CONSPECTUS
Nicotine and methamphetamine are frequently abused in modern society, despite the increasing evidence of their addictive, neuropharmacological, and toxic effects. Tobacco, the most widely abused substance, is the leading cause of preventable death in the United States, killing nearly half a million Americans annually. A methamphetamine epidemic has also spread during the last decade; severe neurotoxicity and addictiveness contribute to the drug's notoriety. Although the majority of research on these two drugs is of pharmacological and neurobiological motivation, further study of these molecules from a chemical perspective may provide novel mechanistic insight into either their addictive potential or their pathological effects. For example, nicotine and methamphetamine share a common structural feature—a secondary amine—suggesting that these molecules could possess similar (or analogous) in vivo reactivity. Discoveries concerning the synthetic requirements for aqueous aldol catalysis and the feasibility of the enamine mechanism under physiological conditions have given rise to the hypothesis that ingested molecules, such as abused drugs, could participate in reactions utilizing an enamine intermediate in vivo.
The chemical reactivity of exogenous drugs with amine functionalities was initially examined in the context of the Maillard reaction, or nonenzymatic browning. The heating of reducing sugars with amino acids yields a brown solution; studies of this reaction were originally applied to food chemistry for the production of distinct flavors and aromas. Further research has since revealed numerous instances in which the in vivo production of advanced glycation end products (AGEs) through the Maillard reaction contribute to the pathology of disease states. Specifically, the modification of long-lived proteins by glycation and glycoxidation and the accumulation of these AGEs compromise the original function of such proteins and change the mechanical properties of affected tissue. In this Account, we summarize our investigations into the capacity for exogenous compounds to initiate the Maillard reaction and the corresponding physiological and immunological impact of the drug-conjugated AGEs that form. Many of the pathological components of diabetes, atherosclerosis, cancer, macular degeneration, Alzheimer's disease, and even the normal aging process are attributable to AGEs and their potential for aggregate formation in the vasculature. A deeper understanding of AGEs—and particularly glycated proteins—will provide fundamental mechanistic insight into disease origins.
Keywords: Aldolase, Enamine, Maillard reaction, Amadori rearrangement, Alzheimer's disease (AD), Advanced glycation end product (AGE), Nornicotine, Methamphetamine, Vascular Endothelial Growth Factor (VEGF)
INTRODUCTION
Substance addiction, which continues to inflict a large economic and social burden on modern society, involves a complex sequence of brain adaptations. The development of drug sensitization and addictive behaviors, which involves neuroplasticity in brain regions subserving learning and memory, is often examined through models of nicotine and psychostimulant abuse. Whereas amphetamines directly activate the mesolimbic dopaminergic circuits, which govern the learnt reward aspect of addiction, nicotine binds nicotinic acetylcholine receptors whose downstream effect includes the modulation of dopamine reactivity within the nucleus accumbens. Thus, both drugs possess a similar capacity to induce severe drug dependence in the user.
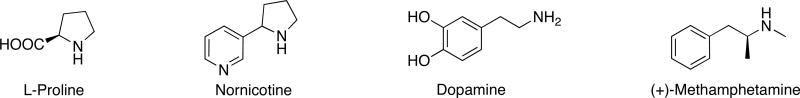
The quest to elucidate the altered psychological and physical pathology of drug addicts and correspondingly to develop better addiction therapies motivates the majority of research on abused substances. Many drugs, nicotine and amphetamines notwithstanding, possess inherently reactive chemical functionalities that would allow them to participate in biologically relevant chemical reactions, however the potential for this in vivo reactivity to induce certain pathologies in addicts is often overlooked in neurobiological research. By contrast, the examination of drug reactivity has been undertaken by chemists, and a classic example is the finding that specific sedatives and depressants promote alkaloid formation in vivo by inhibiting the oxidation of aldehydes.1 Their metabolism generates acetaldehyde, which readily condenses with biogenic amines or amino acids to form imines, and subsequent ring closure may lead to the formation of potentially psychoactive or toxic alkaloids through Schiff base formation by the Pictet-Spengler reaction. We have postulated that nornicotine and methamphetamine (Fig. 1), psychoactive compounds possessing a secondary amine moiety, may mediate complementary enamine-based chemistry in vivo and that this reactivity of abused drugs may provide a mechanistic explanation for certain pathologies observed in addicts.
Figure 1.
Structures of L-proline, nornicotine, dopamine, and (+)-methamphetamine.
THE ENAMINE MECHANISM
Revered by chemists as an essential method to form carbon-carbon bonds with great stereoselective control, the aldol reaction also has prominence within the field of biology with its application to metabolic processes employing aldolases as glycolytic enzymes, the formation of DNA adducts of acetaldehyde, and prebiotic catalysis.2,3 Both natural aldolase and aldolase mimetics, which include both catalytic antibodies and compounds with aldolase activity, have been studied extensively in order to gain improved efficiency, stereoselectivity, and mechanistic understanding of the aldol reaction. Thus, the discovery that a chiral biomolecule proline could catalyze asymmetric aldol reactions represented an important contribution to the field of organocatalysis, particularly with respect to iminium-based and enamine-based mechanisms for catalysis.4,5 This chemical framework was successfully applied to research in aldolase enzyme evolution and small oligopeptide-mediated catalysis of the asymmetric formation of sugars under prebiotic conditions.6 However, consideration of this enamine-based chemistry during investigations into the mechanistic basis of disease pathologies or drug effects in vivo was minimal until small-molecule based aqueous aldol catalysis was proven operable under physiologically relevant conditions.7
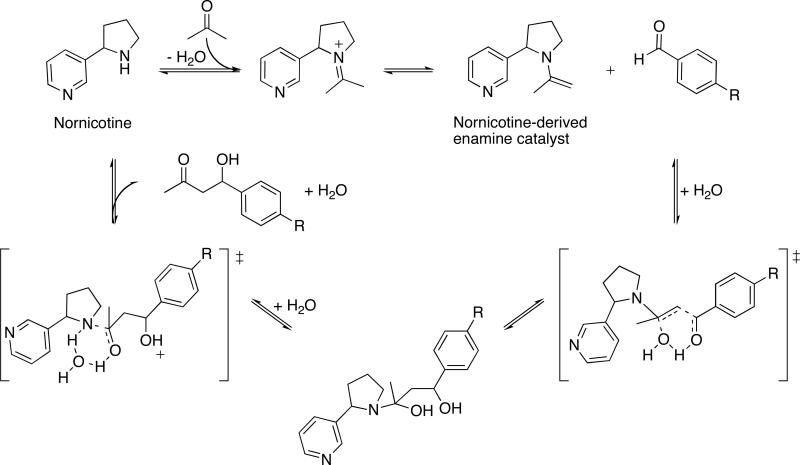
Our research into the reactivity of nicotine metabolites led us to discover that nornicotine, a minor tobacco alkaloid, possessed organocatalytic activity under buffered aqueous conditions, and opposite to the biomolecule proline, such catalysis was lost in common organic solvents (Scheme 1).7 Furthermore, as proven through capturing the hypothesized enamine nucleophile in trapping experiments, nornicotine catalyzed the aqueous aldol reaction involving an activated aldehyde acceptor, while the biomolecule proline failed to mediate such catalysis at physiological pH and temperature. Whereas the nornicotine-derived enamine was relatively stable at pH 7.5-8, the proline-derived enamine was vulnerable to hydrolysis, thus precluding catalysis.8 Contrary to previous studies with proline, nornicotine-based catalysis illustrated the physiological relevance of the enamine mechanism to the aldol reaction (Scheme 2A) and was suggestive of the more general operability of this mechanism in vivo (Scheme 2B).
Scheme 1.
Enamine-based reaction cycle for the covalent catalysis of the aqueous aldol reaction by nornicotine.
Scheme 2.
Catalysis by nornicotine. The enamine mechanism is relevant to (A) the aqueous aldol reaction, and (B) the Maillard reaction.
THE MAILLARD REACTION
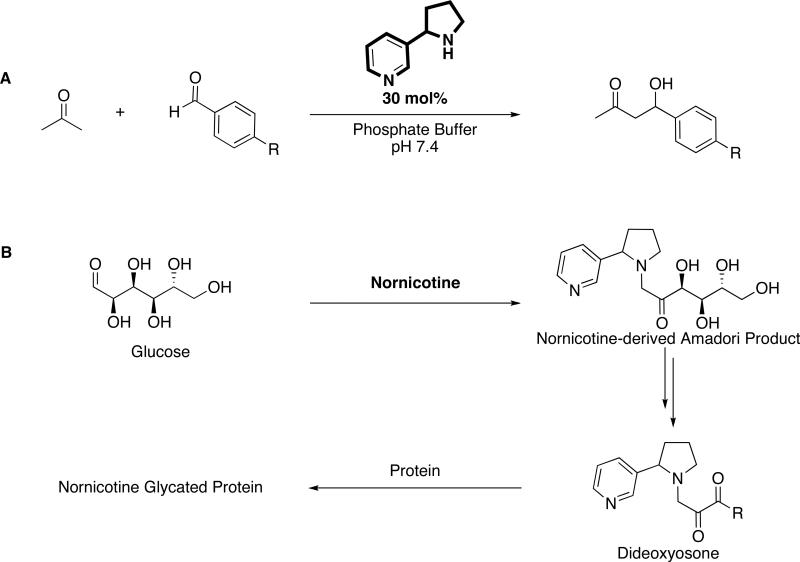
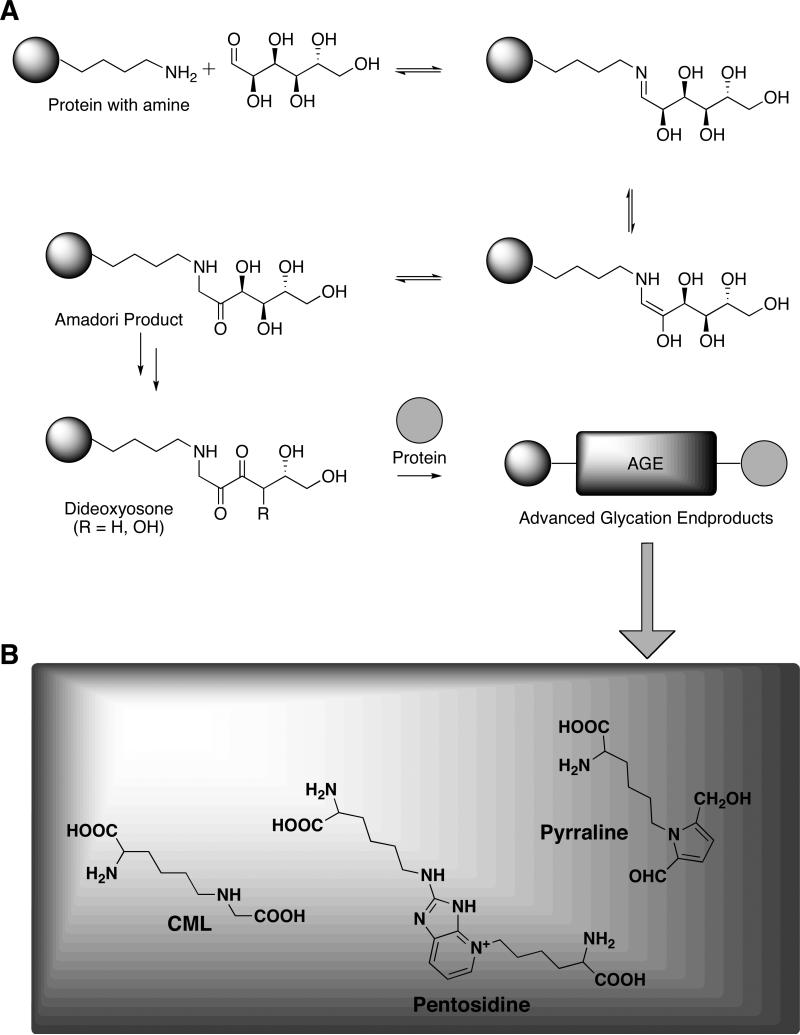
The Maillard reaction, which was first described as the pathway responsible for the color and aroma of certain cooked foods, is initiated by the reversible coupling between a free amine and an aldehyde or ketone moiety of a reducing sugar to form Schiff bases.9 Amadori rearrangement of the Schiff base intermediates and then degradation, fragmentation, or oxidation steps lead to the formation of advanced glycation end products (AGE; Fig. 2A).10 Its scientific relevance has since extended beyond its application to food chemistry to the study of its reaction mechanism and role in disease states. With respect to the latter, the diverse array of glycated proteins are potentially toxic, long-lived, and deleterious to the endogenous role of unmodified protein.11 Specifically, the cross-linking of collagen, a protein with a slow rate of turnover, may precipitate endothelial dysfunction as AGE deposits accumulate on vasculature. Fiber stiffness, thermal denaturation temperature and enzyme resistance comprise a few of the physical properties of proteins compromised by intra- and intermolecular cross-linking.12 Indeed, many of the pathological components of diabetes, atherosclerosis, cancer, macular degeneration, Alzheimer's disease (AD), and even the normal aging process are attributable to AGE and aggregate formation.10,13-16 A second major impact of the Maillard reaction is the side-chain modification of reacting proteins. The altered charge distribution of specific amino acids can abolish the interaction or recognition of the effected molecule with other endogenous biomolecules, a scenario important to degradative enzymes, cell surface receptors, and the cell-collagen interactions mediated by integrin recognition of arginine residues.
Figure 2.
Advanced glycation end product formation. (A) Scheme of the Maillard reaction, depicted by the reaction of glucose and a protein-derived amine, and (B) Structures of common advanced glycation end products (AGEs), in which the ε-amino group of lysine serves as the protein-derived amine.
It should be noted that the Maillard products detected in vivo and implicated in disease states are not formed exclusively by glycation. Indeed, several physiologically relevant pathways contribute to the wide array of immunochemically distinct AGEs. The dehydration and rearrangement of Amadori products may form α-dicarbonyls and the classic AGEs Nε-(carboxymethyl)lysine (CML), pentosidine, and pyrraline (Fig. 2B).17 However, Schiff base fragmentation to generate glycolaldehyde, and rearrangement of Amadori products and glyceraldehyde 3-phosphate also produces α-oxoaldehydes, the most notable of which are the reactive dicarbonyl 3-deoxyglucosone (3-DG) and methylglyoxal.18 Autoxidation reactions of reducing sugars and polyunsaturated fatty acids such as in the formation of glyoxal from glucose emphasizes the ability of various other carbohydrate or lipid-dependent metabolic processes to generate α-oxoaldehydes.19 A distinguishing feature of these Maillard reaction intermediate processes is the role of oxidative reactions (i.e., glycoxidation and nonoxidative glycation).20 Regardless, all intermediates subsequently react with the free amino groups of proteins, phospholipids, and nucleic acids, and thereby contribute to the diversity of late-stage AGEs.
IN VIVO EFFECTS OF AGE FORMATION
The complex kinetics and thermodynamics of the in vivo pathways generating AGEs have impeded the study of AGE formation. Schiff base condensation is a rapid and reversible process amenable to pharmacodynamic modeling. By contrast, the irreversible pathway by which Amadori products rearrange and form cross-links to generate AGEs is complicated by its sensitivity to reaction conditions and by the imperfect characterization of the numerous chemical entities and reaction intermediates produced en route. Even though the Amadori products form within hours to days after the initial reaction of the first sugar, the intermediate products, though measurable, are presumably too short-lived to have dramatic physiological ramifications in contrast to the end products of subsequent glycation and oxidation reactions.21 Nevertheless, detection of in vitro- and in vivo-prepared glycation and oxidation products holds merit in elucidating the kinetics of the early stages of the Maillard reaction.22 The reactivity of the monosaccharide, in which the open-chain conformer of the reducing sugar is conducive to Schiff base condensation, and the concentration of the reducing sugars, which governs the probability that the sugar will encounter a reactive amine, tend to dominate the reaction kinetics.23,24 It follows that hyperglycemic conditions may encourage the forward reaction toward Schiff base and Amadori product formation.25
To elucidate the chemical mechanism by which AGEs arise from Amadori products is nontrivial because it involves analysis of a complex, heterogenous mixture of AGEs forming from an already diverse array of Amadori products. To bypass this hurdle, scientists have focused on identifying pathologies derived from AGE formation and further elucidating how the in vivo occurrence of the Maillard reaction is causally related to them. AGE formation appears to induce a few main biological effects via a distinct progression of events: AGE attachment to and cross-linking of proteins, AGE deposition and subsequent induction of oxidative stress, activation of receptors for AGE (RAGEs), and induction of an immune response against the antigenic AGE-modified proteins.26,27 With respect to the latter, more common AGE motifs such as CML may serve as major immunological epitopes of anti-AGE autoantibodies of broader scope. However, all members of the heterogenous array of AGEs may elicit a unique polyclonal response, which greatly complicates their characterization. AGE-modified proteins are detected through various methods including the use of antibodies to known AGE motifs, visualization of AGE deposits on specific tissues such as vasculature and lens collagen, measurement of the altered fluorescence produced by certain Maillard-type cross-links, and detection of non-fluorescent AGE species through other spectrographic, chromatographic, or immunological methods. Alternatively, RAGE activation, which represents a cell-surface receptor dependent effect of in vivo AGE generation, contributes largely to the pathogenesis of vasculature dysfunction, and its expression is more readily quantified than heterologous AGEs.28
NORNICOTINE-BASED GLYCATION
The characterization of protein adducts and AGEs formed in vivo has illuminated the physiological importance of the Maillard reaction. In conjunction with our elucidation of the enamine mechanism for nornicotine-based aqueous aldol catalysis, we were exploring the propensity of commonly administered drugs containing an amine moiety to participate in other enamine-based reactions such as the Maillard reaction (Scheme 2). 7 The reactive glycation products or glycotoxins present in tobacco and tobacco smoke extracts were previously demonstrated to mediate AGE formation and protein cross-linking in vitro.29 This study also verified that cigarette smoke-derived glycotoxins induced AGE modification of serum proteins of smokers, illustrating the causal link between smoking, tobacco-associated AGE, and vascular disease.29 However, from a mechanistic standpoint, no distinction was made between AGE formation through glycotoxins preformed in tobacco (e.g. during the tobacco curing process) or through tobacco-derived reactive amines that initiated the Maillard reaction in vivo after their inhalation.
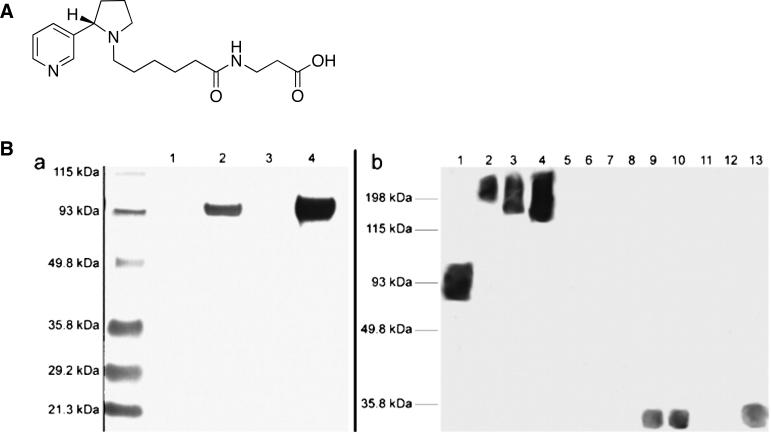
To test whether nornicotine would initiate the Maillard reaction in vitro, the Amadori product of nornicotine was prepared through the incubation of nornicotine and glucose under physiological conditions as well as through synthetic methods (Scheme 2B).30 To verify that this intermediate would react further to generate the glycated proteins associated with smoking, the incubation of nornicotine and glucose was repeated in the presence of protein [ribonuclease A (RNase A), bovine serum albumin (BSA), or human serum albumin (HSA)]. Detection of nornicotine-modified AGEs was accomplished through enzyme-linked immunosorbent assay (ELISA) and western blot analysis using mAb NIC6C12 (Fig. 3). Containing a 3-pyrrolidine-2-yl-pyridine nucleus and an alkyl linker attached to the pyrrolidine nitrogen, the hapten used to generate mAb NIC6C12 was designed to elicit anti-nicotine antibodies with superior recognition for the pyrrolidine nitrogen, and the pyrrolidyl methyl group (Fig. 3A).31 Even though mAb NIC6C12 was originally prepared for the purpose as a passive vaccine, the conservation of the alkaloid nucleus during nicotine metabolism to nornicotine as well as during nornicotine-mediated glycation proved fortuitous in that mAb NIC6C12 displayed good affinity for both nornicotine and nornicotine-modified AGEs.31
Figure 3.
(A) Hapten used to elicit anti-nicotine mAbs. (B) Detection of nornicotine-modified proteins using anti-nornicotine mAb NIC6C12 and nornicotine glycated protein. SDS/PAGE and Western blots: (a) BSA (lane 1), BSA + nornicotine and glucose (lane 2), HSA (lane 3), HSA + nornicotine and glucose (lane 4). (b) HSA + nornicotine and glucose (lane 1), nonsmoker plasma sample A (lanes 2-4; 2.5, 5.0, 10 mg/ml total protein concentration); nonsmoker plasma sample B (lanes 5-7); smoker plasma sample C (lanes 8-10); smoker plasma sample D (lanes 11-13). Reproduced with permission from Proc. Natl. Acad. Sci. USA.30
Though nornicotine represents a minor metabolite of nicotine (~ 8% of peripherally metabolized nicotine), its extended half-life of 8 h permits its accumulation in the serum of smokers.32 Given this and the in vitro stability of experimentally-generated nornicotine glycation products upon extended incubations (> 7 months), we hypothesized that nornicotine-based AGEs would reach detectable levels in serum samples of smokers if the mechanism of nornicotine-based glycation was operable in vivo. Plasma samples of smokers and nonsmokers were assessed by ELISA with mAb NIC6C12, and gratifyingly, nornicotine-modified protein levels were substantially elevated in the plasma of smokers.30
Whereas the formation of nornicotine-modified AGEs validated a hypothesis as to the reactivity of amine-containing compounds, the biological significance of this chemical prediction had yet to be realized. Thus, we embarked upon an investigation into the in vivo ramifications of this nornicotine-mediated glycation. Specifically, the propensity of nornicotine to target lysine during protein glycation and cross-linking resonated with the current research on Alzheimer's disease (AD). Whereas initial investigations into the therapeutic potential of nicotine were based on the upregulation of nicotinic acetylcholine receptors (nAChR) to counteract the nAChR deficiency in the AD brain, alternative mechanisms to explain the documented nicotine-derived protection from AD were still considered.33 The formation of neurotoxic intraneuronal neurofibrillary tangles and amyloid plaques have been shown to be directly proportional to the development of neurological deficits, however the mechanism driving their formation is still debated.34 It follows that a molecule able to prevent aberrant protein aggregation and more specifically avert the “amyloid-initiated cascade” may substantially delay disease progression.35 Thus, to explore an alternative explanation for the nicotine-mediated antagonism of Aβ-peptide toxicity, Salomon et al. examined the effect of nicotine on the different conformations of Aβ-peptide in solution, and concluded that nicotine blocked α-helix to β-sheet conversion through binding the histidine residues of the α-helix.33 The Aβ-peptide normally interconverts between random coil, monomeric α-helical, and oligomeric β-sheet structures, but a shift in this equilibrium toward favoring the β-sheet structure was hypothesized to cause aggregation and precipitation of β-sheet structures as amyloid. This process would then encourage further α-helix to β-sheet conversion. Salomon et al. surmised that the N-CH3 and 5'CH2 pyrrolidine moieties of nicotine mediated the interaction with the α-helix structure, implying that many of the major alkaloidic metabolites of nicotine would behave similarly. This model was subsequently refuted when NMR studies revealed that neither the α-helical nor the random coil conformations of Aβ-peptide were bound by nicotine to an appreciable extent, suggesting that nicotine might interact with the small, soluble Aβ-sheet aggregates to attenuate amyloidosis.33
The folding of Aβ-pleated sheets, the structural building block of Aβ aggregates, relies on the specific KLVFF amino acid sequence.36 The requirement of lysine in Aβ-peptide misfolding provoked the hypothesis that nornicotine-based glycation could target lysine residues of Aβ in a manner reminiscent of glycation.7,30 Through assays using thioflavin T staining of fibrils and diffusion NMR experiments to monitor the progress of Aβ aggregation in incubations of nornicotine, glucose, and either Aβ1–40 or Aβ12–28, we demonstrated that nornicotine is indeed capable of covalently modifying exposed lysine residues such as the Lys-16 of amyloid peptides and soluble Aβ aggregates, and that this modification blocks subsequent peptide aggregation.37
Whereas AGE formation and protein cross-linking have previously been implicated in disease pathologies, the hypothesis that exogenous molecules may participate in and even initiate protein glycation with potentially detrimental effects represents an unexplored application of glycation chemistry. Thus, we postulated that our initial research regarding nornicotine could be extrapolated to other abused molecules containing a secondary amine.
METHAMPHETAMINE-BASED AGEs
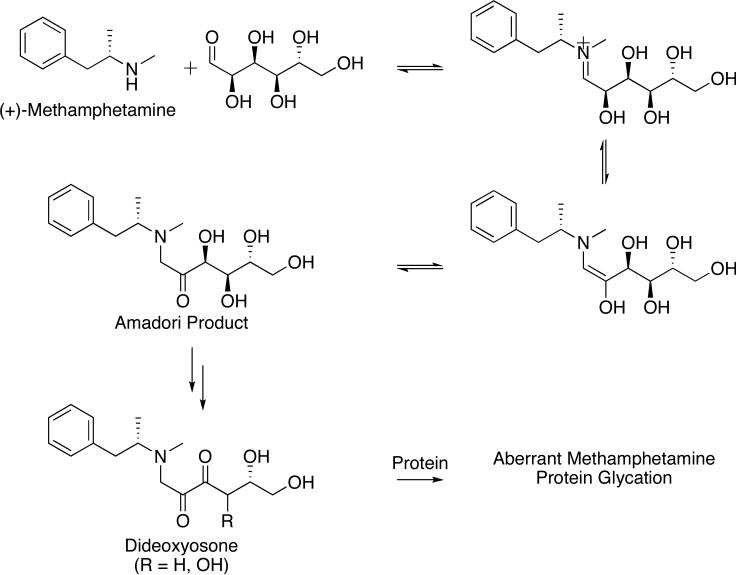
Nicotine and methamphetamine (Fig. 1), two widely abused drugs joined in their high addictive potential and reactive amine moiety, have well-known toxicity regardless of their ability to mediate protein glycation. Upon exposure, methamphetamine spurs excessive release and signaling of all monoamine neurotransmitters within the CNS through a variety of molecular mechanisms.38,39 Acute methamphetamine binges produce diffuse neuronal damage which compromises dopaminergic signaling, however, the ramifications of chronic, low dose exposure and the processes through which methamphetamine causes damage to the cardiovasculature and periphery are ambiguous.38,40,41 Of particular interest is how the long-term pathological consequences of methamphetamine abuse mirror the cardiovascular complications arising from AGE generation. Not only does the amine functionality of methamphetamine permit its participation in the Maillard reaction (Scheme 3), but also its long serum half-life (t1/2 = 12 h) in comparison to other psychostimulants and its frequent administration by addicts support the hypothesis that methamphetamine-derived AGEs are generated at experimentally detectable and pathologically relevant levels.
Scheme 3.
Methamphetamine protein glycation as initiated by methamphetamine and glucose.
The AGEs formed in disease states such as macular degeneration and diabetes mellitus have been linked to autoantibody titers against the glycated proteins, and indeed, the process of attaching nonimmunogenic chemical moieties to a carrier protein is reminiscent of hapten preparation in a research setting for vaccination.27,42 Before chronic methamphetamine abuse could be linked to the appearance of AGE-related pathologies, the generation of methamphetamine-modified AGEs in vivo was illustrated through the detection of a polyclonal response against methamphetamine glycated serum albumin. Protein glycation by methamphetamine was conducted in vitro through the incubation of methamphetamine with glucose at physiological conditions, with the Amadori rearrangement product was detected after 12 h by LC/MS. Addition of BSA to the starting reaction caused the methamphetamine-derived albumin glycation products to form after two weeks, as assessed by ELISA and dot-blot. Immunization of mice with experimentally-prepared methamphetamine-glycated mouse serum albumin (MSA) elicited serum antibodies against methamphetamine-conjugated AGEs (METH-AGEs) that were detectable via ELISA. To verify that this response was indeed specific to the METH-AGEs and distinct from any potentially immunogenic effect of MSA-AGE injected in mice, competition studies with MSA and MSA-AGE were employed to show that serum antibodies failed to recognize these substrates. Furthermore, the polyclonal sera measurably bound METH-AGEs alone (Kd,app ~ 10 mM), suggesting that continued immune system priming with methamphetamine and the sustained production of METH-AGE would serve to augment the polyclonal response as well as initiate an immunoactivated or inflammatory state.
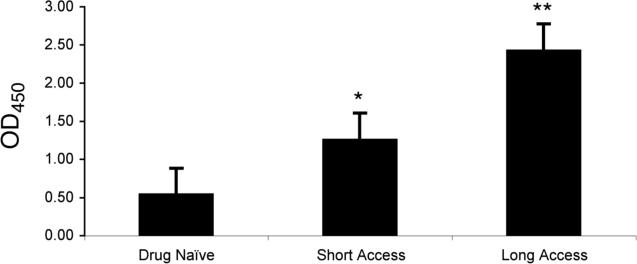
To investigate the potential significance of these results with respect to methamphetamine addiction in humans, a chronic schedule of methamphetamine self-administration was implemented in a rat model.43,44 Two groups of methamphetamine-addicted rats were allowed to self-administer methamphetamine for approximately 3 months in daily drug sessions lasting either 1 h (short access rats: ShA) or 6 h (long access rats: LgA). Gratifyingly, the level of drug intake by the different groups directly correlated with the serum antibody titers against methamphetamine glycated proteins (Fig. 4). Moreover, comparison of ELISA signals between LgA, ShA, and drug naïve (DN) sera samples showed that there was no statistically significant difference in serum antibody binding to either serum albumin or methamphetamine-unmodified glycated protein ELISA substrates. The methamphetamine dose-dependence and relative specificity of the LgA and ShA serum antibodies confirmed that the polyclonal response was attributable to methamphetamine self-administration and subsequent methamphetamine-mediated glycation.
Figure 4.
Quantification of ELISA results for presence of auto-antibodies against methamphetamine-glycated MSA in serum samples of rats self-administering methamphetamine. Bars represent ratio of ELISA signal above negative control background. * p<0.05, ** p<0.0002, comparison to drug naïve. Reproduced with permission from Proc. Natl. Acad. Sci. USA.45
The magnitude of the polyclonal response measured in LgA and ShA rats is suggestive of the in vivo significance of methamphetamine-mediated glycation. Namely, previous work into the neuronal and behavioral repercussions of methamphetamine in rodent models have relied on acute binge doses of drug, oftentimes experimenter-delivered, to evoke the measured phenotype.40 Recently, such studies have attracted scrutiny as scientists have realized the value of translational research in providing insight into the human condition.46 Our dosing regimen, in which LgA rats administered an average drug dose of 7 mg/kg per 6-hour session over a prolonged period of time, was designed to approximate the chronic abuse and level of methamphetamine intake documented for human addicts.46 While the 6-h drug exposure coupled with the half-life of methamphetamine in rats (t1/2 ≈ 1 h) translates to continual immune challenge by methamphetamine relative to 1-h ShA sessions, the low methamphetamine intake of ShA rats was still sufficient to elicit measurable antibody titers, a testament to both the repercussions of more recreational methamphetamine abuse and the relevance of METH-AGE immunogenicity.
IMMUNOMODULATION BY AGEs
Previous work has elucidated the immunosuppressive properties of methamphetamine, which is consistent with many abused drugs, while research into the inflammation or immunoactivation caused by methamphetamine focuses on microgliosis, blood-brain barrier disruption, and neurotoxity from oxidative stress and inflammatory gene upregulation.47,48 We speculated that METH-AGE production would spur a constellation of effects including the corresponding polyclonal response against METH-AGE, METH-AGE deposition on vasculature, and receptor for AGE (RAGE) activation, and that these effects would induce altered cytokine expression levels in the periphery. With respect to the latter effect, the receptor for AGE (RAGE), a member of the immunoglobulin superfamily expressed on most cell types, precipitates the expression of inflammatory and prothrombotic genes upon AGE ligation.26,28 Whereas the impact of RAGE and inflammatory responses have been studied within the context of the chronic vascular disease seen in other disease states (e.g., diabetes, Alzheimer's disease, macular degeneration, nephropathy, retinopathy), this AGE-RAGE hypothesis of immunomodulation and vascular disease has not been investigated as a causative factor of human and nonhuman methamphetamine abuse pathologies.49,50
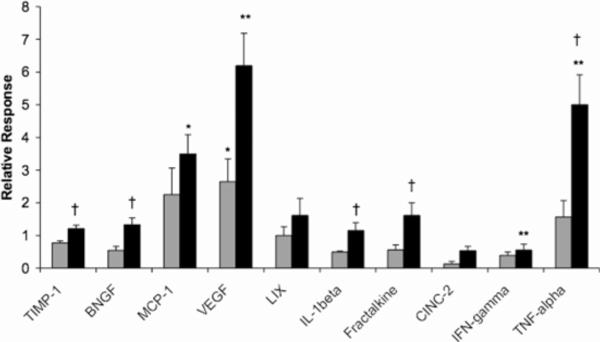
To attribute discrete physiological consequences to a chemical mechanism, namely methamphetamine-mediated glycation, immunomodulation was assessed through measuring the levels of several major cytokines and chemokines in sera from DN, ShA and LgA rats (Fig. 5).45 Both tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), two major proinflammatory cytokines that mediate macrophage activation and vascular permeance, were increased above basal levels in LgA rats. In particular, the substantial fivefold elevation of TNF-α aligns with its known roles in promoting antibody production as well as RAGE expression.51 Methamphetamine self-administration resulted in the dose-dependent upregulation of other cytokine molecules specifically related to AGE exposure. Most notably, the level of vascular endothelial growth factor (VEGF) exhibited a two-fold increase in ShA sera and a six-fold increase in LgA sera as compared to DN sera, an effect not previously reported alongside methamphetamine abuse. VEGF, whose expression is stimulated by AGE production, has been demonstrated to mediate many of the angiogenic activities of AGEs and to play a major role in the development of diabetic retinopathy and nephropathy.52
Figure 5.
Modulation of relevant cytokine and chemokine levels in response to methamphetamine intake. Sera samples analyzed were obtained from ShA rats (grey) and LgA rats (black) self-administering methamphetamine 1 h daily and 6 h daily, respectively, for a period 87 days. Data is expressed as mean fold-increase over drug naïve levels ± SEM. * p<0.05, ** p<0.01, significance in change compared to drug naïve levels. † P<0.05, H0: LgA>ShA. Reproduced with permission from Proc. Natl. Acad. Sci. USA.45
In conjunction with the above major cytokines, the levels of several chemokines were also altered, an indication of the magnitude of the proinflammatory response initiated by chronic drug-taking behavior. This finding mirrors the release of MCP-1 upon AGE exposure and RAGE activation.53 Other chemokines displayed a biphasic behavior with regard to drug dose, a pattern already observed in the varying expression of several cytokines. In sum, the fluctuation in both cytokine and chemokine levels was sensitive to the duration of methamphetamine self-administration, akin to the variance in antibody titers.
CONCLUSION
Our research into the chemical reactivity of exogenous drug molecules highlights how the formation of drug-glycated proteins in vivo becomes a marker of a developing disease pathology. Whereas both endogenous and drug-modified AGEs have been shown to serve as immunological epitopes in vivo, the accumulation of drug-modified AGEs gains additional relevance to the chronic drug addict in its ability to vaccinate the addict against the unmodified drug molecule.
Though the mechanism behind the pathogenesis of certain diseases observed in addicts is undoubtedly multifaceted, the correlation between the generation of AGEs, the polyclonal response to AGEs, and the development of a detectable pathology illustrates the value in turning to chemistry for mechanistic explanations of biological phenomena. Despite potential kinetic and thermodynamic hurdles to the formation of drug-AGEs such as drug availability and conjugation of AGEs to long-lived proteins, there is strong evidence that drug-mediated glycation processes are operable and relevant in vivo. Thus, future work will strive to clarify the causative link between AGE formation and the downstream immunomodulatory and pathological effects. A better understanding of the pharmacodynamics of protein glycation when mediated by exogenous amine-containing compounds is necessary in order for this AGE hypothesis to influence the therapeutic treatment of pathologies attributed to AGEs.
ACKNOWLEDGMENT
We gratefully acknowledge the financial support of the Skaggs Institute for Chemical Biology and the National Institute on Drug Abuse (Grant DA 21939 to J.B.T., Grant DA 98590 to K.D.J.).
Biography
Jennifer B. Treweek received her BS degree in chemistry from Caltech (2004) and conducted research with Prof. Richard Roberts. In 2005, she joined the laboratory of Professor Janda as a graduate student at TSRI where she received a Pfeiffer Research Foundation Grant (2006) and an NIH NRSA Predoctoral Fellowship (2007). Her current research is focused on neuroscience and immunopharmacotherapy.
Tobin J. Dickerson received his BS degree in chemistry from the University of Virginia (1999) and PhD from The Scripps Research Institute (2004). In 2005, he was promoted to Assistant Professor in the Department of Chemistry at TSRI and WIRM. His research interests include the chemical reactivity of drugs of abuse, the interplay between small molecules and the immune system, approaches for the treatment of filarial infections, and technologies that modulate BoNT toxicity.
Kim D. Janda obtained a BS degree from the University of South Florida (1980) and a PhD (1984) from the University of Arizona. He is currently a Professor in the Chemistry and Immunology Departments at TSRI, where he is also a Skaggs Scholar, the Ely R. Callaway, Jr. Chair and Director of WIRM. Among his awards are the Alfred P. Sloan Fellow (1993) and the Arthur Cope Scholar (1999). He was a founder of CombiChem and Drug Abuse Sciences.
REFERENCES
- 1.Davis VE, Walsh MJ, Yamanaka Y. Augmentation of alkaloid formation from dopamine by alcohol and acetaldehyde in vitro. J. Pharmacol. Exp. Ther. 1970;174:401–412. [PubMed] [Google Scholar]
- 2.Wong C-H, Whitesides GM. Enzymes in Synthetic Organic Chemistry. Pergamon; Oxford: 1994. [Google Scholar]
- 3.Fessner W-D, Walter C. Top. Curr. Chem. 1996;184:98–183. [Google Scholar]
- 4.List B, Lerner RA, Barbas CF., III Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000;122:2395–2396. [Google Scholar]
- 5.Mukherjee S, Yang JW, Hoffmann S, List B. Asymmetric enamine catalysis. Chem. Rev. 2007;107:5471–5569. doi: 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]
- 6.List B. Biocatalysis and organocatalysis: asymmetric synthesis inspired by nature. Asymmetric Synth. 2007:161–165. [Google Scholar]
- 7.Dickerson TJ, Janda KD. Aqueous aldol catalysis by a nicotine metabolite. J. Am. Chem. Soc. 2002;124:3220–3221. doi: 10.1021/ja017774f. [DOI] [PubMed] [Google Scholar]
- 8.Chu FL, Yaylayan VA. FTIR monitoring of oxazolidinone formation and decomposition in a glycoaldehyde-phenylalanine model system by isotope labeling techniques. Carbohydr. Res. 2008 doi: 10.1016/j.carres.2008.10.011. in press. [DOI] [PubMed] [Google Scholar]
- 9.Maillard LC, Gautier M. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C. R. Seances Acad. Sci. III. 1912;154:66–68. [Google Scholar]
- 10.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 11.Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 12.Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 13.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA. 1984;91:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stopper H, Schinzel R, Sebekova K, Heidland A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett. 2003;190:151–160. doi: 10.1016/s0304-3835(02)00626-2. [DOI] [PubMed] [Google Scholar]
- 15.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 1998;116:1629–1632. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- 17.Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGE and their interaction with AGE-receptors in vascular disease and diabetes. I. The AGE concept. Cardiovasc. Res. 1998;37:586–600. doi: 10.1016/s0008-6363(97)00233-2. [DOI] [PubMed] [Google Scholar]
- 18.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- 19.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 21.Monnier VM, Sell DR, Dai Z, Nemet I, Collard F, Zhang J. The role of the amadori product in the complications of diabetes. Ann. N. Y. Acad. Sci. 2008;1126:81–88. doi: 10.1196/annals.1433.052. [DOI] [PubMed] [Google Scholar]
- 22.Khalifah RG, Todd P, Booth AA, Yang SX, Mott JD, Hudson BG. Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry. 1996;35:4645–4654. doi: 10.1021/bi9525942. [DOI] [PubMed] [Google Scholar]
- 23.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- 24.Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-amadori glycation pathways. J. Biol. Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 25.McPherson JD, H. SB, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry. 1988;27:1901–1907. doi: 10.1021/bi00406a016. [DOI] [PubMed] [Google Scholar]
- 26.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 27.Turk Z, Ljubic S, Turk N, Benko B. Detection of autoantibodies against advanced glycation endproducts and AGE-immune complexes in serum of patients with diabetes mellitus. Clin. Chim. Acta. 2001;303:105–115. doi: 10.1016/s0009-8981(00)00389-2. [DOI] [PubMed] [Google Scholar]
- 28.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 29.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA. 1997;94:13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickerson TJ, Janda KD. A previously undescribed chemical link between smoking and metabolic disease. Proc. Natl. Acad. Sci. USA. 2002;99:15084–15088. doi: 10.1073/pnas.222561699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isomura S, Wirsching P, Janda KD. An immunotherapeutic program for the treatment of nicotine addiction: Hapten design and synthesis. J. Org. Chem. 2001;66:4115–4121. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- 32.Kyerematen GA, Morgan M, Chattopadhyay B, DeBethizy JD, Vesell ES. Disposition of nicotine and eight metabolites in smokers and nonsmokers: identification in smokers of two metabolites that are longer lived than cotinine. Clin. Pharmacol. Ther. 1998;48:641–651. doi: 10.1038/clpt.1990.208. 48. [DOI] [PubMed] [Google Scholar]
- 33.Salomon AR, Marcinowski KJ, Friedland RP, Zagorski MG. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry. 1996;35:13568–13578. doi: 10.1021/bi9617264. [DOI] [PubMed] [Google Scholar]
- 34.Sisodia SS, Price DJ. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- 35.Zeng H, Zhang Y, Peng L, Shao H, Menon NK, Yang J, Salomon AR, Freidland RP, Zagorski MG. Nicotine and amyloid formation. Biol. Psychiatry. 2001;49:248–257. doi: 10.1016/s0006-3223(00)01111-2. [DOI] [PubMed] [Google Scholar]
- 36.Tjernberg LO, Näslund J, Lindqvist F, Johansson J, Karlström AR, Thyberg J, Terenius L, Nordstedt C. Arrest of β-Amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 1996;271:8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]
- 37.Dickerson TJ, Janda KD. Glycation of the amyloid β-protein by a nicotine metabolite: A fortuitous chemical dynamic between smoking and Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2003;100:8182–8187. doi: 10.1073/pnas.1332847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders C, Ferrer JV, Shi L, Chen J, G. M, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl. Acad. Sci. USA. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itzhak Y, Achat-Mendes C. Methamphetamine and MDMA (ecstasy) neurotoxicity: ‘of mice and men.’. IUBMB Life. 2004;56:249–255. doi: 10.1080/15216540410001727699. [DOI] [PubMed] [Google Scholar]
- 41.Melega WP, Jorgensen MJ, acutean GL, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the Vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- 42.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 44.Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treweek J, Wee S, Koob GF, Dickerson TJ, Janda KD. Self-vaccination by methamphetamine glycation products chemically links chronic drug abuse and cardiovascular disease. Proc. Natl. Acad. Sci. USA. 2007;104:11580–11584. doi: 10.1073/pnas.0701328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- 47.Asanuma M, Miyazaki I, Higashi Y, Tsuji T, Ogawa N. Specific gene expression and possible involvement of inflammation in methamphetamine-induced neurotoxicity. Ann. N. Y. Acad. Sci. 2004;1025:69–75. doi: 10.1196/annals.1316.009. [DOI] [PubMed] [Google Scholar]
- 48.In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J. Toxicol. Environ. Health A. 2005 doi: 10.1080/15287390500177156. [DOI] [PubMed] [Google Scholar]
- 49.He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am. J. Forensic Med. Pathol. 1996;17:155–62. doi: 10.1097/00000433-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Schaiberger PH, Kennedy TC, Miller FC, Gal J, Petty TL. Pulmonary hypertension associated with long-term inhalation of “crank” methamphetamine. Chest. 1993;104:614–616. doi: 10.1378/chest.104.2.614. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycosylation end products is induced by the glycation product themselves and TNF-alpha through nuclear factor kappa B and by 17-beta-estradiol through Sp1 in human vascular endothelial cells. J. Biol. Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 52.Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J. Biol. Chem. 2001;276:43836–43841. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- 53.Yamagishi S.-i., Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, Makita Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J. Biol. Chem. 2002;277:20309–20315. doi: 10.1074/jbc.M202634200. [DOI] [PubMed] [Google Scholar]