Abstract
For decades, extensive research has explored the association between factors related to energy balance and the development of both colorectal cancer and prostate cancer. Physical inactivity, obesity, higher red meat consumption or Western pattern diet, insulin and insulin-like growth factors (IGFs) appear to increase the risk of colorectal cancer while obesity, high animal fat intake, insulin and IGFs have been associated with increasing prostate cancer risk and/or aggressiveness. Recently, there are growing observational data on the relationship between energetic host factors and progression of these cancers. While there are no large randomized trials in either colorectal cancer or prostate cancer assessing these factors on disease progression or disease-related mortality, the data supporting associations between some of these factors and colorectal or prostate cancer survivorship are getting more compelling. This article will evaluate the emerging data on energy balance in patients with colorectal or prostate cancer.
INTRODUCTION
The American Cancer Society has convened three expert panels in the past decade to critically examine the data on nutrition and physical activity in cancer survivors.1–3 In 2001, the panel reported: “For many of the most important nutrition and physical activity questions faced by cancer survivors, the scientific evidence comes only from in vitro and laboratory animal data or anecdotal reports from poorly designed clinical studies.”1 Moreover, the findings from these studies are often contradictory. Very few controlled clinical trials have been done to test the impact of diet, nutritional supplements, or nutritional complementary methods on cancer outcomes among cancer survivors. Only a few observational epidemiologic studies have examined the relationship between nutritional factors and cancer outcomes.1
While randomized trials are still few in number, increasing data from observational studies have allowed the panel to provide more guidance in the last two reports in 2003 and 2006.2,3 Three cancers that have gained appreciably more data are breast, colorectal, and prostate cancer; breast cancer will be discussed in a separate review in this issue, and we have been asked to review the literature related to colorectal and prostate cancer. Colorectal cancer is estimated to affect 146,970 people in the United States4 and 1,023,152 people worldwide5 each year. Prostate cancer is diagnosed in 192,280 men in the United States4 and 679,000 men globally5 annually. In both diseases, greater than 50% of those diagnosed will be long-term survivors from their cancer. Further, both diseases have seen continued improvements in median overall survival in patients with metastatic disease.6,7
Cancer patients and survivors continually seek information on ways to improve their outcomes after diagnosis. Factors that influence energy balance may impact on the outcomes of patients with colorectal and prostate cancer. For this review, we will consider data on energy balance in colorectal cancer and prostate cancer, including physical activity, body mass, change in weight, and dietary factors, as related to disease outcomes. While there are important data on the impact of these factors on quality of life, physical functioning, and tolerance to chemotherapy, this review will be primarily limited to human data on associations between these factors and disease recurrence or progression and mortality.
COLORECTAL CANCER
Epidemiologic and scientific research indicates that diet and other lifestyle factors have a significant influence on the risk of developing colorectal cancer.8 Host factors that influence energy balance, including obesity, certain diets, and physical inactivity, increase one's risk of developing colorectal cancer. Until recently, it was largely unknown if any of these modifiable factors influence the outcomes of patients already diagnosed with colorectal cancer. However, data are emerging that these factors indeed may impact on disease outcomes and mortality in colorectal cancer survivors.
Physical Activity
Extensive research has examined the association between physical activity and the primary risk of colon and/or colorectal cancer. In a recent meta-analysis of 52 studies, Wolin et al9 reported an inverse association between physical activity and primary colon cancer with an overall relative risk of 0.76 (95% CI, 0.72 to 0.81), comparing the most to least active individuals across studies. No association has been observed between physical activity and the primary risk of rectal cancer.10
In contrast, there are only limited data on the impact of exercise on cancer recurrences/progression and mortality in survivors of colorectal cancer. In a study of prediagnosis physical activity and disease outcomes, 526 survivors of colorectal cancer were identified from a cohort of 41,528 Australians that had a previous assessment of physical activity.11 Analyses adjusted for some prognostic factors showed that activity levels were associated with improved disease-specific survival in the entire cohort (hazard ratio [HR], 0.73; 95% CI, 0.54 to 1.00) although the association was largely restricted to stage II and III tumors (HR, 0.49; 95% CI, 0.30 to 0.79). In an ancillary study from the same cohort, survivors of colorectal cancer who were physically active before diagnosis were found to have higher insulin-like growth factor binding protein-3, which was associated with a significant reduction in disease-specific death (HR, 0.52; 95% CI, 0.33 to 0.83).12 The authors suggest that a possible mechanism for the association between physical activity and disease-specific survival in survivors of colorectal cancer may be through the insulin-like growth factor axis, particularly insulin-like growth factor binding protein-3.
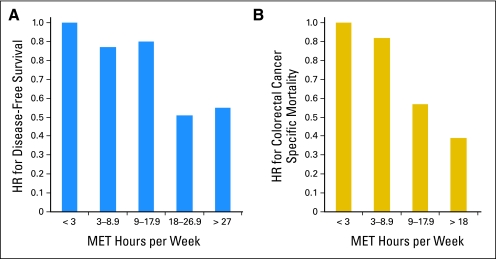
Three studies have examined the association between postdiagnosis physical activity and disease outcomes in survivors of colorectal cancer.13–15 The first is the Cancer and Leukemia Group B (CALGB) observational study of 832 patients with stage III colon cancer enrolled in a randomized adjuvant chemotherapy trial (CALGB 89803) and observed for a median of 3.8 years.15 Higher levels of self-reported physical activity approximately 6 months after completion of chemotherapy (18+ metabolic equivalent task [MET] hours/wk) were associated with superior disease-free, recurrence-free, and overall survival (Fig 1A), adjusted for known prognostic factors, including body mass index (BMI). For reference, 3 MET hours is equivalent to 1 hour average pace walking.
Fig 1.
Physical activity after diagnosis and (A) disease-free survival15 or (B) colorectal cancer–specific mortality.13 HR, hazard ratio; MET, metabolic equivalent task.
The second postdiagnosis observational exercise study utilized the Nurses' Health Study and included 573 women diagnosed with stages I to III colorectal cancer who self-reported leisure-time physical activity before diagnosis and 1 to 4 years after diagnosis.13 Significant 40% to approximately 60% lower risk of both colorectal cancer–specific mortality (Fig 1B) and overall mortality were observed among patients with increasing postdiagnosis physical activity, adjusted for other prognostic factors. In that study, level of physical activity before diagnosis was not associated with mortality. Finally, a study was recently published that demonstrated comparable benefit of exercise in colorectal cancer survivors from the Health Professionals Follow-Up Study.14 Among 668 men with stage I to III colorectal cancer, more than 27 MET hours per week of exercise had an adjusted hazard ratio for colorectal cancer–specific mortality of 0.47 (95% CI, 0.24 to 0.92) compared with men who engaged in 3 or fewer MET hours per week.
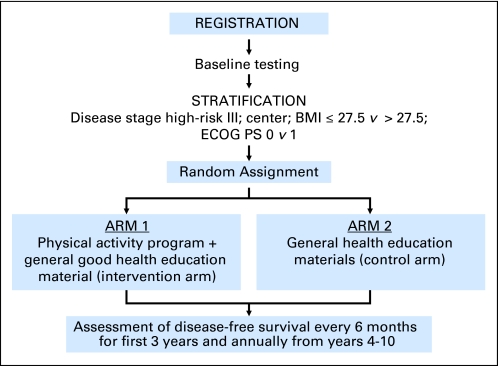
Based on these data, the Colon Health and Life-Long Exercise Change (CHALLENGE) trial was developed as a multinational collaboration between Canada and Australia to determine the effects of a 3-year structured physical activity intervention on disease outcomes in 962 high-risk stage II and III colon cancer survivors who have completed adjuvant chemotherapy within the previous 2 to 6 months (Fig 2).16 The primary end point was disease-free survival and secondary end points included patient-reported outcomes, health-related fitness, biologic correlative markers, and an economic analysis. The trial is currently open to accrual.
Fig 2.
Colon Health and Life-Long Exercise Change Trial (CHALLENGE) schema. BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status.
Several observational studies have shown that higher physical activity levels or meeting physical activity guidelines is associated with better patient-reported quality of life, physical functioning, and fatigue.17–22 Only one randomized trial has investigated the effects of an exercise intervention in colorectal cancer survivors.23 That study suffered from contamination in the comparison group that undermined the intention-to-treat analyses. In a secondary analysis, participants whose fitness increased over the course of the intervention, regardless of group assignment, reported significantly improved quality of life, physical functioning, and psychosocial distress compared to participants whose fitness decreased.
Body Mass and Change in Weight
Despite the relatively consistent evidence that increasing body mass index influences the risk of developing colorectal cancer,24 prospective observational cohort studies regarding the impact of obesity on clinical outcomes in nonmetastatic colorectal cancer has been less certain (Table 1).25–30 In a large randomized trial of adjuvant chemotherapy for stage II and III colon cancer (INT 0089), obese women (BMI ≥ 30 kg/m2) experienced a 24% nonstatistically significant worse disease-free survival compared with normal weight women (BMI, 21 to 24.9 kg/m2); in contrast, BMI was not related significantly to long-term outcomes among male patients in that cohort.26 Among patients enrolled in two different adjuvant chemotherapy trials for colon cancer, very obese patients (BMI ≥ 35 kg/m2) experienced a 27% statistically significant increase in cancer recurrence or death compared with normal weight participants; no sex interaction was detected.28 In a third study using CALGB 89803, patients with BMI ≥ 35 kg/m2 experienced a multivariate hazard ratio for disease-free survival of 1.24 (95% CI, 0.84 to 1.83), compared with normal weight individuals.29
Table 1.
Summary of Studies of BMI in Patients With Colorectal Cancer
| First Author | Year of Diagnosis | No. of Patients and Stage of Disease | Outcome Measure | Hazard Ratio or P (compared with normal weight) | Variables Controlled in Multivariate Analysis |
|---|---|---|---|---|---|
| Tartter25 | 1976-1979 | 279 colon cancer Duke B2, C1, C2 | Recurrence rate | P = .003 for above median weight | Only univariate analyses presented |
| Meyerhardt26 | 1988-1992 | 3,759 colon cancer Duke B2, B3, C | Disease-free survival | 1.11 (95% CI, 0.94 to1.30); BMI ≥ 30 kg/m2 | Age, sex, race, performance status, clinical bowel obstruction or perforation at presentation, stage of disease, presence of peritoneal implants, and whether the patient completed adjuvant therapy |
| Overall survival | 1.11 (95% CI, 0.96 to 1.29) BMI ≥ 30 kg/m | ||||
| Meyerhardt27 | 1990-1992 | 1,792 rectal cancer, stage II and III | Disease-free survival | 1.10 (95% CI, 0.91 to 1.32) BMI ≥ 30 kg/m2 | Age, sex, race, performance status, bowel obstruction, extent of bowel wall invasion, and number of positive lymph nodes , operation type |
| Overall survival | 1.09 (95% CI, 0.90 to 1.33) BMI ≥ 30 kg/m2 | ||||
| Local recurrences | 1.31 (95% CI, 0.91 to 1.88) BMI ≥ 30 kg/m2 | ||||
| Dignam28 | 1989-1994 | 4,288 colon cancer Duke B and C | Disease-free survival | 1.06 (95% CI, 0.93 to 1.21) BMI 30-34.9 kg/m2 | Age, sex, race, number of positive lymph nodes, performance status, presence of bowel obstruction |
| 1.27 (95% CI, 1.05 to 1.53) BMI ≥ 35 kg/m2 | |||||
| Colon cancer events | 1.04 (95% CI, 0.88 to 1.24) BMI 30-34.9 kg/m2 | ||||
| 1.38 (95% CI, 1.10 to 1.73) BMI ≥ 35 kg/m2 | |||||
| Meyerhardt29 | 1999-2001 | 1,053 colon cancer stage III | Disease-free survival | 1.00 (95% CI, 0.72 to 1.40) BMI 30-34.9 kg/m2 | Age, sex, depth of invasion through bowel wall, number of positive lymph nodes, presence of clinical perforation or bowel obstruction at time of surgery, performance status, treatment, weight change approximately 1 year after surgery, smoking status, and physical activity level |
| 1.24 (95% CI, 0.84 to 1.83) BMI ≥ 35 kg/m2 | |||||
| Recurrence-free survival | 0.97 (95% CI, 0.69 to 1.37) BMI 30-34.9 kg/m2 | ||||
| 1.27 (95% CI, 0.85 to 1.89) BMI ≥ 35 kg/m2 | |||||
| Overall survival | 0.90 (95% CI, 0.61 to 1.34) BMI 30-34.9 kg/m2 | ||||
| 0.87 (95% CI, 0.54 to 1.42) BMI ≥ 35 kg/m2 |
Abbreviation: BMI, body mass index.
Only one small study has utilized a measure of adiposity besides BMI. Among 161 patients with resected colorectal cancer, Moon et al30 found that high visceral fat area to subcutaneous fat area ratio as measured from the digital images of patients' computed tomography taken before the surgery resulted in significantly worse disease-free survival (P = .008).
One study has addressed the impact of weight change on recurrence in colorectal cancer survivors.29 In the CALGB 89803 cohort, increasing weight after diagnosis (between time on adjuvant therapy and 6 months after completion of adjuvant therapy) was not associated with disease-free or overall survival.29
Diet
While various dietary factors have been associated with colorectal cancer development, few studies have assessed the influence of diet on colon cancer recurrence and survival.31–33 Using data from 411 patients with colon cancer from two population-based case-control studies, Slattery et al31 observed an improved survival with increasing consumption of calories, fat, and protein. Among 148 patients with colorectal cancer, Dray et al33 reported improved survival with increasing consumption of calories based on dietary information before diagnosis. Both of these two studies were limited by their small sample size, heterogeneous patient population that included all stages of disease, inability to adjust for cancer treatment, and limited capacity to adjust for other prognostic factors.
The largest prospective observational study on diet in colon cancer survivors utilized the cohort from CALGB 89803 in which participants completed a food frequency questionnaire during adjuvant therapy and 6 months after the completion of adjuvant therapy.32 Two major dietary patterns were identified, prudent and Western pattern, by factor analysis. The prudent pattern was characterized by high fruit and vegetable, poultry and fish intakes; the Western pattern was characterized by high meat, fat, refined grains, and dessert intakes. All patients were assigned a relative value along the spectrum of both dietary patterns and the two patterns were not correlated with each other. Compared with patients in the lowest quintile of Western pattern diet, those in the highest quintile experienced worse disease-free survival, with an adjusted HR of 3.25 (95% CI, 2.04 to 5.19; P for trend < .001). In contrast, the prudent pattern diet was not significantly associated to cancer recurrence or mortality.
PROSTATE CANCER
Prostate cancer is the most frequent and second most lethal malignancy in men.4 Survival after prostate cancer diagnosis can often exceed a decade and fewer than 5% of men without metastatic disease at diagnosis will die from prostate cancer within the first 5 to 10 years after diagnosis.34,35 While early cancer detection by prostate-serum antigen (PSA) identifies many patients at early curable stage, overtreatment may also occur among men with indolent cancer that may never progress. There is an urgent need to identify factors (including diet and lifestyle) that may influence the aggressiveness of the tumor.
Adiposity and Clinical Significant Prostate Cancer
More than 20 prospective studies have consistently demonstrated an increased prostate cancer mortality rate among men with higher BMI,36–40 but most did not distinguish whether this association is due to an increased risk of having an aggressive disease at diagnosis or worse outcomes after initial diagnosis. This distinction is especially important because heavy men are less likely to receive PSA or digital rectal exam screening.40 Further, obesity-related hemodilution reduces the sensitivity of PSA testing.41,42 The National Institutes of Health-AARP Diet and Health Study and Health Professionals Follow-Up Study considered PSA screening and reported obese men (≥ 30 kg/m2) had a significant approximately two-fold increase in prostate cancer mortality after controlling for screening.36,40 In a series of men treated with radical prostatectomy by a single surgeon, more than half of the obese men had preoperative PSA velocity ≥ 2 ng/mL per year compared with one third or fewer of men with lower BMI.43 These observations strongly suggest that prostate cancer in obese men may behave more biologically aggressive, independent of screening behavior.
Most,44–51 but not all,52,53 clinical studies among patients undergoing prostatectomy or radiotherapy suggest that obesity at the time of prostate cancer diagnosis is associated with higher risk of post-treatment PSA failure (Table 2).54–58 Among eight studies that have examined the association of BMI with prostate cancer–specific mortality,34,35,46,54–57 five reported a positive association.46,54–58 In Gong et al,56 for example, men with a BMI greater than 30 kg/m2 were 2.6 times more likely to die of prostate cancer after controlling for age at diagnosis, race, smoking status, Gleason grade and clinical stage, and primary treatment.
Table 2.
Summary of Studies of BMI and Prostate Cancer–Specific Mortality
| First Author | Year of Diagnosis | No. of Patient and Prior Treatment | Type and No. of Outcomes | Hazard Ratio or P (compared with normal weight) | Variables Controlled in Multivariate Analysis |
|---|---|---|---|---|---|
| Siddiqui34 | 1990-1999 | 5,135 (prostatectomy) | 290 progression; 151 fatal prostate cancer | BMI was not associated with prostate cancer mortality or total mortality | Gleason score, PSA, surgical margin, seminal vesicle invasion, adjuvant treatment |
| Gong54 | 1993-2004 | 752 (any prior therapy) | 50 fatal prostate cancer | 2.64 (95% CI, 1.18 to 2.92); BMI ≥ 30 kg/m2 | Age, race, smoking, treatment, Gleason score, stage |
| 64 other deaths | 1.62 (95% CI, 0.75 to 3.47); BMI ≥ 30 kg/m2 | ||||
| Palma55 | 1993-2001 | 706 (radiation) | 292 biochemical progression | P = .02 BMI ≥ 30 kg/m2 | Age, PSA, Gleason, tumor stage, risk group, treatment |
| Overall survival (No. of deaths NR) | P = .008 BMI ≥ 30 kg/m2 | ||||
| Efstathiou46 | 1987-1992 | 788 (RTOG 85-31) | 169 fatal prostate cancer | 1.52 (95% CI, 1.02 to 2.28); BMI 25-30 kg/m2 | Age, race, Gleason, stage, treatment |
| 1.64 (95% CI, 1.01 to 2.66) BMI ≥ 30 kg/m2 | |||||
| Merrick56 | 1995-2003 | 1,093 (brachytherapy) | 12 fatal prostate cancer | NS for BMI ≥ 30 kg/m2 | Age and smoking relate to low overall survival |
| 128 total death | NS for BMI ≥30 kg/m2 | ||||
| Smith57 | 1992-2002 | 1,554 (radiation and ADT) | 210 fatal prostate cancer | 1.77 (95% CI, 1.22 to 2.55); highest v lowest tertile of weight | Age, race, Gleason, stage, PSA, treatment, diabetes |
| 765 total death | NS | ||||
| Ma58 | 1982-2007 | 2,546 (any prior therapy) | 281 fatal prostate cancer | 1.57 (95% CI, 1.11 to 2.24); BMI 25-30 kg/m2 | Age, smoking, Gleason, stage Gleason, clinical stage, time between BMI measurement to prostate cancer diagnosis, and competing deaths |
| 2.50 (1.22-5.13) BMI ≥ 30 kg/m2 | |||||
| 1991-2007 | 2,078 (any prior therapy) | 134 fatal prostate cancer | 1.61 (95% CI, 1.12 to 2.32); BMI 25-30 kg/m2 | ||
| 2.23 (95% CI, 1.07 to 4.64) BMI ≥ 30 kg/m2 | |||||
| Davies35 | 1995-2007 | 7,274 (any prior therapy) | 220 fatal prostate cancer | NS for BMI 30-34.9 and BMI ≥ 35 kg/m2 | Age, clinical risk category, diabetes, surgery v no surgery |
| 1,044 total deaths | NS for BMI 30-34.9 and BMI ≥ 35 kg/m2 |
Abbreviations: BMI, body mass index; PSA, prostate serum antigen; NR, not reported; RTOG, Radiation Therapy Oncology Group; NS, not significant; ADT, androgen deprivation therapy.
In contrast, three large, prospective cohort studies have not shown an association between BMI and prostate cancer outcomes.34,35,56 For example, in a multi-institutional cohort of 7,274 men with localized prostate cancer treated with definitive therapy, there were no significant associations between BMI and biochemical progression or the need for secondary treatment, prostate cancer survival, or overall survival.35 Many clinical studies suffer from their inability to account for reverse causality and residual confounding, patient selection biases and competing risks. To better control for the above mentioned issues, Ma et al58 recently assessed the association of prediagnostic BMI collected at baseline and 8 years follow-up in a cohort of 2,546 male participants. Compared with those with baseline BMI under 25 kg/m2, men with BMI 25 to 29.9 kg/m2 and BMI ≥ 30 kg/m2 had 1.4-fold (95% CI, 1.1 to 1.8) and 2.6-fold (95% CI, 1.6 to 4.3) higher risk of prostate cancer mortality, respectively, controlling for age at diagnosis, smoking status, and competing cause of deaths (P trend = .0008).
Despite its effectiveness in the treatment of locally advanced and high-grade localized prostate cancer, long-term androgen deprivation therapy (ADT) causes a number of metabolic consequences including increased fat mass and decreased lean body mass,59,60 and increased fasting glucose and hyperinsulinimia.61 Men undergoing long-term ADT (≥ 12 months) have higher prevalence of diabetes and higher cardiovascular mortality.7,62–66 Use of ADT has nearly doubled over the past two decades.67,68 If obesity and related metabolic changes are considered part of the driving force for aggressive tumor behavior, these findings raise the speculative possibility that ADT-related weight gain and hyperinsulinemia may favor aggressive androgen-independent disease progression. It also underscores the need to develop strategies to prevent these metabolic changes, which may help to prevent not only prostate cancer progression but also ADT treatment-related diabetes and cardiovascular disease.
Physical Activity
A significant amount of research has examined the association between physical activity and the primary risk of prostate cancer, but there are no meta-analyses quantifying this association. In one recent summary of only cohort studies, Johnsen et al69 noted that nine of 22 studies found an inverse association between physical activity and prostate cancer risk, 12 reported no association, and one reported a significant positive association. Based on these inconsistent results, the World Cancer Research Fund and American Institute for Cancer Research concluded that the evidence for an association between physical activity and prostate cancer risk is inconclusive.70
No studies have examined the association between postdiagnosis physical activity and disease outcomes in prostate cancer survivors. Several observational and intervention studies have examined physical activity and health-related fitness or patient-reported outcomes in prostate cancer survivors.71 These studies have consistently demonstrated that physical activity is positively associated with, or has positive effects on, muscular strength, lean body mass, patient-reported quality of life, physical functioning, and fatigue. In a recent trial, Segal et al72 examined the effects of 24 weeks of resistance or aerobic training versus usual care on fatigue, quality of life, physical fitness, body composition, PSA, testosterone, hemoglobin, and lipid levels in 121 patients with prostate cancer receiving radiotherapy. Results showed that both resistance and aerobic exercise improved fatigue over the short-term, however, only resistance exercise improved fatigue over the longer term and provided additional improvements in quality of life, muscular strength, body fat, and triglycerides.
Dietary Factors and Prostate Cancer Progression
Most reviews of diet and prostate cancer progression have suggested that dietary saturated fat is detrimental whereas plant-based diet could be beneficial for prostate cancer progression.73–77 This notion is supported by data from two prospective observational studies, which found that patients with prostate cancer with higher intake of saturated fat had worse disease-specific survival78 or PSA failure after prostatectomy.79 In the later study, higher BMI and intake of saturated fat, but not total calorie intake, were both independent predictors of biochemical failure. Men who were both obese and consumed high saturated fat diet had the shortest biochemical failure–free survival (19 months), and nonobese men who consumed low saturated fat diets had the longest biochemical failure–free survival (46 months; P < .001).79
Of the nine randomized trials using diet and dietary supplement for the prevention of prostate cancer recurrence, only one study investigates the effect of low fat diet.77 In the Prostate Cancer Lifestyle Trial, 93 patients with early-stage, low-grade cancer under watchful waiting were randomly assigned to an experimental arm consisting of counseling toward a low-fat (10% of total calories), plant-based diet, increased exercise, and practice stress management, and group support sessions or to a control arm of usual care. At 1 year, none of the experimental group patients but six control patients underwent conventional treatment due to prostate cancer progression. PSA decreased 4% in the experimental group but increased 6% in the control group (P = .016).80 By 2 years of follow-up, 13 (27%) of 49 control patients and two (5%) of 43 experimental patients had undergone conventional prostate cancer treatment (P < .05). The authors conclude that patients with early-stage prostate cancer choosing active surveillance might be able to avoid or delay conventional treatment for at least 2 years by making changes in their diet and lifestyle.81
POTENTIAL MECHANISMS FOR THE ASSOCIATIONS BETWEEN ENERGY BALANCE AND COLORECTAL AND PROSTATE CANCER OUTCOMES
Increasing evidence supports the hypothesis that energy balance-related host factors influence colorectal and prostate cancer prognosis. For both cancers, one potential mechanism is related to hyperinsulinemia. Insulin and the insulin-like growth factor family have been associated with enhanced tumor growth and antiapoptosis.82 Cancer recurrences are believed to be growth of micrometastases. Thus, an environment that allows such microscopic tumors to proliferate could be detrimental. Cohort studies in colorectal and prostate cancer demonstrating that prediagnostic measurements of C-peptide,58,83 IGFBP 1,83 and adiponectin58 are associated with cancer-related mortality support the notion that aggressive neoplastic behavior may be manipulated by systemic metabolic factors. Further considerations of potential mechanisms are found in other reviews in this issue of the Journal of Clinical Oncology dedicated to host factors and cancer.
NEED FOR RANDOMIZED CONTROLLED TRIALS
To date, studies in colorectal and prostate cancer on energy balance as a host factor in tumor progression are primarily limited to preclinical and observational studies. Although efforts are made in these studies to account for reverse causality, biases, and residual confounding, ultimately randomized controlled trials are needed to provide definitive evidence on the causal effects of energy balance on disease outcomes in these patient populations. A limited number of these studies are developing. We hope that others will achieve funding and reach their accrual goal to further define the role of energetics in colorectal and prostate cancer. Nonetheless, colorectal and prostate cancers are typically diagnosed in older individuals for whom cardiovascular disease, type II diabetes, and hypertension are common comorbidities. Maintaining a healthy body weight, exercising, and controlling obesity-related metabolic risk factors may reduce not only cancer-specific mortality but also total mortality in cancer survivors.
Footnotes
Supported by the Canada Research Chairs Program (K.S.C.); and Grants No. R01 CA118553, P50 CA127003, P01CA87969, and P01 CA55075 (J.A.M.) from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey A. Meyerhardt, Jing Ma, Kerry S. Courneya
Manuscript writing: Jeffrey A. Meyerhardt, Jing Ma, Kerry S. Courneya
Final approval of manuscript: Jeffrey A. Meyerhardt, Jing Ma, Kerry S. Courneya
REFERENCES
- 1.Brown J, Byers T, Thompson K, et al. Nutrition during and after cancer treatment: A guide for informed choices by cancer survivors. CA Cancer J Clin. 2001;51:153–187. doi: 10.3322/canjclin.51.3.153. [DOI] [PubMed] [Google Scholar]
- 2.Brown JK, Byers T, Doyle C, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2003;53:268–291. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- 3.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Mike S, Harrison C, Coles B, et al. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 2006;18:CD005247. doi: 10.1002/14651858.CD005247.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: A meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samad AK, Taylor RS, Marshall T, et al. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 11.Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haydon AM, Macinnis RJ, English DR, et al. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 16.Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: A randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15:271–278. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 18.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3:215–226. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 19.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann Behav Med. 1999;21:171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BL, Trentham-Dietz A, Koltyn KF, et al. Physical activity and function in older, long-term colorectal cancer survivors. Cancer Causes Control. 2009;20:775–784. doi: 10.1007/s10552-008-9292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch BM, Cerin E, Owen N, et al. Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes Control. 2007;18:735–742. doi: 10.1007/s10552-007-9016-6. [DOI] [PubMed] [Google Scholar]
- 22.Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51:1242–1248. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 23.Courneya KS, Friedenreich CM, Quinney HA, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E. Diet, body weight, and colorectal cancer: A summary of the epidemiologic evidence. J Womens Health (Larchmt) 2003;12:173–182. doi: 10.1089/154099903321576574. [DOI] [PubMed] [Google Scholar]
- 25.Tartter PI, Slater G, Papatestas AE, et al. Cholesterol, weight, height, Quetelet's index, and colon cancer recurrence. J Surg Oncol. 1984;27:232–235. doi: 10.1002/jso.2930270407. [DOI] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 27.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: Findings from Intergroup trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 28.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 29.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 2008;15:1918–1922. doi: 10.1245/s10434-008-9891-4. [DOI] [PubMed] [Google Scholar]
- 31.Slattery ML, French TK, Egger MJ, et al. Diet and survival of patients with colon cancer in Utah: Is there an association? Int J Epidemiol. 1989;18:792–797. doi: 10.1093/ije/18.4.792. [DOI] [PubMed] [Google Scholar]
- 32.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 33.Dray X, Boutron-Ruault MC, Bertrais S, et al. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52:868–873. doi: 10.1136/gut.52.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006;107:521–529. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 35.Davies BJ, Smaldone MC, Sadetsky N, et al. The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol. 2009;182:112–117. doi: 10.1016/j.juro.2009.02.118. discussion 182:117, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Giovannucci E, Liu Y, Platz EA, et al. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–1279. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 38.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 40.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 41.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 42.Freedland SJ, Sun L, Kane CJ, et al. Obesity and oncological outcome after radical prostatectomy: Impact of prostate-specific antigen-based prostate cancer screening: Results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102:969–974. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 43.Loeb S, Yu X, Nadler RB, et al. Does body mass index affect preoperative prostate specific antigen velocity or pathological outcomes after radical prostatectomy? J Urol. 2007;177:102–106. doi: 10.1016/j.juro.2006.08.097. discussion 177:106, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 45.Bassett WW, Cooperberg MR, Sadetsky N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: Data from CaPSURE. Urology. 2005;66:1060–1065. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer: Analysis of RTOG 85-31. Cancer. 2007;110:2691–2699. doi: 10.1002/cncr.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 48.Mallah KN, DiBlasio CJ, Rhee AC, et al. Body mass index is weakly associated with, and not a helpful predictor of, disease progression in men with clinically localized prostate carcinoma treated with radical prostatectomy. Cancer. 2005;103:2030–2034. doi: 10.1002/cncr.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strom SS, Kamat AM, Gruschkus SK, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 50.Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11:6889–6894. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 51.Stroup SP, Cullen J, Auge BK, et al. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–1009. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 52.Chun FK, Briganti A, Graefen M, et al. Body mass index does not improve the ability to predict biochemical recurrence after radical prostatectomy. Eur J Cancer. 2007;43:375–382. doi: 10.1016/j.ejca.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 53.Gallina A, Karakiewicz PI, Hutterer GC, et al. Obesity does not predispose to more aggressive prostate cancer either at biopsy or radical prostatectomy in European men. Int J Cancer. 2007;121:791–795. doi: 10.1002/ijc.22730. [DOI] [PubMed] [Google Scholar]
- 54.Gong Z, Agalliu I, Lin DW, et al. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 55.Palma D, Pickles T, Tyldesley S. Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU Int. 2007;100:315–319. doi: 10.1111/j.1464-410X.2007.06897.x. [DOI] [PubMed] [Google Scholar]
- 56.Merrick GS, Galbreath RW, Butler WM, et al. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol. 2007;30:588–596. doi: 10.1097/COC.0b013e318068b506. [DOI] [PubMed] [Google Scholar]
- 57.Smith MR, Bae K, Efstathiou JA, et al. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92-02. J Clin Oncol. 2008;26:4333–4339. doi: 10.1200/JCO.2008.16.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 60.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 61.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 62.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 63.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 64.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 65.Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 66.Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 67.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 69.Johnsen NF, Tjonneland A, Thomsen BL, et al. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2009;125:902–908. doi: 10.1002/ijc.24326. [DOI] [PubMed] [Google Scholar]
- 70.World Cancer Res Fund/American Institute for Cancer Res . Washington DC: AICR; 2007. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. [Google Scholar]
- 71.Thorsen L, Courneya KS, Stevinson C, et al. A systematic review of physical activity in prostate cancer survivors: Outcomes, prevalence, and determinants. Support Care Cancer. 2008;16:987–997. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]
- 72.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 73.Kenfield SA, Chang ST, Chan JM. Diet and lifestyle interventions in active surveillance patients with favorable-risk prostate cancer. Curr Treat Options Oncol. 2007;8:173–196. doi: 10.1007/s11864-007-0034-0. [DOI] [PubMed] [Google Scholar]
- 74.Berkow SE, Barnard ND, Saxe GA, et al. Diet and survival after prostate cancer diagnosis. Nutr Rev. 2007;65:391–403. doi: 10.1111/j.1753-4887.2007.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 75.Demark-Wahnefried W. Dietary interventions in prostate cancer. Curr Urol Rep. 2008;9:217–225. doi: 10.1007/s11934-008-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22:187–199. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 77.Van Patten CL, de Boer JG, Tomlinson Guns ES. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: A review of the randomized controlled trial evidence. J Urol. 2008;180:2314–2321. doi: 10.1016/j.juro.2008.08.078. discussion 180:2721-2722, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Meyer F, Bairati I, Shadmani R, et al. Dietary fat and prostate cancer survival. Cancer Causes Control. 1999;10:245–251. doi: 10.1023/a:1008913307947. [DOI] [PubMed] [Google Scholar]
- 79.Strom SS, Yamamura Y, Forman MR, et al. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer. 2008;122:2581–2585. doi: 10.1002/ijc.23414. [DOI] [PubMed] [Google Scholar]
- 80.Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174:1065–1069. doi: 10.1097/01.ju.0000169487.49018.73. discussion 174:1069-1070, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: Results from two years of follow-up. Urology. 2008;72:1319–1323. doi: 10.1016/j.urology.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 82.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 83.Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]