Abstract
Background
MUTYH-associated polyposis is a recessively inherited disorder characterized by a lifetime risk of colorectal cancer that is up to 100%. Because specific histological and molecular genetic features of MUTYH-associated polyposis colorectal cancers might influence tumor behavior and patient survival, we compared survival between patients with MUTYH-associated polyposis colorectal cancer and matched control patients with colorectal cancer from the general population.
Methods
In this retrospective multicenter cohort study from Europe, 147 patients with MUTYH-associated polyposis colorectal cancer were compared with 272 population-based control patients with colorectal cancer who were matched for country, age at diagnosis, year of diagnosis, stage, and subsite of colorectal cancer. Kaplan–Meier survival and Cox regression analyses were used to compare survival between patients with MUTYH-associated polyposis colorectal cancer and control patients with colorectal cancer. All statistical tests were two-sided.
Results
Five-year survival for patients with MUTYH-associated polyposis colorectal cancer was 78% (95% confidence interval [CI] = 70% to 84%) and for control patients was 63% (95% CI = 56% to 69%) (log-rank test, P = .002). After adjustment for differences in age, stage, sex, subsite, country, and year of diagnosis, survival remained better for MUTYH-associated polyposis colorectal cancer patients than for control patients (hazard ratio of death = 0.48, 95% CI = 0.32 to 0.72).
Conclusions
In a European study cohort, we found statistically significantly better survival for patients with MUTYH-associated polyposis colorectal cancer than for matched control patients with colorectal cancer.
CONTEXT AND CAVEATS
Prior knowledge
Individuals with MUTYH-associated polyposis, a recessively inherited disorder, have a lifetime risk for colorectal cancer that approaches 100%. It is not known whether specific histological and molecular genetic features of such cancers influence tumor behavior and patient survival.
Study design
Characteristics and survival of European patients with MUTYH-associated polyposis colorectal cancer and matched population-based control patients with colorectal cancer were compared.
Contribution
The survival was statistically significantly better for patients with MUTYH-associated polyposis colorectal cancer than for control patients, even after adjustment for differences in age, stage, sex, subsite, country, and year of diagnosis.
Implications
Prospective studies are needed to further investigate this survival difference between MUTYH-associated colorectal cancer patients and colorectal cancer patients from the general population and to investigate whether disease-specific interventions, such as timing and type of surgery and chemotherapy, are warranted.
Limitations
Several types of bias are possible, including selection, lead-time, and length-time biases. Treatment was not reported for many patients in this study.
From the Editors
Worldwide, colorectal cancer accounted for about one million newly diagnosed cancers in 2002, representing approximately 10% of all new cancers (1). Estimated 5-year survival for colorectal cancer is approximately 54% in Western Europe (1). Tumors in patients with inherited cancer syndromes may arise through distinct molecular genetic pathways and show histological features that are different from those in most sporadic tumors. These differences might, at least in part, influence tumor behavior and patient survival. For instance, mismatch repair–deficient tumors (associated with Lynch syndrome or sporadic microsatellite instability) have been reported to have a decreased likelihood of metastasizing, and patients with such tumors have better survival than patients with sporadic colorectal cancer (2–8), although some reports have not confirmed this finding (9–12).
In 2002, the first autosomal recessive inherited form of colorectal cancer, MUTYH-associated polyposis (Mendelian Inheritance in Man #608456), was described (13). MUTYH-associated polyposis is believed to be responsible for 0.3%–1% of all colorectal cancers (14,15).
The MUTYH protein is a base excision repair glycosylase that is involved in the repair of DNA damage resulting from the oxidation of guanine nucleotides. The oxidation product of guanine, 8-oxo-7,8-dihydro-2′-deoxyguanosine can mispair with adenine, leading to a transversion in which a G:C base pair is replaced with a T:A base pair. The MUTYH protein prevents these transversions by scanning the newly synthesized DNA strand for any mispaired adenines, with guanines or 8-oxo-7,8-dihydro-2′-deoxyguanosines, and excising them.
The risk of colorectal cancer in individuals with biallelic MUTYH mutations is high. The penetrance of colorectal cancer in patients with MUTYH-associated polyposis at age 60 years was estimated to be 100% in one study (16) and 43% in another (14).
We hypothesized that survival of patients with MUTYH-associated polyposis and colorectal cancer might differ from that of colorectal cancer patients from the general population because of the distinct mutational mechanism underlying MUTYH-associated polyposis. The purpose of this study was to compare survival between patients with MUTYH-associated polyposis colorectal cancer and matched control patients with colorectal cancer from the general population.
Subjects and Methods
Study Population
This multicenter study was collaboration between three research groups from the Institute of Human Genetics (University of Bonn, Bonn, Germany), the Institute of Medical Genetics (School of Medicine, Cardiff University, Cardiff, United Kingdom), and the Department of Clinical Genetics (Leiden University Medical Center, Leiden, the Netherlands). The study population contained 147 patients with MUTYH-associated polyposis colorectal cancer and 272 matched patients with colorectal cancer from the general population. Informed consent was obtained according to protocols approved by the appropriate national and/or local ethic review boards (the Multi-Centre Research Ethics Committee for Wales, ref. 06/MRE09/19; University of Bonn Ethics Review Board No. 063/04; and Leiden University Medical Center Ethics Review Board No. P01.019). The Patients with MUTYH-associated polyposis were all biallelic MUTYH mutation carriers and included 113 index patients and 34 of their affected siblings. Siblings were selected and tested for MUTYH mutations in case they had developed colorectal cancer and/or polyps. Genotyping was performed as described previously (17–19) [see the Leiden Open Variation Database database for all reported MUTYH mutations (20)]. The time of diagnoses ranged from June 15, 1967, through August 13, 2001, for Dutch patients; from October 15, 1977, through March 10, 2006, for German patients; and from February 12, 1970, through February 14, 2006 for patients from the United Kingdom.
Colon cancer was defined by use of the code C18 and rectal cancer was defined by use of the codes C19–C20, according to the International Classification of Diseases for Oncology, Edition 3 (21). Tumor localization was categorized by the following anatomical subsites: proximal colon (consisting of the cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure; C18.0–C18.5), distal colon (consisting of descending colon and sigmoid; C18.6–C18.7), colon not otherwise specified (C18.8–C18.9), and rectum (consisting of rectosigmoid and rectum; C19.9–C20.9). Tumor stage was classified according to pathological TNM stage (22). When the pathological stage was unknown, clinical stage was used. For most patients in this study, treatment information was not known and could, therefore, not be included as a determinant influencing survival. Year of diagnosis was used as a proxy of treatment because treatment changed during the study period. Survival time was defined as the time from the date of diagnosis until death or the end of the study (July 1, 2006). Patients who were still alive at the end of the study were censored on July 1, 2006.
The control patients from the general population were patients who were diagnosed with colorectal cancer and whose data were derived from the Saarland Cancer Registry in Germany, the Eindhoven Cancer Registry in the Netherlands, or the Northern and Yorkshire Cancer Registry and Information Service in the United Kingdom. The Saarland Cancer Registry is the only population-based cancer registry in Germany, and it has provided internationally accepted high-quality data throughout the past 35 years (23). Saarland is a state located in southwestern Germany with a population of approximately 1.1 million or approximately 1.3% of the total German population. The population structure and the health-care system in Saarland are very similar to Germany as a whole. The Eindhoven Cancer Registry is the oldest population-based cancer registry in the Netherlands that collects data from an area of 2.4 million inhabitants in southern Netherlands (24). The Northern and Yorkshire Cancer Registry and Information Service is one of the 11 UK registries and collects data from a population of 6.6 million in the center of the United Kingdom.
We aimed to select two control patients with colorectal cancer for each patient with MUTYH-associated polyposis colorectal cancer who were matched for country, stage at diagnosis, age at diagnosis, year of diagnosis, and cancer subsite. The age of diagnosis in the matched German and Dutch control patients was between 7 years younger and 7 years older than that in the case patient. The age of diagnosis in the matched UK control patients was between 4 years younger and 4 years older than that in the case patient. Cancer subsite was defined as either colon or rectum for German and Dutch control patients or as one of the first three characters of International Classification of Diseases for Oncology coding—C18, C19, and C20—for UK control patients. Patients with MUTYH-associated polyposis colorectal cancer from the United Kingdom were matched by the year of diagnosis for the period from January 1, 1996, through December 31, 2004. For UK patients with MUTYH-associated polyposis colorectal cancer who were diagnosed before 1996 (n = 19), we used control patients who were diagnosed in 1996 because the Northern and Yorkshire Cancer Registry and Information Service did not have data before 1996. Also, no control data were available for patients with MUTYH-associated polyposis colorectal cancer who were diagnosed after 2004, and so these patients were matched with control patients from 2004. We selected only control patients without second tumors because otherwise control patients might be included with a possible inheritable form of colorectal cancer that might influence the outcome of the survival analysis.
Statistical Analysis
Differences in patient and tumor characteristics between patients with MUTYH-associated polyposis colorectal cancer and control patients were analyzed by use of the χ2 test. Survival analysis was performed with Kaplan–Meier curves and Cox regression. The Cox model accounted for the clustering effect of sibling pairs. Hazard ratios (HRs) and 95% confidence intervals (CIs) were produced with robust standard errors by comparing patients with MUTYH-associated polyposis with control patients. Regression analysis was adjusted for the matching variables (ie, age, period of diagnosis, site of colon tumor, center, and stage). Moreover, all analyses were adjusted for sex. Stratified analyses were performed by adjusting for the same set of variables (ie, age as continuous variable, period of diagnosis [1967–1979, 1980–1989, 1990–1999, or 2000–2006], site of colon tumor [colon or rectum], center [Germany, United Kingdom, or the Netherlands], stage [I, II, III, or IV], and sex [male or female]). We used STATA software, version 10.0 (Stata Corp LP, College Station, TX). The proportional hazard assumption of MUTYH-associated polyposis was evaluated by applying Kaplan–Meier curves. The effect of MUTYH-associated polyposis over time satisfied the assumption of proportionality because the graphs of the log[−log(survival)] vs log(survival time) resulted in graphs with parallel lines. All statistical tests were two-sided.
Results
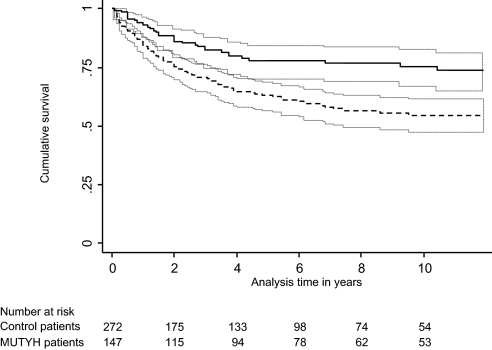
Crude survival for patients with MUTYH-associated polyposis was statistically significantly better than for control patients with colorectal cancer from the general population (log-rank test 5-year survival, P = .002) (Figure 1). Five-year survival was 78% (95% CI = 70% to 84%) for patients with MUTYH-associated polyposis colorectal cancer compared with 63% (95% CI = 56% to 69%) for control patients with colorectal cancer.
Figure 1.
Crude survival of patients with MUTYH-associated polyposis colorectal cancer and control patients with colorectal cancer in the United Kingdom, Germany, and the Netherlands (including a total of 419 participants, 147 patients with MUTYH-associated polyposis colorectal cancer and 272 control patients). Survival estimates and the corresponding 95% confidence intervals (gray dotted lines) for MUTYH-associated polyposis patients with colorectal cancer (black continuous line) and control patients with colorectal cancer (black dotted line). After adjustment for differences in age, stage, sex, subsite, country, and year of diagnosis, survival remained better for MUTYH-associated polyposis colorectal cancer patients than for control patients (hazard ratio of death = 0.48, 95% CI = 0.32 to 0.72, P < .001).
Perfect matching of all patients with MUTYH-associated polyposis and control patients was not feasible. There were some differences between patients with MUTYH-associated polyposis and control patients, including the number of positive lymph nodes (N stage), for which 69 (25%) of the 272 control patients had mismatches or missing information; whether metastasis occurred (M stage), in which 81 (30%) had mismatches or missing information; tumor subsite for which 33 (12%) had mismatches; exact year of diagnosis, for which 76 (28%) had mismatches; and sex, for which 128 (47%) had mismatches. Among the 272 control patients, there was a larger proportion of unknown N or M stage (39% or 106 patients) than among the 147 patients with MUTYH-associated polyposis (10% or 14 patients). In addition, patients with MUTYH-associated polyposis had statistically significantly more tumors located in the proximal colon (52% or 76 patients) than control patients (39% or 107 patients) (P = .015) and diagnosis before 1989 (19% or 28 patients vs 16% or 44 patients, respectively) (P = .046) (Table 1).
Table 1.
Characteristics of the total study population (n = 419), including 147 patients with MUTYH-associated polyposis colorectal cancer (MAP CRC) and 272 control patients with CRC
| Characteristic | Patients with MAP CRC | Control CRC patients | Total population |
| Male, No. (%) | 82 (56) | 124* (46)† | 206 (49) |
| Index patient, No. (%) | 113 (77) | 272 | 385 |
| Siblings, No. (%) | 34 (23) | 0 | 34 |
| Method of detection CRC | |||
| Symptomatic, No. (%) | 109 (74) | 272 | 381 |
| Surveillance, No. (%) | 34 (23) | 0 | 34 |
| Unknown, No. (%) | 4 (3) | 0 | 4 |
| Median age (range), y | 54.0 (32.1–81.1) | 52.1 (28.5–79.1) | 53.1 (28.5–81.1) |
| Location, No. (%) | |||
| Proximal colon | 76 (52) | 107 (39)‡ | 183 (44) |
| Distal colon | 21 (14) | 73 (27) | 94 (22) |
| Rectum | 38 (26) | 65 (24) | 103 (25) |
| Colon, not otherwise specified | 12 (8) | 18 (7) | 30 (7) |
| Unknown | 0 (0) | 9 (3) | 9 (2) |
| T stage, No. (%) | |||
| 0 or in situ | 6 (4) | 6 (2) | 12 (3) |
| 1 | 15 (10) | 33 (12) | 48 (12) |
| 2 | 22 (15) | 38 (14) | 60 (14) |
| 3 | 50 (34) | 118 (43) | 168 (40) |
| 4 | 8 (5) | 18 (7) | 26 (6) |
| Unknown | 46 (31) | 59(22) | 105 (25) |
| N stage, No. (%) | |||
| 0 | 85 (58) | 136 (50) | 221 (53) |
| 1 | 33 (23) | 54 (20) | 87 (21) |
| 2 | 15 (10) | 29 (10) | 44 (11) |
| Unknown | 14 (10) | 53 (20) | 67(16) |
| M stage, No. (%) | |||
| 0 | 125 (85) | 161 (59)§ | 286 (68) |
| 1 | 12 (8) | 25 (9) | 37 (9) |
| Unknown | 10 (7) | 86 (32) | 96 (23) |
| Period of diagnosis, No. (%) | |||
| 1967–1979 | 12 (8) | 20 (7) | 32 (8) |
| 1980–1989 | 16 (11) | 24 (9) | 40 (10) |
| 1990–1999 | 69 (47) | 136 (50) | 205 (49) |
| 2000–2006 | 50 (34) | 92 (34) | 142 (34) |
| Country, No. (%) | |||
| Germany | 55 (37) | 106 (39) | 161 (38) |
| United Kingdom | 42 (29) | 66 (24) | 108 (26) |
| the Netherlands | 50 (34) | 100 (37) | 150 (36) |
Unknown for six patients.
P = .046. χ2 test was used. All statistical tests were two-sided.
P = .015.
P < .001.
After adjustment for age, country, period of diagnosis, stage, subsite, and sex, risk of death was statistically significantly lower among patients with MUTYH-associated polyposis colorectal cancer than among control patients (HR = 0.48, 95% CI = 0.32 to 0.72, P < .001) (Table 2).
Table 2.
Cox regression analysis for patients with MUTYH-associated polyposis (MAP) colorectal cancer (CRC) compared with control CRC patients*
| Analysis | Hazard ratio (95% confidence interval) | Hazard ratio (95% confidence interval) |
| All patients (n = 419) | Symptomatic patients (n = 381)† | |
| Overall (MAP vs control CRC) | 0.48 (0.32 to 0.72) | 0.48 (0.32 to 0.73) |
| Stratified | ||
| Stage | ||
| I or II | 0.45 (0.23 to 0.91) | 0.47 (0.21 to 1.04) |
| III or IV | 0.64 (0.34 to 1.20) | 0.64 (0.33 to 1.22) |
| Site of diagnosis | ||
| Colon | 0.42 (0.26 to 0.67) | 0.40 (0.24 to 0.66) |
| Rectum | 0.48 (0.22 to 0.1.02) | 0.55 (0.24 to 1.25) |
| Country | ||
| United Kingdom | 0.66 (0.22 to 1.97) | 0.76 (0.25 to 2.32) |
| Germany | 0.28 (0.10 to 0.74) | 0.28 (0.10 to 0.82) |
| the Netherlands | 0.49 (0.31 to 0.79) | 0.45 (0.27 to 0.74) |
| Calendar period | ||
| 1967–1989 | 0.49 (0.20 to 1.17) | 0.40 (0.15 to 1.03) |
| 1990–2006 | 0.51 (0.30 to 0.85) | 0.52 (0.31 to 0.88) |
Data are from the Cox model with robust standard errors. The model was adjusted for the matching variables, age, country, period of diagnosis, stage, and site of colorectal cancer, and also for sex.
Symptomatic patients are MUTYH-associated patients who underwent colon screening because of symptoms (eg, anemia, nausea, diarrhea, or blood in the stool) and had colorectal cancer at presentation. MUTYH-associated patients in whom colorectal cancer was detected during surveillance because of a positive family history or previously identified polyps were excluded from this analysis.
When the analysis was stratified by stage, the survival benefit was higher among patients with stage I and II disease (HR = 0.45, 95% CI =0.23 to 0.91) than for stage III and IV disease (HR = 0.64, 95% CI = 0.34 to 1.20) (Table 2). The survival benefit was similar among patients with MUTYH-associated polyposis colorectal cancer whose tumor was in the colon (HR = 0.42, 95% CI = 0.26 to 0.67) and among those whose tumor was in the rectum (HR = 0.48 for rectum, 95% CI = 0.22 to 1.02). Increased survival was observed among patients with MUTYH-associated polyposis from all three countries (compared with control patients), with that for the German group being the highest. When the analysis was stratified by the period of diagnosis, similar survival benefits were observed for the period 1967–1989 (HR = 0.49, 95% CI = 0.20 to 1.17) and 1990–2006 (HR = 0.51, 95% CI = 0.30 to 0.85).
In this study, colorectal cancer was detected during surveillance in 25 of the 113 index patients with MUTYH-associated polyposis and in nine of the 34 siblings with MUTYH-associated polyposis. Colon surveillance was initiated in these 25 index patients and nine siblings because of previously identified polyps that caused symptoms, including constipation, diarrhea, or blood in the stool (n = 16), or because of a family history of colorectal cancer, most often in a parent (n = 18).
In four patients (three index patients and one sibling), the mode of detection of colorectal cancer was not known. When we excluded patients with MUTYH-associated polyposis colorectal cancer detected during surveillance from the analysis, we still observed statistically significant better survival among MUTYH-associated polyposis patients than among control patients with colorectal cancer (Table 2).
Discussion
In a European cohort, survival of MUTYH-associated polyposis patients with colorectal cancer was statistically significantly better than that of control patients with colorectal cancer. This advantage in survival remained statistically significant after adjustments for age, stage, colon site, period of diagnosis, country, and sex. In a stratification analysis for early-stage (ie, stages I and II) vs late stage (ie, stages III and IV) cancers, the survival benefit for patients with MUTYH-associated polyposis colorectal cancer compared with control patients with colorectal cancer was slightly higher among patients with early-stage colorectal cancer (HR = 0.45, 95% CI = 0.23 to 0.91) than among those with later stage colorectal cancer (HR = 0.64, 95% CI = 0.34 to 1.20).
To our knowledge, this is the first study to examine survival of MUTYH-associated polyposis patients with colorectal cancer compared with that of matched control patients with colorectal cancer from the general population. Patients with MUTYH-associated polyposis colorectal cancer were recruited from the largest MUTYH-associated polyposis cohort so far assembled.
Given the retrospective character of the study, there are many possible biases and limitations that might lead to an overestimation or an underestimation of survival benefits (eg, selection, lead-time, and length-time biases). Treatment was not reported for many patients in this study.
Selection Bias
It can be expected that patients from families with several affected members who survived their cancer may be more likely to come to the attention of clinical geneticists than those from families in which all affected members died from their disease. Therefore, cohorts of patients who are recruited through genotyping studies could be biased toward those with better prognosis. This form of bias may have been operating in previous studies of Lynch syndrome–specific survival (9,25). However, a number of observations are counter to this argument. First, Hampel et al. (26) reported that index patients from Lynch syndrome families are younger at diagnosis of colorectal cancer than other mutation-positive patients in their family. Therefore, patients who come to medical attention through genetic testing do not necessarily have a milder phenotype. Second, although patients who die young or shortly after their diagnosis of colorectal cancer might not come to the immediate attention of clinical geneticists, the nonaffected members of their family may be referred for genetic counseling. MUTYH genotyping can be done on DNA isolated from archived formaldehyde-fixed tumor tissue (27) of a deceased patient or in the DNA isolated from blood of parents and/or siblings who are still alive. Third, patients with MUTYH-associated polyposis who have a relatively mild phenotype (eg, nonaggressive colorectal cancer at a later age) are likely be underrepresented in our cohort of patients with MUTYH-associated polyposis because the likelihood that they could have inherited a predisposition toward colorectal cancer may be lower.
Another selection bias might be that patients with MUTYH-associated polyposis who have no polyps or only a few polyps (eg, 0–10 polyps) are likely to be underrepresented in our cohort, particularly when there is no family history of colorectal cancer in a sibling. MUTYH mutation screening in population-based colorectal cancer patients has shown that one-third of biallelic mutation carriers with colorectal cancer have no or only few polyps (eg, 0–10 polyps) (15,28). Such patients are less frequently referred for molecular genetic analysis than patients with more florid forms of polyposis (eg, more than 10 polyps, numerous or multiple polyps). It is not known whether prognosis of patients with no or only few polyps (eg, 0–10 polyps) differs from that of patients with MUTYH-associated polyposis with colorectal cancer and polyposis.
Finally, for UK patients with MUTYH-associated polyposis colorectal cancer who were diagnosed before 1996 (n = 19), we used control patients who were diagnosed in 1996 because the Cancer Registry did not have data before 1996. This procedure could have lead to better survival in control patients because treatment of cancer is expected to have improved between 1970 and 1996, the period in which the 19 UK case patients were diagnosed with colorectal cancer. However, after adjustment for date period in the multivariable Cox regression analysis, results remained unchanged.
Lead-Time and Length-Time Bias
Heightened awareness among and surveillance of high-risk patients lead to diagnoses at an earlier stage of disease (lead-time bias) and might account for an apparent survival advantage. Length-time bias is also a consideration if screening tests lead to detection of asymptomatic indolent tumors. Patients with MUTYH-associated polyposis who are enrolled in surveillance programs could gain a survival benefit by early detection.
As expected, there are differences in stage between patients whose disease was detected by surveillance and those whose disease was detected symptomatically; stage I disease was diagnosed in 14 (41%) of the 34 patients during surveillance and in 21 (19%) of the 109 patients diagnosed symptomatically. It should be noted that, after adjustment for other factors including stage, exclusion of patients with MUTYH-associated polyposis diagnosed during surveillance, survival benefit (ie, hazard ratio) did not change (Table 2, second column).
Other Possible Biases
Another explanation of the better survival of patients with MUTYH-associated polyposis compared with that of control patients might be that patients with MUTYH-associated polyposis receive more extensive surgery because they usually have more polyps. However, the overall survival of patients with MUTYH-associated polyposis might actually be worse because they are prone to develop multiple cancers. Indeed, 46 (31%) of the 147 patients with MUTYH-associated polyposis in this study actually had two or more colorectal cancers at the time of diagnosis or developed a second colorectal cancer later on in life.
Immune Response Differences and Survival Advantage
An active immune response (represented by a high number of tumor-infiltrating lymphocytes) is strongly associated with better survival rates in control patients with colorectal cancer (29–31). It has been proposed that the immune system of patients with high microsatellite instability and mismatch repair–deficient tumors might be more active than that of colorectal cancer patients in the general population, which would lead to better survival (32,33). Because of a defect in the DNA repair, more mutant proteins are expected in the mismatch repair–deficient tumors than in sporadic colon tumors. As a result, more peptide fragments of mutant proteins might be presented at the cell surface of the mismatch repair–deficient cancer cells, which activate the immune system. Furthermore, the enhanced mutation rate in these tumors may also induce a mutation burden that is not compatible with tumor cell survival.
We have previously shown (34) that MUTYH-associated polyposis colorectal cancers share similar characteristics with mismatch repair–deficient cancers, including a preferential proximal location, a high rate of mucinous morphology, and an increased level of tumor-infiltrating lymphocytes. The disruption of MUTYH protein function in MUTYH-associated polyposis carcinoma cells might lead to more oxidative DNA damage and generation of mutant peptides that could be presented to cytotoxic T cells through the expression of HLA class I receptors. It has, indeed, been shown that loss of expression of HLA class I receptors has been frequently identified in MUTYH-associated polyposis colorectal cancers and in mismatch repair–deficient colorectal tumors (35,36,37), indicating that these tumors may be subject to strong selective pressure that favors outgrowth of cancer cells that acquire an immune-evasive phenotype (37).
In conclusion, in this study, patients with MUTYH-associated polyposis colorectal cancer had statistically significantly better survival than matched control patients. The reasons for this difference remain unknown, but a compromised base excision repair system could render MUTYH-associated polyposis colorectal cancers more immunogenic than sporadic colorectal cancers, which are characterized predominantly by chromosomal instability. This survival difference may have implications for clinical decision making in relation to the timing and type of interventions required, such as surgery and chemotherapy. Future prospective studies are needed to confirm this survival difference between MUTYH-associated colorectal cancer patients and colorectal cancer patients from the general population.
Funding
This study was supported by the Cancer Research Wales, the Wales Office of Research and Development through the Wales Gene Park; the German Cancer Aid (grant 106244); and the Dutch Digestive Diseases Foundation (grant MWO 0355). Neither of these funds had any role in the design and conduct of the study; collection, analysis, or interpretation of the data; the decision to submit the manuscript for publication, and the writing of the manuscript. The authors had full responsibility for all activities above.
Footnotes
We thank the patients and families who agreed to participate in the study and all the doctors who assisted with recruitment. The authors also thank the Mallorca Group of European experts on hereditary gastrointestinal tumors for their helpful discussions.
The authors have no conflicts of interest to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(3):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sankila R, Aaltonen LA, Jarvinen HJ, et al. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology. 1996;110(3):682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- 3.Watson P, Lin KM, Rodriguez-Bigas MA, et al. Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer. 1998;83(2):259–266. [PubMed] [Google Scholar]
- 4.Lynch HT, Bardawil WA, Harris RE, et al. Multiple primary cancers and prolonged survival: familial colonic and endometrial cancers. Dis Colon Rectum. 1978;21(3):165–168. doi: 10.1007/BF02586560. [DOI] [PubMed] [Google Scholar]
- 5.Stigliano V, Assisi D, Cosimelli M, et al. Survival of hereditary non-polyposis colorectal cancer patients compared with sporadic colorectal cancer patients. J Exp Clin Cancer Res. 2008;27:39. doi: 10.1186/1756-9966-27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 7.Myrhoj T, Bisgaard ML, Bernstein I, et al. Hereditary non-polyposis colorectal cancer: clinical features and survival. Results from the Danish HNPCC register. Scand J Gastroenterol. 1997;32(6):572–576. doi: 10.3109/00365529709025102. [DOI] [PubMed] [Google Scholar]
- 8.Tomoda H, Baba H, Akazawa K. Prolonged survival in hereditary nonpolyposis colorectal cancer. Oncol Rep. 1999;6(2):321–324. doi: 10.3892/or.6.2.321. [DOI] [PubMed] [Google Scholar]
- 9.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354(26):2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 10.Feeley KM, Fullard JF, Heneghan MA, et al. Microsatellite instability in sporadic colorectal carcinoma is not an indicator of prognosis. J Pathol. 1999;188(1):14–17. doi: 10.1002/(SICI)1096-9896(199905)188:1<14::AID-PATH323>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Percesepe A, Benatti P, Roncucci L, et al. Survival analysis in families affected by hereditary non-polyposis colorectal cancer. Int J Cancer. 1997;71(3):373–376. doi: 10.1002/(sici)1097-0215(19970502)71:3<373::aid-ijc12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Salahshor S, Kressner U, Fischer H, et al. Microsatellite instability in sporadic colorectal cancer is not an independent prognostic factor. Br J Cancer. 1999;81(2):190–193. doi: 10.1038/sj.bjc.6690676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Tassan N, Eisen T, Maynard J, et al. Inherited variants in MYH are unlikely to contribute to the risk of lung carcinoma. Hum Genet. 2004;114(2):207–210. doi: 10.1007/s00439-003-1033-2. [DOI] [PubMed] [Google Scholar]
- 14.Lubbe SJ, Di Bernardo MC, Chandler IP, et al. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol. 2009;27(24):3975–3980. doi: 10.1200/JCO.2008.21.6853. [DOI] [PubMed] [Google Scholar]
- 15.Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst. 2004;96(21):1631–1634. doi: 10.1093/jnci/djh288. [DOI] [PubMed] [Google Scholar]
- 16.Farrington SM, Tenesa A, Barnetson R, et al. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005;77(1):112–119. doi: 10.1086/431213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP) J Med Genet. 2005;42(9) doi: 10.1136/jmg.2005.033217. e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer. 2006;119(4):807–814. doi: 10.1002/ijc.21905. [DOI] [PubMed] [Google Scholar]
- 19.Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362(9377):39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- 20.Out AA, Tops CM, Nielsen M, et al. Leiden open variation database of the MUTYH gene. Hum Mutat. 2010 doi: 10.1002/humu.21343. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organisation; 2000. [Google Scholar]
- 22.UICC. TNM Classification of Malignant Tumours. New York, NY: Wiley-Liss; 2002. [Google Scholar]
- 23.Brenner H, Stegmaier C, Ziegler H. Estimating completeness of cancer registration in Saarland/Germany with capture-recapture methods. Eur J Cancer. 1994;30A:1659–1663. doi: 10.1016/0959-8049(94)00259-8. [DOI] [PubMed] [Google Scholar]
- 24.Janssen-Heijnen MLG, Louwman WJ, van de Poll-Franse LV, et al. Results of 50 Years Cancer Registry in the South of the Netherlands: 1955–2004 (in Dutch) Eindhoven, the Netherlands: Eindhoven Cancer Registry; 2005. [Google Scholar]
- 25.Clark AJ, Barnetson R, Farrington SM, et al. Prognosis in DNA mismatch repair deficient colorectal cancer: are all MSI tumours equivalent? Fam Cancer. 2004;3(2):85–91. doi: 10.1023/B:FAME.0000039915.94550.cc. [DOI] [PubMed] [Google Scholar]
- 26.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Van Puijenbroek M, Nielsen M, Tops CM, et al. Identification of patients with (atypical) MUTYH-associated polyposis by KRAS2 c.34G > T prescreening followed by MUTYH hotspot analysis in formalin-fixed paraffin-embedded tissue. Clin Cancer Res. 2008;14(1):139–142. doi: 10.1158/1078-0432.CCR-07-1705. [DOI] [PubMed] [Google Scholar]
- 28.Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology. 2009;136(4):1251–1260. doi: 10.1053/j.gastro.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 30.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494. [PubMed] [Google Scholar]
- 31.Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182(3):318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Guidoboni M, Gafa R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159(1):297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. [PubMed] [Google Scholar]
- 34.Nielsen M, de Miranda NF, van PM, et al. Colorectal carcinomas in MUTYH-associated polyposis display histopathological similarities to microsatellite unstable carcinomas. BMC Cancer. 2009;9:184. doi: 10.1186/1471-2407-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloor M, Becker C, Benner A, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65(14):6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 36.Dierssen JW, de Miranda NF, Ferrone S, et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Miranda NF, Nielsen M, Pereira D, et al. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression-a common event amongst DNA-repair-deficient colorectal cancers. J Pathol. 2009;219(1):69–76. doi: 10.1002/path.2569. [DOI] [PubMed] [Google Scholar]