induced activation of nuclear factors-kappaB, activated protein-1 and apoptosis in human lung cancer cells. Oncogene. 2000;19:2110–2119. doi: 10.1038/sj.onc.1203547. [DOI] [PubMed] [Google Scholar]
- Marasa BS, Rao JN, Zou T, Liu L, Keledjian KM, Zhang AH, Xiao L, Chen J, Turner DJ, Wang JY. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-kappaB activation through Ca2+ influx. Biochem J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, Payne CM, Powell MB, Gerner EW, Earnest DL. Different bile acids exhibit distinct biological effects: The tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111–118. doi: 10.1080/01635589809514689. [DOI] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Merchant NB, Rogers CM, Trivedi B, Morrow J, Coffey RJ. Ligand-dependent activation of the epidermal growth factor receptor by secondary bile acids in polarizing colon cancer cells. Surgery. 2005;138:415–421. doi: 10.1016/j.surg.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Milovic V, Teller IC, Faust D, Caspary WF, Stein J. Effects of deoxycholate on human colon cancer cells: Apoptosis or proliferation. Eur J Clin Invest. 2002;32:29–34. doi: 10.1046/j.0014-2972.2001.00938.x. [DOI] [PubMed] [Google Scholar]
- Nagengast FM, Grubben MJAL, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–1070. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]
- Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974;53:1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Narisawa T, Fukaura Y, Terada K, Sekiguchi H. Prevention of N-methylnitrosourea-induced colon tumorigenesis by ursodeoxycholic acid in F344 rats. Jpn J Cancer Res. 1998;89:1009–1013. doi: 10.1111/j.1349-7006.1998.tb00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai R, Tarnawski AS, Tran T. Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell. 2004;15:2156–2163. doi: 10.1091/mbc.E03-12-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi DS, Loftus EV, Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Lengauer C, Velculescu VE. Colorectal cancer: Mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- Radeff-Huang J, Seasholtz TM, Chang JW, Smith JM, Walsh CT, Brown JH. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. J Biol Chem. 2007;282:863–870. doi: 10.1074/jbc.M601698200. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Narasawa T, Weisburger JH, Wynder EL. Promoting effect of sodium deoxycholate on colon adenocarcinomas in germfree rats. J Natl Cancer Inst. 1976;56:441–442. doi: 10.1093/jnci/56.2.441. [DOI] [PubMed] [Google Scholar]
- Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA. 2002;99:1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust C, Karnitz LM, Paya CV, Moscat J, Simari RD, Gores GJ. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2000;275:20210–20216. doi: 10.1074/jbc.M909992199. [DOI] [PubMed] [Google Scholar]
- Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G. Epinephrine Protects Cancer Cells from Apoptosis via Activation of cAMP-dependent Protein Kinase and BAD Phosphorylation. J Biol Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- Schlottman K, Wachs FP, Krieg RC, Kullmann F, Scholmerich J, Rogler G. Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res. 2000;60:4270–4276. [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X (L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem. 2004;279:22539–22547. doi: 10.1074/jbc.M314337200. [DOI] [PubMed] [Google Scholar]