Abstract
OBJECTIVE
This report presents data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network on care of and morbidity and mortality rates for very low birth weight infants, according to gestational age (GA).
METHODS
Perinatal/neonatal data were collected for 9575 infants of extremely low GA (22–28 weeks) and very low birth weight (401–1500 g) who were born at network centers between January 1, 2003, and December 31, 2007.
RESULTS
Rates of survival to discharge increased with increasing GA (6% at 22 weeks and 92% at 28 weeks); 1060 infants died at ≤ 12 hours, with most early deaths occurring at 22 and 23 weeks (85% and 43%, respectively). Rates of prenatal steroid use (13% and 53%, respectively), cesarean section (7% and 24%, respectively), and delivery room intubation (19% and 68%, respectively) increased markedly between 22 and 23 weeks. Infants at the lowest GAs were at greatest risk for morbidities. Overall, 93% had respiratory distress syndrome, 46% patent ductus arteriosus, 16% severe intraventricular hemorrhage, 11% necrotizing enterocolitis, and 36% late-onset sepsis. The new severity-based definition of bronchopulmonary dysplasia classified more infants as having bronchopulmonary dysplasia than did the traditional definition of supplemental oxygen use at 36 weeks (68%, compared with 42%). More than one-half of infants with extremely low GAs had undetermined retinopathy status at the time of discharge. Center differences in management and outcomes were identified.
CONCLUSION
Although the majority of infants with GAs of ≥24 weeks survive, high rates of morbidity among survivors continue to be observed.
Keywords: extremely low gestation, very low birth weight, morbidity, death
Over the previous 2 decades, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) has monitored trends in morbidity and mortality rates among very low birth weight (VLBW) infants born at the university centers that constitute the NRN.1–6 Increased VLBW infant survival rates have paralleled improvements in prenatal, obstetric, and neonatal care.7,8 NRN data suggest that a plateau in VLBW infant survival rates might have been reached, despite increased use of prenatal corticosteroid treatment, prenatal antibiotic treatment, and early neonatal surfactant treatment.6 Previous NRN reports presented patient characteristics, interventions, and outcomes according to birth weight (BW), with an upper limit of 1500 g. Such BW-specific data may be skewed by more-mature infants with growth restriction. The aim of this study was to evaluate management, hospital complications, and mortality rates among infants with gestational ages (GAs) of 22 to 28 weeks who were born at NRN centers between 2003 and 2007.
METHODS
Study Population and Clinical Outcomes
Infants born alive at NRN centers in 2003–2007 with GAs of 22 0/7 to 28 6/7 weeks and BWs of 401 to 1500 g were studied, including those with congenital anomalies. These infants were part of the NRN VLBW registry.1–6
Research personnel collected maternal pregnancy/delivery data soon after birth and infant data from birth to death, discharge/transfer, or 120 days of age (“status”). For infants with prolonged hospitalizations, limited information was collected up to 1 year. Definitions for maternal and infant characteristics were provided in a manual of operations. GA was determined as the best obstetric estimate by using ultrasonography and/or the date of the last menstrual period. Intrauterine growth restriction, defined as BW of <10th percentile for gender and GA, was determined by using growth charts published by Alexander et al.9 Morbidities were defined in earlier publications,1–6,10,11 including respiratory distress syndrome, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), early-onset and late-onset sepsis, necrotizing enterocolitis, patent ductus arteriosus, and retinopathy of prematurity (ROP). Respiratory distress syndrome was defined on the basis of clinical features and oxygen or respiratory support for ≥6 of the first 24 hours.
Three definitions of BPD were used, namely, traditional BPD (supplemental oxygen use at postmenstrual age [PMA] of 36 weeks); BPD determined by using the National Institutes of Health Workshop severity-based diagnostic criteria,12 and BPD determined according to physiologic definition.13 Surviving infants who were discharged or transferred before PMA of 36 weeks were classified on the basis of their status at 36 weeks, if status information was available, or oxygen use at discharge/transfer, if status information was not available. Unless noted otherwise, BPD refers to the traditional definition.
Revisions to data collection in 2006 included questions about maternal chorioamnionitis, placental pathologic conditions, nitric oxide use, and ibuprofen use and expanded data collection on birth resuscitation and neurologic, pulmonary, and ophthalmologic outcomes. In addition to ophthalmologic examination results and interventions, the following outcomes, defined in the manual of operations, were recorded: favorable in both eyes, severe ROP in either eye, or undetermined in either eye without severe ROP in either eye. Complete definitions are included in a footnote to Table 6. The registry was approved by the institutional review boards at each center.
TABLE 6.
Rates of Infections and Other Morbidities According to GA for VLBW Infants Who Were Born in NRN Centers Between January 1, 2003, and December 31, 2007, and Survived >12 Hours After Birth
| Characteristi | % (Range) | |||||||
|---|---|---|---|---|---|---|---|---|
| 22 wk (N = 62) |
23 wk (N = 496) |
24 wk (N = 1223) |
25 wk (N = 1426) |
26 wk (N = 1530) |
27 wk (N = 1811) |
28 wk (N = 1967) |
Total (N = 8515) |
|
| Early-onset sepsisa | 6 (0–67) | 4 (0–20) | 4 (0–9) | 2 (0–7) | 2 (0–6) | 2 (0–6) | 1 (0–4) | 2 (<1–4) |
| Meningitisb | 0 (0–0) | 5 (0–25) | 3 (0–12) | 4 (0–15) | 3 (0–9) | 1 (0–5) | 1 (0–5) | 3 (0–8) |
| Late-onset sepsisa,c | 58 (0–100) | 62 (0–86) | 55 (29–74) | 46 (24–67) | 35 (14–53) | 27 (15–52) | 20 (4–36) | 36 (18–51) |
| NECb | 5 (0–33) | 12 (0–50) | 15 (0–22) | 13 (5–24) | 9 (0–25) | 10 (0–21) | 8 (3–20) | 11 (4–19) |
| NEC managed medicallyd | 67 (50–100) | 31 (0–100) | 39 (0–100) | 52 (10–100) | 48 (17–100) | 47 (23–75) | 58 (0–100) | 48 (21–100) |
| NEC treated surgicallyd | 33 (0–50) | 69 (0–100) | 61 (0–100) | 48 (0–90) | 52 (0–83) | 53 (25–77) | 42 (0–100) | 52 (0–79) |
| PDAa | 55 (13–100) | 54 (21–100) | 60 (31–80) | 55 (25–92) | 48 (21–88) | 42 (14–80) | 32 (13–60) | 46 (26–78) |
| Indomethacin therapy for PDAe,f | 82 (0–100) | 73 (0–100) | 76 (25–96) | 72 (29–94) | 69 (7–94) | 68 (37–94) | 67 (31–95) | 71 (33–91) |
| Surgical treatment of PDAa,e | 50 (0–100) | 43 (0–77) | 40 (13–62) | 33 (13–65) | 24 (6–44) | 16 (0–33) | 12 (0–33) | 27 (10–41) |
| Infants in hospital at 28 d | N = 30 | N = 277 | N = 874 | N = 1197 | N = 1386 | N = 1683 | N = 1866 | N = 7313 |
| ROP examination performeda,g | 93 (50–100) | 91 (71–100) | 93 (50–100) | 94 (78–100) | 96 (67–100) | 95 (87–100) | 92 (68–99) | 94 (82–99) |
| ROP diagnoseda,h | 96 (50–100) | 88 (0–100) | 89 (50–100) | 79 (29–94) | 65 (20–81) | 49 (18–75) | 32 (5–56) | 59 (35–75) |
| ROP stage ≥3a,h | 57 (0–100) | 48 (0–100) | 42 (25–77) | 25 (11–54) | 14 (0–29) | 7 (0–14) | 3 (0–11) | 16 (6–28) |
| Intervention/surgical treatment for ROPa,h | 50 (0–100) | 40 (0–100) | 35 (17–58) | 17 (0–40) | 8 (0–21) | 4 (0–9) | 2 (0–7) | 12 (4–22) |
| Infants in hospital with weight measured at PMA of 36 wk | N = 24 | N = 215 | N = 736 | N = 976 | N = 1106 | N = 1231 | N = 1204 | N = 5492 |
| Growth failure at 36 wka,i | 92 (50–100) | 91 (0–100) | 85 (67–100) | 83 (63–100) | 79 (33–98) | 76 (42–98) | 73 (44–96) | 79 (59–97) |
| Cranial ultrasonography performed within 28 d after birth | 85 (50–100) | 92 (67–100) | 95 (87–100) | 97 (85–100) | 98 (92–100) | 98 (94–100) | 98 (90–100) | 97 (93–100) |
| Sonogram findings within 28 da,j | N = 53 | N = 454 | N = 1163 | N = 1385 | N = 1499 | N = 1781 | N = 1925 | N = 8260 |
| Normal | 32 (0–100) | 41 (13–74) | 49 (14–70) | 57 (30–84) | 65 (36–90) | 70 (50–83) | 77 (50–91) | 64 (43–79) |
| IVH grade 1 | 13 (0–40) | 9 (0–50) | 11 (0–43) | 9 (0–17) | 11 (0–23) | 10 (5–24) | 10 (0–32) | 10 (5–23) |
| IVH grade 2 | 13 (0–50) | 9 (0–25) | 9 (0–29) | 8 (2–19) | 5 (0–14) | 5 (0–14) | 4 (0–25) | 6 (2–12) |
| IVH grade 3 | 8 (0–33) | 15 (0–47) | 12 (5–20) | 8 (0–15) | 7 (0–14) | 6 (0–15) | 4 (0–10) | 7 (3–13) |
| IVH grade 4 | 30 (0–67) | 21 (0–50) | 14 (0–33) | 13 (3–36) | 7 (0–31) | 5 (1–17) | 3 (0–15) | 9 (4–23) |
| Ventriculomegaly, no IVH | 4 (0–33) | 3 (0–13) | 3 (0–6) | 3 (0–6) | 2 (0–9) | 2 (0–6) | 1 (0–5) | 2 (0–4) |
| PVL within 28 db,k | 6 (0–33) | 4 (0–25) | 3 (0–11) | 4 (0–18) | 3 (0–8) | 2 (0–8) | 2 (0–5) | 3 (<1–6) |
| Infants born in 2006–2007 | N = 19 | N = 174 | N = 438 | N = 547 | N = 566 | N = 728 | N = 754 | N = 3226 |
| PDAa,l | 53 (0–100) | 52 (13–100) | 56 (0–100) | 55 (20–100) | 51 (12–100) | 43 (0–80) | 34 (0–63) | 47 (23–78) |
| Ibuprofen therapy for PDAl | 0 (0–0) | 13 (0–64) | 16 (0–50) | 16 (0–60) | 13 (0–64) | 12 (0–44) | 11 (0–60) | 13 (0–52) |
| Infants in hospital at 28 d | N = 11 | N = 92 | N = 320 | N = 471 | N = 508 | N = 678 | N = 718 | N = 2798 |
| ROP examination performedf,m | 91 (50–100) | 91 (71–100) | 92 (50–100) | 94 (75–100) | 95 (67–100) | 96 (82–100) | 93 (68–100) | 94 (82–100) |
| ROP outcome at statusa,n | ||||||||
| Determined, favorable in both eyes | 10 (0–100) | 27 (0–100) | 28 (0–62) | 31 (0–86) | 38 (0–100) | 46 (0–100) | 46 (5–100) | 39 (8–83) |
| Determined, severe ROP in either eye | 30 (0–100) | 30 (0–100) | 21 (0–67) | 11 (0–38) | 5 (0–25) | 3 (0–9) | <1 (0–9) | 7 (0–20) |
| Undetermined ROP status in either eye (neither had severe ROP) | 60 (0–100) | 43 (0–100) | 51 (0–82) | 58 (14–100) | 57 (0–100) | 51 (0–100) | 53 (0–95) | 53 (8–88) |
Ranges are across all participating NRN centers. Proportions are among all infants who survived >12 hours, except as noted. Information was missing as follows: patent ductus arteriosus, 4 infants; indomethacin therapy for patent ductus arteriosus, 38 infants; surgical treatment for patent ductus arteriosus, 2 infants, necrotizing enterocolitis, 1 infant; early-onset sepsis, 1 infant; meningitis, 1 infant; late-onset sepsis, 2 infants; ROP examination performed, 1 infant; ROP, 1 infant; ROP stage ≥3, 7 infants; intervention/surgical treatment for ROP, 32 infants. P values were determined with the Wald χ2 test for differences according to GA, with adjustment for center and BW or, for early-onset sepsis, meningitis, and ibuprofen therapy for patent ductus arteriosus, with adjustment for BW only. PDA indicates patent ductus arteriosus; NEC, necrotizing enterocolitis.
P ≤ .001.
P ≤ .01.
Proportions among infants who survived >3 days after birth.
Proportions among infants with necrotizing enterocolitis.
Proportions among infants with patent ductus arteriosus.
P ≤ .05.
Proportions among infants who were still in the hospital at 28 days of life.
Proportions among infants who were still in the hospital at 28 days and underwent a ROP examination.
Growth failure was defined as <10th percentile for gender at PMA of 36 weeks. Proportions were determined among infants who were alive and in the hospital at PMA of 36 weeks and who had weight measurements at PMA of 35 to 37 weeks. Among those in the hospital at 36 weeks, weight data were missing for 101 infants with other measurements taken at PMA of 36 weeks, weight was measured but not in the 35- to 37-week period for 21 infants, and growth failure could not be determined for 2 infants with missing gender information.
From the sonogram with the most-severe findings. Proportions were determined among those who had sonograms performed within 28 days after birth. Categories shown are mutually exclusive. Some infants with IVH also had PVL, that is, 18 (2%) of 832 infants with grade 1 IVH, 21 (4%) of 506 infants with grade 2 IVH, 31 (5%) of 598 infants with grade 3 IVH, and 117 (16%) of 710 infants with grade 4 IVH. Ten (6%) of 173 infants with ventriculomegaly and no IVH also had PVL.
Proportions indicate the proportions of infants with PVL overall, with and without IVH, among infants who had sonograms performed within 28 days after birth.
Proportions were determined among infants born in 2006–2007. Ibuprofen treatment proportions are among infants who had patent ductus arteriosus. Information was missing as follows: patent ductus arteriosus, 1 infant; ibuprofen treatment, 1 infant.
Proportions among infants born in 2006–2007 who were still in the hospital at 28 days of life. Information was missing for ROP examination for 1 infant.
Proportions among infants born in 2006 –2007 who were still in the hospital at 28 days and had ROP examinations performed. An assessment of ophthalmologic outcome at the time of status was made as follows:(1) favorable, defined as one of the following in each eye: vessels mature, vessels in zone III in 2 consecutive examinations, acute ROP of stage 1 or 2 in zone III in 2 consecutive examinations, or ROP in zone II or zone III but determined to be clearly regressing; (2) unfavorable, defined as severe ROP on the basis of one of the following in either eye: received surgical treatment for ROP, met criteria for undergoing surgery, or retinal detachment resulting from ROP; or (3) undetermined outcome, defined as one of the following in either eye: immature vessels in zone I or zone II, immature vessels reaching zone III in any single examination, stage 1 or 2 ROP in zone III at any single examination, stage 2 or 3 ROP in zone II or III not regressing, or active ROP in zone I or zone II. Data on outcome at status was missing for 13 infants.
Statistical Analyses
All infants were studied for assessment of maternal characteristics, neonatal demographic features, interventions performed soon after birth, and survival. Infants who died at ≤12 hours were excluded from analyses focused on morbidities diagnosed at >12 hours. For determination of rates of survival without morbidity, morbidity was defined as severe IVH (≥grade 3), PVL, BPD, necrotizing enterocolitis, ≥stage 3 ROP, or infection (early-onset sepsis, late-onset sepsis, or meningitis).
Statistical significance for unadjusted comparisons was determined by using χ2 or Wilcoxon tests. Logistic or linear regression models were used to assess associations with GA, with adjustment for study center and infant BW, with statistical significance determined by using Wald χ2 or F tests. Generalized logit regression models were used for comparisons involving categorical variables with >2 levels.
Risk of death and changes in clinical practice during the study period were assessed by using robust Poisson regression models14 to produce correct SEs for the estimated relative risks (RRs). Additional adjustments for clustering according to center were not made because study center was treated as a fixed effect in these models, which also included effects for BW and GA. To assess linear trends, year was included as a continuous variable, with adjusted RRs for the change per year being reported. Initial models included terms for interactions between each GA and year, to assess whether yearly trends varied according to GA. Nonsignificant interactions were removed, and the models were rerun.
Participating NRN Study Centers
The numbers of infants included from each center were as follows: University of Alabama, 805 infants; Brown University, 616 infants; University of California, San Diego, 528 infants; Case Western Reserve University, 415 infants; University of Cincinnati, 974 infants; Duke University, 426 infants; Emory University, 516 infants; Indiana University, 720 infants; University of Iowa, 99 infants; University of Miami, 515 infants; University of New Mexico, 97 infants; University of Rochester, 243 infants; Stanford University, 334 infants; University of Texas Southwestern Medical Center at Dallas, 488 infants; University of Texas Health Science Center at Houston, 765 infants; Tufts University, 137 infants; University of Utah, 269 infants; Wake Forest University, 465 infants; Wayne State University, 637 infants; Yale University, 526 infants.
RESULTS
Study Group
A total of 9575 infants with GAs of 22 to 28 weeks and BWs of 401 to 1500 g were born at NRN centers between January 1, 2003, and December 31, 2007, and are included in this study. Overall, 25% of the cohort subjects were multiple births.
Maternal and Infant Characteristics, Delivery Room Interventions, and Early Deaths
Rates of prenatal steroid use increased with increasing GA, from 13% at 22 weeks to 53% at 23 weeks and 85% to 87% at 24 to 28 weeks (Table 1). Rates of prenatal antibiotic use were lowest for mothers who delivered at 22 weeks (51%) and highest for those who delivered at 24 to 25 weeks (73%). Chorioamnionitis was documented more frequently in maternal records and confirmed more commonly by placental histologic findings at lower GAs. Overall, 59% of infants were born through cesarean section, with the steepest increase in cesarean section delivery rates between GAs of 22 and 24 weeks (7% at 22 weeks and 60% at 24 weeks).
TABLE 1.
Maternal Demographic Features and Perinatal Information According to GA for VLBW Infants Born in NRN Centers Between January 1, 2003, and December 31, 2007 (Including Infants Who Died Within 12 Hours After Birth)
| Characteristic | 22 wk (N = 421) |
23 wk (N = 871) |
24 wk (N = 1370) |
25 wk (N = 1498) |
26 wk (N = 1576) |
27 wk (N = 1838) |
28 wk (N = 2001) |
Total (N = 9575) |
|---|---|---|---|---|---|---|---|---|
| Mother’s age, ya | ||||||||
| Mean (range) | 27 (22–32) | 27 (25–32) | 27 (25–31) | 27 (23–30) | 27 (25–30) | 27 (25–31) | 27 (25–32) | 27 (25–31) |
| SD (range) | 6.9 (4.2–9.9) | 6.4 (0.0–7.0) | 6.5 (4.7–8.9) | 6.6 (4.9–7.2) | 6.5 (4.6–7.6) | 6.7 (5.1–7.5) | 6.8 (5.2–7.5) | 6.6 (5.7–7.0) |
| High school degree, % (range) | 68 (0–100) | 69 (25–100) | 72 (40–100) | 70 (33–94) | 72 (25–90) | 70 (0–100) | 72 (41–89) | 71 (36–88) |
| Medical insurance, % (range)b | ||||||||
| Medicaid/public insurance | 51 (20–100) | 42 (20–100) | 50 (15–71) | 53 (33–69) | 50 (29–71) | 50 (24–81) | 49 (19–78) | 49 (29–69) |
| Private insurance | 35 (0–67) | 45 (0–67) | 39 (9–76) | 38 (6–62) | 40 (5–69) | 40 (3–67) | 42 (4–79) | 40 (6–63) |
| Self-pay/uninsured | 13 (0–40) | 13 (0–51) | 11 (0–42) | 8 (0–38) | 9 (0–30) | 9 (0–22) | 8 (0–31) | 9 (<1–31) |
| Other | <1 (0–13) | <1 (0–11) | <1 (0–10) | <1 (0–11) | <1 (0–10) | 1 (0–15) | <1 (0–15) | <1 (0–13) |
| ≥1 prenatal visit, % (range)b | 88 (33–100) | 92 (84–100) | 93 (85–100) | 94 (86–100) | 93 (80–100) | 95 (85–100) | 95 (89–100) | 94 (85–100) |
| Diabetes mellitus, % (range)c | 3 (0–20) | 3 (0–17) | 3 (0–10) | 4 (0–8) | 5 (2–15) | 5 (0–11) | 6 (1–15) | 5 (3–8) |
| Hypertension, % (range)b | 8 (0–26) | 10 (0–28) | 14 (0–40) | 20 (9–38) | 25 (13–43) | 27 (15–42) | 31 (11–47) | 22 (14–37) |
| Prepartum hemorrhage, % (range)b | 21 (0–67) | 27 (0–76) | 21 (9–40) | 22 (5–56) | 19 (0–54) | 17 (5–35) | 16 (6–23) | 20 (9–32) |
| Prenatal steroid treatment, % (range)b | 13 (0–100) | 53 (10–100) | 85 (49–100) | 86 (62–100) | 86 (48–100) | 87 (57–100) | 86 (46–100) | 80 (45–97) |
| Prenatal antibiotic treatment, % (range)b | 51 (21–92) | 65 (0–88) | 73 (56–90) | 73 (48–94) | 68 (45–100) | 66 (51–85) | 64 (50–89) | 67 (55–85) |
| ROM >24 h before delivery, % (range)b | 22 (0–45) | 22 (0–42) | 25 (8–40) | 26 (13–40) | 28 (15–50) | 25 (14–32) | 24 (16–34) | 25 (18–32) |
| Mode of delivery, % (range)b | ||||||||
| Vaginal, vertex | 60 (20–100) | 53 (0–75) | 32 (20–56) | 31 (19–44) | 33 (11–43) | 30 (14–44) | 30 (12–48) | 34 (18–43) |
| Vaginal, breech | 32 (0–80) | 23 (0–56) | 7 (0–22) | 4 (0–13) | 3 (0–13) | 2 (0–4) | 2 (0–5) | 6 (0–12) |
| Vaginal, not otherwise specified | <1 (0–33) | <1 (0–6) | 0 (0–0) | <1 (0–5) | <1 (0–2) | <1 (0–1) | <1 (0–1) | <1 (0–1) |
| Cesarean section | 7 (0–33) | 24 (3–100) | 60 (24–80) | 65 (54–79) | 65 (52–89) | 68 (55–86) | 68 (47–88) | 59 (47–81) |
| Infants born in 2006–2007 | N = 159 | N = 321 | N = 493 | N = 573 | N = 583 | N = 732 | N = 771 | N = 3632 |
| Chorioamnionitis documented in mother’s medical record, % (range)b | 28 (0–100) | 26 (0–100) | 20 (0–39) | 19 (0–56) | 19 (0–44) | 15 (0–28) | 14 (0–22) | 18 (7–29) |
| Placental pathologic evaluation performed, % (range) | 77 (36–100) | 86 (50–100) | 82 (50–100) | 83 (62–100) | 80 (46–100) | 80 (44–100) | 83 (0–100) | 82 (58–100) |
| Placental pathologic evaluation | N = 123 | N = 272 | N = 401 | N = 475 | N = 461 | N = 585 | N = 634 | N = 2951 |
| Histologic chorioamnionitis, % (range)b | 70 (25–100) | 61 (0–100) | 59 (0–100) | 51 (25–100) | 48 (0–73) | 41 (23–61) | 34 (8–57) | 48 (26–73) |
Ranges are across all participating NRN centers. Information was missing as follows: mother’s age, 4 infants; mother’s education, 2834 infants; mother’s medical insurance, 300 infants; prenatal care, 8 infants; diabetes mellitus, 8 infants; hypertension, 10 infants; prepartum hemorrhage, 8 infants; prenatal steroid treatment, 27 infants; prenatal antibiotic treatment, 30 infants; rupture of membranes date and/or time, 228 infants; mode of delivery, 9 infants; chorioamnionitis, 9 infants; placental pathologic evaluation, 26 infants; histologic chorioamnionitis, 17 infants. P values were determined with the Wald χ2 test for differences according to GA, with adjustment for center and BW. ROM indicates rupture of membranes.
P ≤ .05.
P ≤ .001.
P ≤ .01.
With adjustment for center and BW, there were no differences in racial distribution according to GA (Table 2). Early neonatal interventions differed according to GA (Table 2). At 22 weeks, only 19% of infants underwent intubation and ventilation in the delivery room. Intubation rates increased to 68% at 23 weeks and 87% at 24 weeks and decreased at >24 weeks. Of 856 infants who received resuscitation drugs and/or chest compressions, 96% also underwent intubation. Rates of surfactant therapy increased from 17% at 22 weeks to 63% at 23 weeks and 90% at 24 weeks. The proportion of infants who died at ≤ 12 hours decreased with increasing GA, from 85% at 22 weeks to 1% to 2% at 27 to 28 weeks (Table 3). Risk of early death was significantly elevated for infants born at 22 to 24 weeks, compared with infants born at 28 weeks (22 weeks, adjusted RR: 15.76 [95% confidence interval [CI]: 10.13–24.52]; 23 weeks, adjusted RR: 9.88 [95% CI: 6.48 –15.08]; 24 weeks, adjusted RR: 2.90 [95% CI: 1.90–4.43]), but not for infants born at 25 to 27 weeks.
TABLE 2.
Infant Demographic Features and Delivery Information According to GA for VLBW Infants Born in NRN Centers Between January 1, 2003, and December 31, 2007 (Including Infants Who Died Within 12 Hours After Birth)
| Characteristic | 22 wk (N = 421) |
23 wk (N = 871) |
24 wk (N = 1370) |
25 wk (N = 1498) |
26 wk (N = 1576) |
27 wk (N = 1838) |
28 wk (N = 2001) |
Total (N = 9575) |
|---|---|---|---|---|---|---|---|---|
| BW, ga | ||||||||
| Mean (range) | 511 (473–621) | 581 (549–639) | 651 (609–677) | 744 (709–791) | 854 (737–891) | 960 (919–1009) | 1082 (1022–1207) | 836 (789–903) |
| SD (range) | 66.9 (30.4–122) | 92.0 (55.4–139) | 105 (90.6–125) | 135 (107–162) | 163 (133–183) | 189 (164–218) | 206 (160–229) | 241 (218–259) |
| Male, % (range)a | 58 (0–93) | 55 (43–100) | 53 (40–70) | 53 (46–81) | 53 (45–63) | 55 (37–66) | 51 (36–58) | 53 (47–58) |
| Race/ethnicity, % (range) | ||||||||
| Black, non-Hispanic | 45 (0–100) | 38 (0–81) | 41 (0–85) | 41 (0–81) | 39 (4–86) | 38 (2–89) | 36 (0–87) | 39 (3–84) |
| Black, Hispanic | 0 (0–0) | 1 (0–10) | <1 (0–10) | <1 (0–6) | <1 (0–5) | <1 (0–3) | <1 (0–5) | <1 (0–3) |
| White, non-Hispanic | 30 (0–80) | 37 (0–63) | 34 (4–70) | 34 (0–71) | 36 (4–62) | 40 (3–79) | 41 (5–88) | 37 (5–71) |
| White, Hispanic | 19 (0–67) | 20 (0–100) | 18 (0–76) | 19 (0–88) | 19 (0–73) | 18 (<1–74) | 17 (0–67) | 18 (1–70) |
| American Indian/Alaska native | <1 (0–20) | 0 (0–0) | <1 (0–40) | <1 (0–13) | <1 (0–28) | <1 (0–10) | <1 (0–30) | <1 (0–20) |
| Asian/Pacific islander | 4 (0–43) | 3 (0–54) | 3 (0–37) | 3 (0–23) | 3 (0–21) | 3 (0–19) | 3 (0–23) | 3 (0–27) |
| >1 race/other | 1 (0–19) | 1 (0–14) | 2 (0–26) | 1 (0–21) | 2 (0–22) | <1 (0–9) | 1 (0–11) | 1 (0–17) |
| Intrauterine growth restriction, % (range)a | 0 (0–0) | 4 (0–16) | 6 (0–30) | 8 (0–14) | 8 (1–20) | 10 (5–15) | 9 (0–15) | 8 (5–10) |
| Multiple birth, % (range)a | 28 (0–48) | 30 (11–100) | 25 (7–32) | 21 (6–40) | 22 (8–40) | 25 (0–40) | 28 (16–37) | 25 (18–34) |
| Delivery room resuscitation, % (range) | ||||||||
| Endotracheal intubationa | 19 (0–100) | 68 (10–100) | 87 (53–100) | 82 (53–98) | 75 (32–92) | 65 (31–90) | 47 (10–82) | 67 (41–85) |
| Resuscitation druga | 3 (0–20) | 8 (0–32) | 9 (0–32) | 6 (0–28) | 5 (0–22) | 4 (0–19) | 2 (0–7) | 5 (1–16) |
| Chest compressiona | 3 (0–40) | 10 (0–24) | 13 (0–40) | 10 (1–37) | 7 (0–22) | 6 (0–15) | 4 (0–14) | 8 (2–19) |
| Apgar score of ≤3, % (range)a | ||||||||
| At 1 mina | 89 (0–100) | 73 (50–100) | 53 (30–71) | 44 (25–63) | 36 (22–53) | 32 (17–48) | 23 (12–30) | 42 (29–53) |
| At 5 mina | 86 (0–100) | 49 (0–89) | 20 (0–40) | 12 (3–25) | 8 (0–22) | 7 (1–14) | 4 (0–9) | 16 (3–25) |
| Admission temperature, °Ca,b | ||||||||
| Mean (range) | 34.7 (31.3–37.0) | 35.0 (33.2–36.6) | 35.4 (34.2–37.0) | 35.8 (34.8–36.9) | 36.1 (35.1–37.0) | 36.2 (35.1–37.1) | 36.2 (35.1–37.2) | 35.9 (34.8–37.0) |
| SD (range) | 1.7 (0.1–3.2) | 1.7 (0.1–1.9) | 1.4 (0.7–1.5) | 1.1 (0.6–1.3) | 1.0 (0.5–1.2) | 0.9 (0.5–1.1) | 0.9 (0.4–1.2) | 1.2 (0.7–1.3) |
| Surfactant therapy, % (range)a | 17 (0–100) | 63 (10–100) | 90 (58–100) | 88 (72–100) | 85 (56–100) | 78 (43–94) | 65 (41–86) | 76 (58–88) |
Ranges are across all participating NRN centers. Information was missing as follows: gender, 2 infants; race/ethnicity, 24 infants; intrauterine growth restriction, 2 infants; endotracheal intubation, 9 infants; resuscitation drug, 13 infants; chest compression, 13 infants; Apgar score at 1 minute, 78 infants; Apgar score at 5 minutes, 76 infants; temperature, 1097 infants.
P ≤ .001 from the Wald χ2 test for differences according to GA, with adjustment for center and BW. Differences in BW were adjusted for center effects only. Race/ethnicity was tested as black, white, or other.
Infant temperature at initial admission to the nursery for infants born in 2003–2005 and first temperature reading obtained within 60 minutes after birth for infants born in 2006 –2007.
TABLE 3.
Mortality Rates According to GA for VLBW Infants Born in NRN Centers Between January 1, 2003, and December 31, 2007
| % (Range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 22 wk (N = 421) |
23 wk (N = 871) |
24 wk (N = 1370) |
25 wk (N = 1498) |
26 wk (N = 1576) |
27 wk (N = 1838) |
28 wk (N = 2001) |
Total (N = 9575) |
||
| Survived | 6 (0–50) | 26 (2–53) | 55 (20–100) | 72 (50–90) | 84 (61–100) | 88 (76–100) | 92 (88–100) | 72 (55–95) | |
| Died | 94 (50–100) | 74 (47–98) | 45 (0–80) | 28 (10–50) | 16 (0–39) | 12 (0–24) | 8 (0–12) | 28 (5–45) | |
| Time of deatha | |||||||||
| ≤12 h | 85 (0–100) | 43 (0–90) | 11 (0–44) | 5 (0–19) | 3 (0–11) | 1 (0–5) | 2 (0–7) | 11 (1–25) | |
| >12–24 h | 2 (0–6) | 3 (0–7) | 2 (0–6) | <1 (0–3) | <1 (0–2) | <1 (0–2) | <1 (0–1) | 1 (0–2) | |
| >1–3 d | 1 (0–8) | 9 (0–30) | 6 (0–11) | 3 (0–25) | 2 (0–8) | 1 (0–6) | <1 (0–4) | 3 (0–7) | |
| 4–7 d | 2 (0–23) | 4 (0–20) | 4 (0–11) | 3 (0–7) | 1 (0–8) | 1 (0–6) | <1 (0–2) | 2 (0–5) | |
| 8–14 d | 2 (0–50) | 5 (0–50) | 5 (0–20) | 3 (0–9) | 2 (0–6) | 2 (0–19) | <1 (0–5) | 3 (1–8) | |
| 15–28 d | 1 (0–15) | 4 (0–16) | 7 (0–15) | 4 (0–8) | 3 (0–11) | 2 (0–5) | 2 (0–7) | 3 (0–6) | |
| ≥29 d | 1 (0–8) | 6 (0–17) | 10 (0–30) | 8 (0–15) | 5 (0–10) | 4 (0–8) | 2 (0–5) | 5 (1–9) | |
| Survived | N = 25 | N = 226 | N = 748 | N = 1078 | N = 1319 | N = 1616 | N = 1847 | N = 6859 | |
| Survived without morbidityb | 0 (0–0) | 8 (0–14) | 9 (0–18) | 20 (0–43) | 34 (0–49) | 44 (19–65) | 57 (6–74) | 37 (7–50) | |
| Died | N = 396 | N = 645 | N = 622 | N = 420 | N = 257 | N = 222 | N = 154 | N = 2716 | |
| Respiratory support withheld/withdrawn before deathc | 82 (40–100) | 77 (0–100) | 66 (21–96) | 68 (0–100) | 73 (42–100) | 66 (20–100) | 60 (0–100) | 72 (29–95) | |
| Died at ≤12 h | N = 359 | N = 375 | N = 147 | N = 72 | N = 46 | N = 27 | N = 34 | N = 1060 | |
| Respiratory support withheld/withdrawn before deathd | 85 (40–100) | 85 (43–100) | 79 (0–100) | 86 (0–100) | 78 (0–100) | 85 (0–100) | 79 (25–100) | 84 (53–100) | |
Ranges are across all participating NRN centers.
Proportions among all infants including survivors.
Proportions among infants who survived. Morbidities included severe IVH, PVL, BPD, necrotizing enterocolitis, infections, and ROP stage ≥3.
Proportions among infants who died. Data on respiratory support withheld/withdrawn were missing for 52 infants.
Proportions among infants who died within 12 hours. Data on respiratory support withheld/withdrawn were missing for 2 infants.
Changes in Clinical Practices
Rates of prenatal steroid use increased by ~1% per year during the study period, and rates of cesarean section delivery increased by ~2% per year (Table 4). Rates of prenatal antibiotic use decreased by ~3% per year. These trends did not vary according to GA (year-GA interaction: for prenatal steroid therapy, P = .47; for cesarean section delivery, P = .37; for prenatal antibiotic treatment, P=.66). Rates of endotracheal intubation in the delivery room and surfactant therapy varied according to GA (year-GA interaction: P < .01 for each). Rates of intubation and surfactant therapy decreased for infants born at 28 weeks. During the study period, the proportion of infants receiving continuous positive airway pressure (CPAP) therapy at 24 hours increased among infants of ≥24 weeks, as did the proportion of infants who never underwent intubation. Although the adjusted RR for BPD decreased over time among infants who survived to PMA of 36 weeks, the change was clinically insignificant.
TABLE 4.
Clinical Practice Indicators and Survival Rates According to Birth Year for 9575 VLBW Infants Born in NRN Centers Between January 1, 2003, and December 31, 2007
| Characteristic | Percent | Adjusted RR (95% CI) |
P | ||||
|---|---|---|---|---|---|---|---|
| 20003 (N = 1919) |
2004 (N = 1992) |
2005 (N = 2032) |
2006 (N = 1900) |
2007 (N = 1732) |
|||
| Prenatal steroid treatment, all infants | 81 | 76 | 80 | 79 | 83 | 1.01 (1.00–1.02) | .01 |
| Prenatal antibiotic treatment, all infants | 72 | 68 | 68 | 63 | 66 | 0.97 (0.96–0.98) | <.001 |
| Cesarean section, all infants | 57 | 58 | 62 | 60 | 60 | 1.02 (1.00–1.03) | .01 |
| Delivery room endotracheal intubation | |||||||
| 22 wk | 17 | 22 | 21 | 16 | 19 | 0.98 (0.85–1.13) | .8 |
| 23 wk | 69 | 67 | 67 | 68 | 67 | 0.99 (0.96–1.02) | .6 |
| 24 wk | 89 | 89 | 88 | 83 | 86 | 0.98 (0.97–1.00) | .04 |
| 25 wk | 86 | 86 | 79 | 81 | 77 | 0.97 (0.95–0.99) | <.001 |
| 26 wk | 83 | 78 | 68 | 76 | 69 | 0.95 (0.94–0.97) | <.001 |
| 27 wk | 69 | 70 | 64 | 58 | 61 | 0.96 (0.94–0.98) | <.001 |
| 28 wk | 54 | 58 | 45 | 41 | 38 | 0.90 (0.87–0.93) | <.001 |
| All infants | 71 | 72 | 66 | 63 | 62 | ||
| Surfactant therapy | |||||||
| 22 wk | 18 | 17 | 21 | 13 | 18 | 0.97 (0.83–1.13) | .7 |
| 23 wk | 65 | 66 | 63 | 63 | 59 | 0.98 (0.94–1.01) | .2 |
| 24 wk | 91 | 88 | 89 | 88 | 93 | 1.00 (0.99–1.01) | .9 |
| 25 wk | 89 | 89 | 85 | 91 | 87 | 0.99 (0.98–1.00) | .2 |
| 26 wk | 89 | 83 | 84 | 87 | 82 | 0.99 (0.97–1.00) | .045 |
| 27 wk | 78 | 80 | 75 | 77 | 78 | 1.00 (0.98–1.01) | .6 |
| 28 wk | 73 | 70 | 61 | 62 | 59 | 0.94 (0.92–0.96) | <.001 |
| All infants | 78 | 77 | 74 | 75 | 74 | ||
| Survived to discharge | |||||||
| 22 wk | 6 | 7 | 5 | 3 | 8 | ||
| 23 wk | 27 | 21 | 33 | 27 | 21 | ||
| 24 wk | 56 | 53 | 55 | 55 | 54 | ||
| 25 wk | 71 | 72 | 70 | 75 | 71 | ||
| 26 wk | 82 | 87 | 82 | 83 | 84 | ||
| 27 wk | 88 | 86 | 88 | 88 | 91 | ||
| 28 wk | 94 | 89 | 93 | 93 | 92 | ||
| All infants | 72 | 70 | 71 | 73 | 72 | 1.00 (0.99–1.01) | .3 |
| Survived >12 h | N = 1709 | N = 1762 | N = 1818 | N = 1689 | N = 1537 | ||
| Never intubated, all infants who survived >12 ha | 8 | 8 | 10 | 10 | 11 | 1.12 (1.07–1.18) | <.001 |
| Necrotizing enterocolitis, all infants who survived >12 h | 9 | 11 | 11 | 11 | 12 | 1.04 (0.99–1.09) | .1 |
| Survived >24 h | N = 1685 | N = 1738 | N = 1785 | N = 1678 | N = 1532 | ||
| CPAP therapy at 24 h | |||||||
| 22 wk | 0 | 0 | 0 | 0 | 0 | ||
| 23 wk | 5 | 4 | 4 | 1 | 1 | 0.73 (0.51–1.05) | .09 |
| 24 wk | 5 | 7 | 11 | 6 | 8 | 1.09 (0.95–1.24) | .2 |
| 25 wk | 12 | 14 | 22 | 18 | 23 | 1.16 (1.07–1.25) | <.001 |
| 26 wk | 27 | 32 | 32 | 25 | 34 | 1.01 (0.96–1.07) | .6 |
| 27 wk | 32 | 35 | 35 | 37 | 40 | 1.05 (1.0–1.09) | .04 |
| 28 wk | 36 | 36 | 36 | 41 | 40 | 1.04 (1.0–1.08) | .06 |
| All infants who survived >24 h | 23 | 25 | 26 | 27 | 29 | ||
| Survived >72 h | N = 1626 | N = 1672 | N = 1721 | N = 1631 | N = 1478 | ||
| Late-onset sepsis, all infants who survived >72 h | 36 | 38 | 37 | 36 | 33 | 0.98 (0.96–1.0) | .1 |
| Cranial sonography performed within 28 d after birth | N = 1660 | N = 1708 | N = 1749 | N = 1646 | N = 1497 | ||
| Severe IVH, all infants with sonograms | 16 | 16 | 14 | 17 | 16 | 0.96 (0.93–1.0) | .05 |
| Infants who underwent cranial imaging before and/or after 28 d | N = 1665 | N = 1714 | N = 1752 | N = 1651 | N = 1500 | ||
| PVL, all infants with imaging findings | 4 | 5 | 5 | 4 | 5 | 0.93 (0.87–1.0) | .06 |
| Survived to PMA of 36 wk | N = 1426 | N = 1455 | N = 1483 | N = 1421 | N = 1280 | ||
| BPD, infants who survived to PMA of 36 wk | 43 | 42 | 40 | 43 | 43 | 0.94 (0.92–0.95) | <.001 |
| Survived to discharge | N = 1385 | N = 1403 | N = 1445 | N = 1383 | N = 1243 | ||
| Survived without morbidityb | |||||||
| 22 wk | 0 | 0 | 0 | 0 | 0 | ||
| 23 wk | 14 | 10 | 3 | 5 | 9 | ||
| 24 wk | 5 | 10 | 10 | 8 | 11 | ||
| 25 wk | 22 | 20 | 21 | 17 | 20 | ||
| 26 wk | 32 | 38 | 34 | 31 | 34 | ||
| 27 wk | 44 | 44 | 46 | 42 | 44 | ||
| 28 wk | 58 | 55 | 62 | 55 | 54 | ||
| All infants who survived to discharge | 37 | 37 | 38 | 35 | 36 | 1.04 (1.02–1.06) | <.001 |
Information was missing as follows: prenatal steroid treatment, 27 infants; prenatal antibiotic treatment, 30 infants; cesarean section delivery, 9 infants; delivery room endotracheal intubation, 9 infants; surfactant therapy, 10 infants; never intubated, 3 infants; necrotizing enterocolitis, 1 infant; CPAP therapy, 14 infants; late-onset sepsis, 2 infants; severe IVH, 9 infants; PVL, 1 infant; BPD, 42 infants; survived without morbidity, 32 infants. RRs and P values were determined for the change per year from a modified Poisson model that included effects for study center, infant BW and GA, and year and, where significant, effects for the year-GA interaction (delivery room intubation, surfactant therapy and CPAP therapy at 24 h). RRs are shown for all infants overall in cases in which the year-GA interaction was not significant and separately for infants born at each GA in cases in which the interaction was significant. The year-GA interaction could not be assessed for the category of never intubated because of small sample sizes.
Never used conventional or high-frequency ventilator or underwent nasal synchronized intermittent mandatory ventilation.
Morbidities included BPD, severe IVH, PVL, necrotizing enterocolitis, ROP stage ≥3, and infections (early-onset sepsis, late-onset sepsis, or meningitis). Proportions were determined among survivors. Of the 25 surviving infants born at GA of 22 weeks, none survived without major morbidity.
Neonatal Characteristics and Morbidities Among Infants Who Survived >12 Hours
Overall, 89% of infants born at GAs of 22 to 28 weeks survived >12 hours. Substantially more early survivors born at 22 to 24 weeks received resuscitation efforts (intubation, drug treatment, and/or chest compression) in the delivery room, compared with infants born at 22 to 24 weeks who died at ≤12 hours (22 weeks, 90% vs 7%; 24 weeks, 91% vs 59%). Significant differences in resuscitation efforts between those who survived >12 hours and those who did not were not seen among infants with GAs of 25 to 27 weeks. Among infants born at 28 weeks, a smaller proportion of those who survived >12 hours received resuscitation efforts in the delivery room, compared with those who died within 12 hours (48% vs 65%; P =.05).
Infants at the lowest GAs were at the greatest risk for morbidities of prematurity (Tables 5 and 6). Overall, 93% infants experienced respiratory distress. Rates of mechanical ventilation at 24 hours decreased from 96% at 22 weeks to 40% at 28 weeks, and rates of CPAP therapy at 24 hours increased from 0% at 22 weeks to 3% at 23 weeks, 8% at 24 weeks, and 38% at 28 weeks. The risk of BPD was inversely related to GA at birth. Because of the inclusion of infants with mild BPD (oxygen therapy for ≥28 days but use of room air at 36 weeks), more infants were classified as having BPD with the new, severity-based, definition of BPD (new definition, 68%; traditional definition, 42%; physiologic definition, 40%).
TABLE 5.
Pulmonary Morbidities According to GA for VLBW Infants Who Were Born in NRN Centers Between January 1, 2003, and December 31, 2007, and Survived >12 Hours After Birth
| Characteristi | % (Range) | |||||||
|---|---|---|---|---|---|---|---|---|
| 22 wk (N = 62) |
23 wk (N = 496) |
24 wk (N = 1223) |
25 wk (N = 1426) |
26 wk (N = 1530) |
27 wk (N = 1811) |
28 wk (N = 1967) |
Total (N = 8515) |
|
| Respiratory distress syndromea | 95 (75–100) | 98 (75–100) | 98 (64–100) | 97 (77–100) | 94 (61–100) | 90 (50–100) | 86 (55–100) | 93 (60–99) |
| Surfactant therapya | 97 (50–100) | 97 (90–100) | 95 (83–100) | 90 (72–100) | 86 (58–100) | 78 (43–95) | 65 (41–86) | 82 (64–93) |
| Pneumothorax | 15 (0–40) | 11 (0–33) | 11 (0–23) | 9 (4–20) | 7 (0–15) | 5 (0–14) | 4 (0–9) | 7 (3–13) |
| Pulmonary hemorrhageb | 16 (0–50) | 15 (0–50) | 13 (6–40) | 10 (3–28) | 7 (2–20) | 4 (0–14) | 3 (0–7) | 7 (3–18) |
| Postnatal steroid treatmenta | 15 (0–50) | 18 (0–50) | 20 (0–60) | 14 (0–44) | 9 (0–30) | 6 (0–14) | 2 (0–6) | 10 (0–24) |
| Never intubateda,c | 0 (0–0) | <1 (0–6) | <1 (0–2) | 2 (0–8) | 5 (0–14) | 12 (0–40) | 23 (6–44) | 9 (2–22) |
| Respiratory support at 24 h for infants who survived >24 h | N = 55 | N = 471 | N = 1192 | N = 1414 | N = 1520 | N = 1804 | N = 1962 | N = 8418 |
| Conventional or high-frequency ventilationa,d | 96 (0–100) | 94 (83–100) | 89 (71–100) | 76 (57–95) | 61 (43–92) | 49 (21–74) | 40 (20–61) | 62 (47–83) |
| Nasal SIMVb,d | 0 (0–0) | <1 (0–6) | 2 (0–16) | 3 (0–20) | 3 (0–18) | 2 (0–12) | 3 (0–16) | 3 (0–14) |
| CPAP therapya,d | 0 (0–0) | 3 (0–10) | 8 (0–29) | 18 (5–30) | 30 (5–49) | 36 (12–79) | 38 (17–66) | 26 (8–46) |
| Use of oxygen alonea,d | 2 (0–100) | 1 (0–6) | 1 (0–7) | 2 (0–7) | 2 (0–13) | 3 (0–10) | 5 (0–15) | 3 (<1–9) |
| Infants who survived to PMA of 36 wk | N = 27 | N = 241 | N = 790 | N = 1121 | N = 1344 | N = 1648 | N = 1852 | N = 7023 |
| BPD (oxygen use at 36 wk)a,e | 85 (0–100) | 73 (35–100) | 69 (31–100) | 55 (20–100) | 44 (19–100) | 34 (13–76) | 23 (9–88) | 42 (20–89) |
| Infants in hospital at PMA of 36 wk or discharged/transferred at 33–36 wk | N = 27 | N = 231 | N = 774 | N = 1088 | N = 1284 | N = 1565 | N = 1739 | N = 6708 |
| Severity-based BPDa,f | ||||||||
| Mild BPD | 15 (0–100) | 26 (0–50) | 26 (0–67) | 37 (0–62) | 35 (0–58) | 28 (0–52) | 16 (0–35) | 27 (5–38) |
| Moderate BPD | 30 (0–100) | 35 (0–100) | 34 (0–68) | 29 (9–70) | 26 (5–71) | 20 (4–55) | 15 (0–57) | 23 (8–60) |
| Severe BPD | 56 (0–100) | 39 (0–100) | 37 (0–100) | 26 (3–86) | 17 (4–44) | 13 (0–30) | 8 (0–29) | 18 (3–40) |
| Infants born in 2006–2007 | N = 19 | N = 174 | N = 438 | N = 547 | N = 566 | N = 728 | N = 754 | N = 3226 |
| Inhaled nitric oxide treatmentb,g | 11 (0–50) | 8 (0–50) | 10 (0–54) | 8 (0–27) | 7 (0–25) | 3 (0–12) | 3 (0–14) | 6 (0–19) |
| Infants who survived to PMA of 36 wk | N = 9 | N = 83 | N = 274 | N = 422 | N = 482 | N = 650 | N = 691 | N = 2611 |
| BPD by physiologic definitiona,h | 89 (50–100) | 70 (0–100) | 68 (0–100) | 55 (19–100) | 44 (6–100) | 31 (0–100) | 22 (0–100) | 40 (15–82) |
Ranges are across all participating NRN centers. Proportions are among all infants who survived >12 hours, except as noted. Information was missing as follows: respiratory distress syndrome, 5 infants; surfactant treatment, 7 infants; pulmonary hemorrhage, 2 infants; postnatal steroid treatment, 41 infants; never intubated, 3 infants; ventilator use at 24 hours, 13 infants; nasal synchronized intermittent mandatory ventilation at 24 hours, 14 infants; CPAP at 24 hours, 14 infants; oxygen alone at 24 hours, 14 infants; nitric oxide use, 1 infant. P values were determined with the Wald χ2 test for differences according to GA, with adjustment for center and BW or, for nasal synchronized intermittent mandatory ventilation and inhaled nitric oxide use, with adjustment for BW only. SIMV indicates synchronized intermittent mandatory ventilation.
P ≤ .001.
P ≤ .05.
Never used conventional or high-frequency ventilator or underwent nasal synchronized intermittent mandatory ventilation.
Proportions among infants who survived >24 hours after birth. Use of oxygen alone at 24 hours was defined as receiving supplemental oxygen without conventional or high-frequency ventilation, nasal synchronized intermittent mandatory ventilation, or CPAP therapy.
Proportions among infants who survived to PMA of 36 weeks and had nonmissing outcome data (N values shown). BPD could not be determined for 42 infants.
Proportions among infants who were still in the hospital at PMA of 36 weeks or, if discharged or transferred before 36 weeks, were in the hospital for ≥28 days and until PMA of 33 weeks. N values are shown for infants with nonmissing outcome data. Severity-based BPD could not be determined for 68 infants. More information about severity-based BPD is presented in the text.
Proportions among infants born in 2006–2007.
Proportions among infants born in 2006–2007 who survived to PMA of 36 weeks and had nonmissing outcome data (N values shown). BPD according to the physiologic definition could not be determined for 90 infants. Information on how BPD was determined according to the physiologic definition is presented in the text.
Most infants who survived >12 hours underwent ≥1 cranial ultrasound evaluation within 28 days; 64% of results were normal (Table 6). Overall, 10% of sonograms indicated grade 1 IVH, 6% grade 2 IVH, 7% grade 3 IVH, 9% grade 4 IVH, 2% ventriculomegaly without IVH, and 2% other abnormalities. PVL was observed for 3% of infants with sonograms performed in the first 28 days and 4% with sonograms performed after 28 days. Rates of abnormal ultrasound findings decreased with increasing GA.
Sepsis was diagnosed more frequently at the lowest GA (rates of early-onset sepsis were 6% at 22 weeks and 1% at 28 weeks, and rates of late-onset sepsis were 58% at 22 weeks and 20% at 28 weeks); 11% of infants developed necrotizing enterocolitis (Table 6). Patent ductus arteriosus was diagnosed for 46% of infants, of whom 71% were treated with indomethacin, 13% ibuprofen (2006–2007), and 27% surgical closure. Among 7313 infants who were still in the hospital at 28 days, 94% underwent an ophthalmologic examination before hospital discharge, death, or transfer. Of the 6866 with examination findings, 59% were diagnosed as having ROP (96% at 22 weeks and 32% at 28 weeks), and 12% underwent treatment for ROP (50% at 22 weeks and 2% at 28 weeks). A total of 2630 infants evaluated in 2006–2007 had ROP outcomes recorded at the time of discharge or 120 days of age. Among those infants, 39% had favorable outcomes, 7% had unfavorable outcomes with severe ROP requiring treatment, and 53% had undetermined ROP outcomes (ie, had not reached the threshold for surgery or were still immature and required further examination) (Table 6).
Survival and Morbidity Rates (All 9575 Infants)
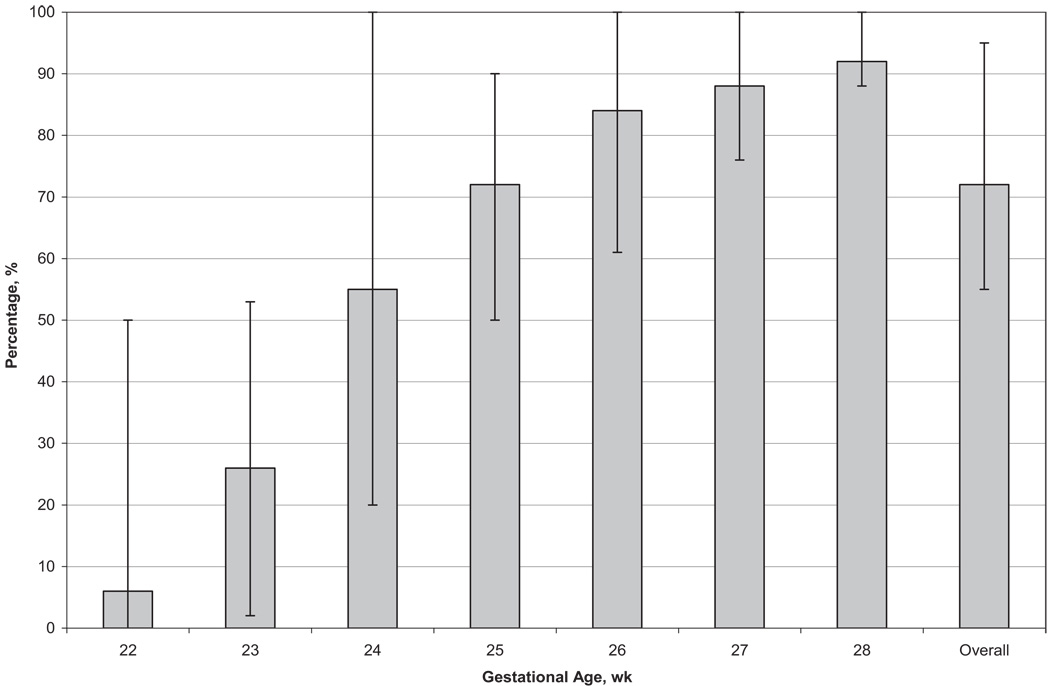
Rates of survival to discharge increased with increasing GA, from 6% at 22 weeks to 92% at 28 weeks (72% overall) (Fig 1 and Table 3). Infants born at 22 to 23 weeks had >3 times the risk of death, compared with infants born at 28 weeks (22 weeks, adjusted RR: 3.88 [95% CI: 3.18–4.73]; 23 weeks, adjusted RR: 3.56 [95% CI: 2.95–4.30]). RRs decreased but remained significant for infants born at 24 to 27 weeks, compared with 28 weeks (24 weeks, adjusted RR: 2.52 [95% CI: 2.10–3.04]; 27 weeks, adjusted RR: 1.23 [95% CI: 1.01–1.49]). Rates of survival to discharge according to GA did not change during the study period (Table 4).
FIGURE 1.
Survival to discharge according to GA among 9575 VLBW infants born in NICHD NRN centers between January 1, 2003, and December 31, 2007. The thin lines indicate ranges across centers.
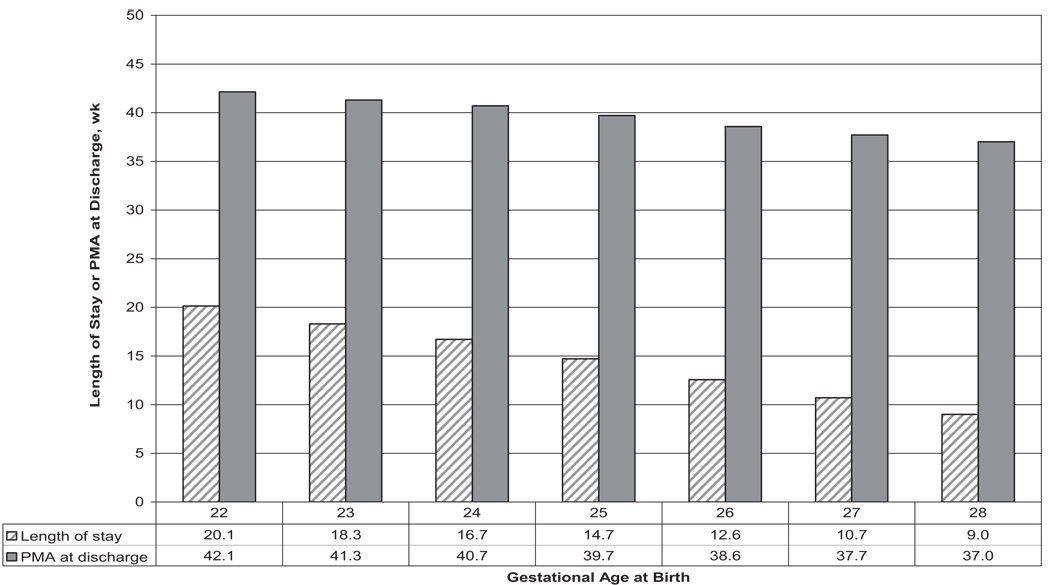
Neonatal morbidities occurred frequently among survivors. Rates of survival with morbidity decreased from 100% at 22 weeks to 92% at 23 weeks, 91% at 24 weeks, 80% at 25 weeks, 66% at 26 weeks, 56% at 27 weeks, and 43% at 28 weeks. Infection and BPD were the most-frequent morbidities. Although unadjusted rates of survival without major morbidity seemed unchanged, the adjusted RR for survival without morbidity increased over time (Table 4). The median length of hospital stay among survivors was 84 days, and lengths of stay decreased with increasing GA, from 141 days at 22 weeks to 63 days at 28 weeks (P <.001). PMA at discharge decreased from 42 weeks for surviving infants born at GAs of 22 weeks to 37 weeks for those born at 28 weeks (Fig 2).
FIGURE 2.
Median length of hospitalization (in weeks) and median PMA at discharge (in weeks) according to GA at birth among 6859 VLBW infants who were born in NICHD NRN centers between January 1, 2003, and December 31, 2007, and survived to discharge.
DISCUSSION
Although VLBW infant mortality rates in the United States decreased substantially in the 1980s and early 1990s,3–6, 15–18 most reports, including findings for this cohort, failed to demonstrate further progress in reducing neonatal morbidity and mortality rates.6,16–19 In contrast, a population cohort of all preterm infants born at GAs of <27 weeks in Sweden in 2004 –2007 demonstrated survival rates higher than rates reported for other countries or reported previously for Sweden. 20 Our study reviewed neonatal morbidity and mortality rates for a large cohort of extremely preterm infants, to evaluate changes in clinical practice and contemporary outcomes at US academic centers. Although previous reports from the NRN used BW as the reference for morbidity and survival rates, the current study assessed outcomes according to GA. Appreciation of GA-based outcomes is particularly valuable for prenatal counseling and physician/family decision-making.
The decisions to provide active obstetric care and to initiate neonatal intensive care for the most-immature infants remain controversial. Center differences in obstetric/early neonatal interventions were identified, but we did not collect sufficiently detailed information on decision-making processes to help explain differences. In our cohort, rates of active obstetric intervention, as indicated by prenatal steroid administration and cesarean section delivery, increased markedly after 23 weeks of gestation. Prenatal steroid use was almost twice as frequent for infants born at GAs of 24 to 28 weeks, compared with infants born earlier. Similarly, rates of neonatal interventions and intensive care, measured as active resuscitation with ventilation in the delivery room, increased substantially between 22 and 23 weeks (19% vs 68%). Rates of death at ≤12 hours, which in part reflect willingness to provide intensive care to the most-immature infants, decreased with increasing GA, from 85% of infants at 22 weeks to 2% of infants at 28 weeks.
In-hospital morbidity rates remain high among extremely preterm infants, and morbidities contribute to adverse neurodevelopmental outcomes. The majority of infants studied experienced a major complication during the initial hospitalization, with the risk of morbidity being inversely related to GA at birth. Center differences in the proportions of infants with specific morbidities were noted. At the lowest GAs (22–24 weeks), small numbers of infants at some centers contributed to the variability. The registry does not collect data on the reasons behind the choice of interventions for individual infants and has limited data on the severity of illness at birth, information that might permit more-detailed evaluation and understanding of center differences. Reducing the high rates of in-hospital morbidity among extremely low GA infants who are provided ongoing intensive care remains a challenge for clinicians and investigators.
To reduce rates of BPD, attention is being paid to avoidance of intubation, less prophylactic use of surfactant, and alternative modes of respiratory support. Rates of endotracheal intubation in the delivery room decreased in recent years among infants of >24 weeks, with a corresponding increase in CPAP therapy use at 24 hours of life. At GA of 28 weeks, use of surfactant decreased in the most-recent years. Furthermore, the proportion of infants who survived >12 hours without ever undergoing intubation and ventilation increased with increasing GA and more-recent year of birth. With substantially increased use of CPAP therapy, it was surprising that overall rates of BPD were unchanged, although the adjusted RR for BPD decreased over the study period.
This is the first study to report ophthalmologic status as favorable, unfavorable, or undetermined at the time of the last in-hospital examination. Although 7% of all infants had severe ROP, the rate was 30% for infants with GAs of 22/23 weeks. Of concern, 53% of infants had undetermined ophthalmologic status at the last examination before discharge. This finding has implications for discharge planning and underscores the importance of a medical home, to ensure careful ophthalmologic follow-up monitoring of these vulnerable infants after discharge home or transport to a community hospital.
Although ours is not a population-based study, we included all extremely low gestation births at 20 academic centers across the United States that together represent >110 000 live births per year, an annual birth cohort equal in size to the Swedish national cohort described recently.20 The rate of extremely low gestation birth was fivefold higher in our NRN cohort (~10 births at <27 weeks per 1000 infants) than in the Swedish cohort (2.3 births at <27 weeks per 1000 infants). This remarkable difference may be explained in part by Sweden’s universal health insurance, with free prenatal care and associated social services, as well as an ethnically more homogeneous and somewhat older pregnant population. The high rates of prematurity in our cohort underscore the importance of the current health care debate in the United States. Survival rates for extremely low gestation infants born at NRN centers are lower than those reported from Sweden. For nearly all infants in the Swedish cohort, GA was estimated on the basis of ultrasound findings. The authors of the Swedish study noted that a limitation of the use of ultrasonography to determine GA is that erroneously low GAs might be estimated for infants with growth restriction. Given the decrease in mortality rates with increasing GA, underestimation of GA by as little as 1 week might explain in part the difference in mortality rates between the 2 cohorts. Greater use of prenatal steroid treatment at all GAs and of surfactant therapy at 22 to 23 weeks also might have contributed to differences between the 2 cohorts.
During the 5-year study period, there was no substantial improvement in rates of survival to discharge for extremely low gestation infants born at NRN centers. However, each additional week of GA at birth had substantial survival advantage; the most marked changes were between GAs of 22 and 25 weeks, with survival rates increasing from 6% to 72%. Furthermore, rates of survival to discharge without major morbidities increased dramatically between 22 and 25 weeks, with continued steady improvement for each additional week of gestation. PMA at discharge for VLBW infants, a proxy measure of length of stay and a reflection of the cost of care, was inversely related to GA at birth. Each additional week of GA at birth reduced PMA at discharge by almost 1 week and total length of hospital stay by ~2 weeks, a reflection of both severity of illness and complications of prematurity among these very immature infants. Although adjusted RRs for survival without morbidity increased over time, the burden of in-hospital complications remained high. Retrospective analyses of center differences and benchmarking studies to identify best performance have been unable to identify modifiable practices that consistently improve outcomes, which underscores the need for hypothesis-driven clinical trials to assess the efficacy of current neonatal interventions. 21–24 Clinicians and investigators are challenged to identify and to test currently available interventions and resources that yield consistently lower morbidity and mortality rates at some centers, so that we can improve rates of survival without major morbidities and reduce long-term neurodevelopmental impairments for all infants.
WHAT’S KNOWN ON THIS SUBJECT: The NICHD NRN has published periodic evaluations of morbidity and mortality rates for VLBW infants. Increased VLBW survival has paralleled improvements in prenatal, obsteric and neonatal care, but recent data suggest that a plateau in survival may have been reached.
WHAT THIS STUDY ADDS: This study is the first NRN study to report outcomes on the basis of GA-specific information, which should be particularly valuable to obstetricians and pediatricians as they counsel parents of high-risk infants.
ACKNOWLEDGMENTS
The National Institutes of Health provided grant support for the NRN Generic Database Study (Recruitment 2003–2007). This study was supported in part by PHS grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee chairs: Alan H. Jobe, MD, PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, Pritzker School of Medicine, University of Chicago (2006–2007); Case Western Reserve University: Rainbow Babies and Children’s Hospital (National Institutes of Health grants GCRC M01 RR80 and U10 HD21364): Avroy A. Fanaroff, MD; Cincinnati Children’s Hospital Medical Center: University of Cincinnati Hospital and Good Samaritan Hospital (National Institutes of Health grants GCRC M01 RR8084 and U10 HD27853): Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Marcia Worley Mersmann, RN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN; Duke University: University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (National Institutes of Health grants GCRC M01 RR30 and U10 HD40492): C. Michael Cotten, MD, MHS; Kathy J. Auten, MSHS; Melody B. Lohmeyer, RN, MSN; Emory University: Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (National Institutes of Health grants UL1RR025008 and U10 HD27851): David P. Carlton, MD; Ann M. Blackwelder, RNC, BS, MS; Michelle Tidwell, BSN; Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA; Indiana University: Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (National Institutes of Health grants GCRC M01 RR750 and U10 HD27856): James A. Lemons, MD; Diana D. Appel, RN, BSN; Dianne E. Herron, RN; Lucy C. Miller, RN, BSN, CCRC; Leslie Richard, RN; Leslie D. Wilson, BSN, CCRC; Faithe Hamer, BS; RTI International (National Institutes of Health grant U01 HD36790): W. Kenneth Poole, PhD; Betty K. Hastings; Elizabeth M. McClure, MEd; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN, BSN; Stanford University: Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (National Institutes of Health grants GCRC M01 RR70 and U10 HD27880): David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS, CCRC; Melinda S. Proud, RCP; Andrew W. Palmquist, RN; Tufts Medical Center: Floating Hospital for Children (National Institutes of Health grants GCRC M01 RR54 and U10 HD53119): Brenda L. MacKinnon, RNC; Ellen Nylen, RN, BSN; University of Alabama at Birmingham: Health System and Children’s Hospital of Alabama (National Institutes of Health grants GCRC M01 RR32 and U10 HD34216): Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; University of California, San Diego: Medical Center and Sharp Mary Birch Hospital for Women and Newborns (National Institutes of Health grant U10 HD40461): Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Chris Henderson, RCP, CRTT; Wade Rich, BSHS, RRT; University of Iowa: Children’s Hospital (National Institutes of Health grants GCRC M01 RR59 and U10 HD53109): John A. Widness, MD; Karen J. Johnson, RN, BSN; University of Miami: Holtz Children’s Hospital (National Institutes of Health grants GCRC M01 RR16587 and U10 HD21397): Ruth Everett-Thomas, RN, MSN; University of New Mexico: Health Sciences Center (National Institutes of Health grants GCRC M01 RR997 and U10 HD53089): Conra Backstrom Lacy, RN; University of Rochester: Golisano Children’s Hospital at Strong (National Institutes of Health grants GCRC M01 RR44 and U10 HD40521): Linda J. Reubens, RN, CCRC; Erica Burnell, RN; University of Tennessee (National Institutes of Health grant U10 HD21415): Sheldon B. Korones, MD; University of Texas Southwestern Medical Center at Dallas: Parkland Health and Hospital System and Children’s Medical Center Dallas (National Institutes of Health grants GCRC M01 RR633 and U10 HD40689): Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; University of Texas Health Science Center at Houston: Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (National Institutes of Health grant U10 HD21373): Jon E. Tyson, MD, MPH; Esther G. Akpa, RN, BSN; Patty A. Cluff, RN; Anna E. Lis, RN, BSN; Georgia E. Mc-David, RN; Claudia I. Franco, RNC, MSN; Beverly Foley Harris, RN, BSN; Sarah Martin, RN, BSN; Maegan C. Simmons, RN; Patti Pierce Tate, RCP; University of Utah: University Hospital, Latter Day Saints Hospital, and Primary Children’s Medical Center (National Institutes of Health grants GCRC M01 RR64 and U10 HD53124): Bradley A. Yoder, MD; Karen A. Osborne, RN, BSN, CCRC; Jennifer J. Jensen, RN, BSN; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN, BSN; Wake Forest University: Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (National Institutes of Health grants GCRC M01 RR7122 and U10 HD40498): Robert G. Dillard, MD; Nancy J. Peters, RN, CCRP; Wayne State University: Hutzel Women’s Hospital and Children’s Hospital of Michigan (National Institutes of Health grant U10 HD21385): Rebecca Bara, RN, BSN; Geraldine Muran, RN, BSN; Women and Infants’ Hospital of Rhode Island (National Institutes of Health grant U10 HD27904): William Oh, MD; Angelita M. Hensman, RN, BSN; Yale University: Yale-New Haven Children’s Hospital (National Institutes of Health grants CTSA UL1 RR24139, GCRC M01 RR6022, and U10 HD27871): Patricia Gettner, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. We thank Avroy Fanaroff for thoughtful review of the manuscript and Mazie Tinsley for manuscript preparation.
ABBREVIATIONS
- VLBW
very low birth weight
- BPD
bronchopulmonary dysplasia
- BW
birth weight
- CI
confidence interval
- GA
gestational age
- IVH
intraventricular hemorrhage
- ROP
retinopathy of prematurity
- RR
relative risk
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- CPAP
continuous positive airway pressure
- PVL
periventricular leukomalacia
- PMA
postmenstrual age
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
Reprints Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87(5):587–597. [PubMed] [Google Scholar]
- 2.Hack M, Wright LL, Shankaran S, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Network, November 1989 to October 1990. Am J Obstet Gynecol. 1995;172(2):457–464. doi: 10.1016/0002-9378(95)90557-x. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Wright LL, Stevenson DK, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991–December 1992. Am J Obstet Gynecol. 1995;173(5):1423–1431. doi: 10.1016/0002-9378(95)90628-2. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179(6):1632–1639. doi: 10.1016/s0002-9378(98)70037-7. [DOI] [PubMed] [Google Scholar]
- 5.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network. January 1995 through December 1996. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e1. Available at: http://www.pediatrics.org/cgi/content/full/107/1/e1. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 8.Iams JD, Romero R, Culhane JF, Goldenberg RL. Preterm birth: primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 9.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in VLBW neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 11.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 13.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR EPICure Study Group. The EPICure Study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106(4):659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 16.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110(1):143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 17.Chan K, Ohlsson A, Synnes A, et al. Survival, morbidity, and resource use of infants of 25 weeks’ gestational age or less. Am J Obstet Gynecol. 2001;185(1):220–226. doi: 10.1067/mob.2001.115280. [DOI] [PubMed] [Google Scholar]
- 18.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity: moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadow W, Lee G, Lin K, Lantos J. Changes in mortality for extremely low birth weight infants 1990s: implications for treatment decisions and resource use. Pediatrics. 2004;113(5):1223–1229. doi: 10.1542/peds.113.5.1223. [DOI] [PubMed] [Google Scholar]
- 20.EXPRESS Group. Fellman V, Hellström-Westas L, Norman M, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 21.Vohr BR, Wright LL, Dusick AM, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 22.Lee SK, Chan K, Ohlsson A, et al. Variations in treatment decisions and outcomes of infants ≤25 weeks gestation: evidence from Canada. Pediatr Res. 1999;45(4):247A. [Google Scholar]
- 23.Synnes AR, Chien L-Y, Peliowski A, Baboolal R, Lee SK. Canadian NICU Network. Variations in intraventricular hemorrhage incidence rates among Canadian neonatal ICUs. J Pediatr. 2001;138(4):525–531. doi: 10.1067/mpd.2001.111822. [DOI] [PubMed] [Google Scholar]
- 24.Walsh M, Laptook A, Kazzi SN, et al. A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics. 2007;119(5):876–890. doi: 10.1542/peds.2006-2656. [DOI] [PubMed] [Google Scholar]