Abstract
The medial prefrontal cortex plays a role in anticipation of rewards and goal orientation, properties that are influenced by cocaine administration. Single unit firing was measured in the medial prefrontal cortex (mPFC) of 7 male rats during the expression of approach responses towards a sexually receptive female. Nose-poking in male rats was used as a measure of approach behavior during the following periods: a baseline, first exposure to a female, a second baseline two hours later and a second exposure to female 10 minutes after cocaine (15 mg kg−1 i.p.). Two types of excitatory responses were identified. First, a subset of cells (23%) showed increased firing activity during nose poke behavior upon presentation of the female, but not before. Another subset of cells (12%) showed increased firing in the presence of the female only after cocaine was administered. The present results provide preliminary evidence for neurons in the mPFC that are involved in sexually motivated approach behavior and that are modulated by cocaine.
Keywords: prelimbic, medial prefrontal cortex, male rat, sex, sexual motivation, nosepoke, female receptivity, mesocortical dopamine system, glutamatergic neurons, regular spiking neurons, cortical, cocaine, reward, microwire electrode arrays
Introduction, Methods, Results, Discussion
Mounting evidence implicates several extra-hypothalamic structures in appetitive or preparatory components of sexual behavior (Everitt, 1990). The prefrontal cortex is particularly interesting because of its role in organizing goal-directed behaviors and its physiological and anatomical connectivity with mesencephalic dopamine neurons (Watanabe, 1996). Activity of neurons in the medial prefrontal cortex (mPFC) of the rat has been associated with behaviors directed at obtaining natural and artificial rewards. Medial PFC neurons fire in a spatially dependent manner when rats are placed into an arena where they have been trained to search for a food reward (Hok et al., 2005). Elevated discharge patterns are also observed upon delivery of a liquid reward following a sequence of predictive stimuli (Cowen and McNaughton, 2007). Neurons of the mPFC showed both increases and decreases in activity during or immediately following lever press responding for water reward (Peters et al., 2005). Similar correlations between activity of mPFC neurons and lever pressing for cocaine and heroin have been reported (Chang et al., 1998). There is a lack of studies exploring single unit firing in this cortical region during sexually motivated behavior in rodents. However, there is behavioral evidence indicating that the mPFC does play a role in sexual behavior in male and female rats (Afonso, et al., 2007; Agmo and Villalpando, 1995; Agmo et al., 1995; Balfour et al., 2006; Hernandez-Gonzalez et al., 2007). Extensive dorsal prefrontal/anterior cingulate lesions, that included portions of the prelimbic area, dramatically reduced male copulatory performance in general (Agmo et al., 1995). It was noted in the latter study that the role of the mPFC in sexual motivation and sexual performance might be to process sensory feedback from females. In the absence of such processing, as with a focal lesion in this area sexual behavior is not initiated. Therefore, if true, distal presentation of a sexually receptive female should activate neurons within this cortical area. The present study used a simple measure of nosepoke behavior, which is easily carried out by rodents, to test whether mPFC neurons of male rats change their firing patterns during the approach towards a sexually receptive female. It is known that both sexually receptive females and cocaine are strong hedonic stimuli in male rats. Rats will lever press for cocaine and also for access to a receptive female and they also have been shown to develop conditioned place preference to both stimuli (Everitt, 1990). In order to investigate the interactions between sexual motivation and cocaine at the level of the prefrontal cortex, rats were tested in both the absence and presence of cocaine administration.
Seven Long-Evans male rats (325–400 g; 70–102 days old; Charles River Laboratories, Wilmington, MA) were housed individually in a temperature-humidity and light dark cycle controlled room (lights on 0700 hr–1900 hr). Water and Purina rat chow were provided ad libitum. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study. Surgical implantation of electrodes was carried out 3–7 days before experiments. Microwire electrode arrays (Plexon Inc, TX) were advanced into the right mPFC using standard stereotaxic procedures (Bregma coordinates AP: +3.2 mm to +3.3, ML: −0.5 to −0.75 mm and DV: −4 mm; intraraural line was set at −3.4 mm). Electrodes consisted of eight Teflon-coated stainless steel wires with a 50 μm outer diameter arranged in a 2 × 4 array. Impedance across electroplated wires was 240–500kOhms (Impedance Check Unit, FHC, Bowdoin, ME). Electrodes were then anchored to the skull with dental cement and 4 miniature stainless steel self-tapping screws (Thread 0.06 in. 1/8 in. length; J.I. Morris Precision Screws, Southbridge, MA). A ground wire was secured around 2 screws. Sexually naïve males were used for these studies. The specific day of testing after surgical procedures was dependent on availability of randomly selected sexually receptive females from the animal colony. Prior to recording sessions (0800 – 1100 hrs), females were placed into a cage with a male breeder and observed for hops, darts and ear wiggling and lordotic display with male mounting. In vivo recordings were carried out in a clear Plexiglas arena (chamber size 24″ L × 12″ cm W × 12″ H). The behavioral test cage used for neurophysiological recordings is shown in Figure 1B. The test box contained a removable wall, custom-made of black foam display board (0.25-inch thick), which divided it into two compartments. A 0.5-inch diameter hole was made through the dividing wall at the level of a rats snout. This allowed rats to easily make exploratory nosepokes. The chamber was located inside a custom designed sound attenuation box (30″ H × 27″ L × 23″ W, Med-Associates, St. Albans, VT) with an electrical commutator device near a digital camera centralized over the test arena. The entire chamber was located inside a Faraday cage (TMC Mfg., Peabody, MA). Digital video streams were acquired at 30 Hz and synchronized with neural signals. Rats were tested once clearly defined units were detected on the recording channels. Nosepoking behavior was identified and time-stamped using Cineplex markup software (Plexon Inc). The time stamps were used to align behavior and spike events for analysis.
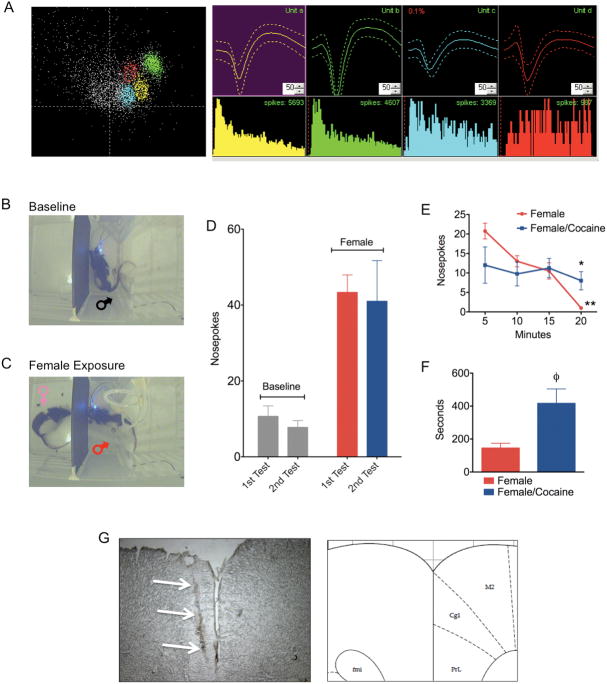
Figure 1.
Single unit recordings in mPFC of awake behaving male rats presented with a sexually receptive female. A) Representative single units isolated from the medial prefrontal cortex. B) Nosepoke responses before and C) following presentation of a sexually receptive female. D) Total amounts of nosepokes made during two baseline sessions and two female presentation sessions. E) Nosepokes over time during the presentation of a receptive female rats before and after cocaine administration (*significant difference between female and female/cocaine; **significant difference between 20 and 5 minutes). F) Latency in seconds (± standard error) to initiate nosepoke behavior after the introduction of a receptive female on the other side of the dividing wall (φ significant difference between female and female/cocaine). G) Cresyl violet stained section of the mPFC showing an example electrode lesion and its corresponding anatomical location according the Paxinos and Watson atlas of the rat brain.
Males were acclimated for 30 minutes before recordings. During this time, online sorting procedures were carried out. This was followed by a 20-minute recording session where the animal was allowed to explore the cage in the absence of the dividing wall. The dividing wall was then placed and baseline nose pokes were measured during an initial baseline (10 minutes) and an initial female exposure (20 minutes). Animals were returned to their home cages after the first session and re-tested 2 hours later. Four out of the 7 rats were re-tested. Therefore data for the re-test phase corresponds to 4 rats. During the re-testing phase, rats were again placed into the compartment and nose pokes measured during a second baseline (10 minutes). They were then given an intraperitoneal injection of cocaine (15 mg kg−1) and placed into the compartment for a third baseline (10 minutes) followed by re-exposure to the female (20 minutes). This dose of cocaine has been shown to produce sensitization to the locomotor effects of cocaine with changes in prefrontal dopamine receptor function (Febo et al., 2003), conditioned place preference that is blocked by prelimbic prefrontal lesions (Tzschentke and Schmidt, 1999), as well as increase extracellular concentrations of dopamine in the medial prefrontal cortex of the rat (Williams and Steketee, 2005). The re-test following cocaine injection was carried out to examine the role of monoamine transmission on prefrontal cell activity and behavior.
Neural data were collected using a multichannel acquisition processor system (MAP, Plexon, Dallas, TX). A 50–60 μV waveform amplitude cutoff was applied, along with a 1.2ms spike refractory period. Spikes were sorted online using a template matching algorithm (Nicolelis et al., 1997) and classification of individual units per channel was confirmed online from their clustering in 2D PCA space (Figure 1A). To maintain consistency across test session, the waveform detection parameters were stored on the first session and used for the 2nd session. Neural data were imported to Offline Sorter (Plexon Inc., TX) for resorting and classification. A template matching routine was used to drop waveforms that did not fall under the user-specified fit tolerance for waveform shape and amplitude (Nicolelis et al., 1997). Timestamps for the classified spikes and behavioral event data were then exported to Neuroexplorer for single unit analysis. Perievent time histograms (PETH) and raster plots that aligned nosepokes to spike timestamps were generated. This allowed inspection of unit firings around the time of behavior. Spikes were binned at 100 msec intervals and analyzed around a 6 second window flanking the behavioral event of interest. Significant changes in firing rates were considered above or below 99% confidence intervals (2 or more spikes surpassing 99% C.I.). Unit firing rates were converted to Z-scores as an additional method of detecting significant changes in unit firing (spikes were considered significant if above or below 2.5σ). A non-parametric Kruskall-Wallis ANOVA confirmed significant changes in firing rates of single units for the different behavioral events. Number of nosepokes was quantified over the various epochs (baselines, female presentation before and after cocaine). Total counts, nosepoke rate (events per minute) and latency to first nosepoke were analyzed (Figure 1D–F). All behavior, single unit and population statistical analysis was done using Statistical Package for the Social Sciences (SPSS, Chicago, IL) and GraphPad Prism (La Jolla, CA). After studies, animals were sacrificed and brains stored in formalin solution for verification of electrode placement site using standard histological techniques (Figure 1G).
Rats reliably showed nosepoke behavior during baseline and female presentation periods (Figure 1B–F). No differences were observed between the first and second sessions, but a greater amount of nosepokes was observed with the presentation of females than in their absence (Figure 1D). Cocaine treatment did not increase the total amount of nosepoking behavior in the presence of the female (Figure 1D). However, a closer inspection of the temporal dynamics of nosepoke behavior indicates that during the first presentation, rats showed a high rate of nosepoking that declined over time to 0 nosepokes at 20 minutes (Figure 1E; p = 0.001, Friedman non-parametric ANOVA). On the other hand, following cocaine administration, rats showed stable nosepoking behavior throughout the 20-minute testing period (Figure 1E). It was also noted that rats did not begin nosepoking immediately following cocaine injection, during the 10-minute cocaine period, but only at a latency of 400 seconds (Figure 1F) after the female was reintroduced. Although rats immediately following cocaine treatment made no nosepokes, an increase in general motor activity was observed.
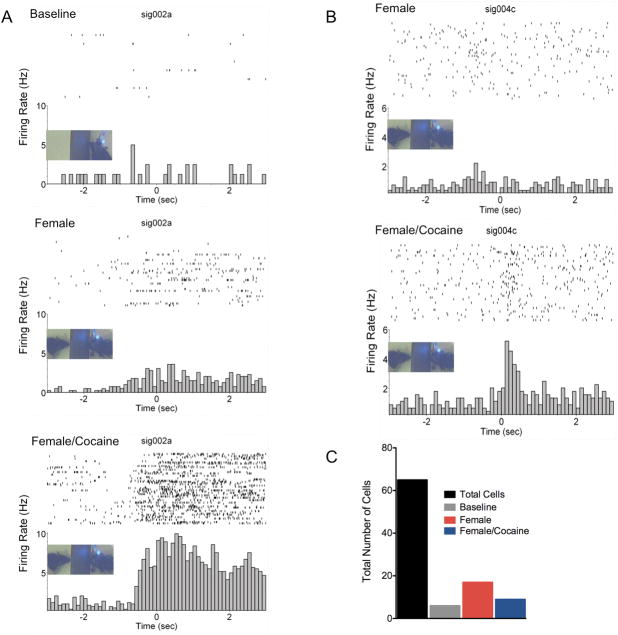
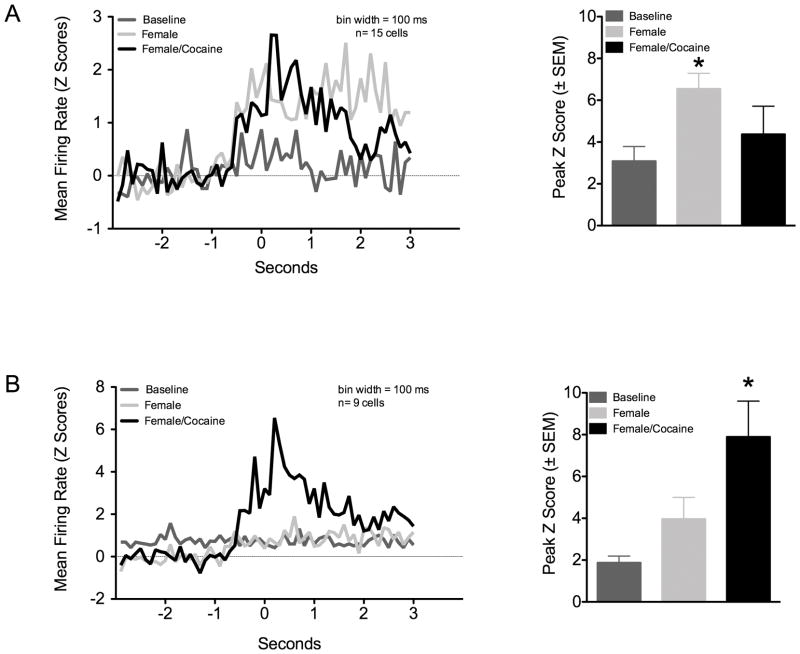
Sixty-five total neurons from 7 rats were classified as single units. An average 9 ± 6 S.D. units were recorded per rat; however, the sample distribution was unequal across the animals. Activity of the 65 neurons was measured across 38 out 56 of total active wires implanted on the 7 rats (mean number of active wires per rat was 5.4 ± 1.7S.D. out of 8 total per rat). The overall mean firing rate was 1.6 ± 0.2Hz (range: 0.1–13 Hz). Peaks in the interspike interval distribution were observed at 10–20 ms (mode ISI = 24 ms; Figure 1A). Both excitatory and inhibitory responses were observed across all trials, but mostly excitatory responses were observed to be associated with the presence or absence of female. Six units were found to increase firing during 1st baseline nosepoking, 8 during the 2nd baseline, 18 units were active during 1st exposure to female and 12 units only after cocaine administration. Out of the 65 cells, 15 (23% recorded from 7 rats) were observed to selectively increase firing with the initial presentation of the female and an additional 8 (12% recorded from 4 rats) showed increased firing only after exposure to cocaine (Figure 2C). An additional 4 cells (3 female pre-cocaine, 1 female post-cocaine) showed increases in firing activity after exposure to females, but these also fired either during baseline nosepoking or before cocaine administration and were thus non-selective for the trials. PETH’s and raster plots in Figure 2 shows example excitatory responses. A subset of cells that did not increase firing activity during baseline nosepoking did so upon presentation of females. This was observed as an increased probability of firing just prior to the nosepoke responses. In the cell shown in Figure 2A, increased spiking was still observed during re-test baseline (2nd baseline) before reintroducing the female rat (this was observed in 3 out of 65 cells; data not shown). Reintroducing the female following a single injection of cocaine dramatically increased the probability of firing during nosepoking. On the other hand, the cell presented in Figure 2B showed increased firing activity during nosepoking only after a cocaine injection. Analysis of the total group of cells showing these patterns of activity is shown in Figure 3. Two main firing modes were identified. A non-parametric Kruskall-Wallis ANOVA was used to test for differences in peak firing across trials (α = 0.05; Bartlett’s test for equal variances indicated significant differences). One subset of cells showed increased peak firing activity upon presentation of female rats (Kruskall Wallis H stat = 12, p = 0.008). These cells did not show further increases in firing rate following cocaine administration. Another subset of cells showed an increased firing during the presence of the female (p = 0.054), that was further increased following cocaine administration (Kruskall Wallis H stat = 13, p = 0.004 vs. baseline). These cells also showed a significant difference when comparing activity during the 2nd and 1st baseline period before presentation or re-exosure to the female, respectively (p < 0.05). Bivariate correlations between neuronal firing rates and nosepoke rates over the course of the female presentation epochs were performed but not significant correlations were observed (data not shown).
Figure 2.
Perievent time histograms and overlying raster plots for two representative excitatory neuronal responses in mPFC. All plots have a bin width of 100 ms. The 0 second mark aligns spikes around the nosepoke event. Data presented as spikes second−1 (Hz). A) Activity of a cell during nosepoke behavior during a baseline, presentation of a sexually receptive female and re-exposure to the female following cocaine administration. B) Activity of a cell that fired during nosepoking behavior in the presence of a sexually receptive female, but only after administration of cocaine. C) Summary of the proportions of total cells sampled and those categorized as responding during baseline and receptive female presentations.
Figure 3.
Perievent histograms for subsets of mPFC cells showing two modes of firing activity during nosepoke responses in the presence of a sexually receptive female. The 0 second mark aligns spikes around the nosepoke event. A) Cells that predominantly increased firing activity during presentation of a sexually receptive female. B) Cells that mostly showed increased firing in the presence of the female only after cocaine administration. *Significantly different from baseline cell firing (p < 0.05, Kruskall-Wallis ANOVA).
The present pilot results are consistent with the notion that neurons in the mPFC are somehow involved in approach responses in the presence of a sexually motivating stimulus. Firing activity of neurons in mPFC increased during nosepoke behavior in the presence, but not the absence of a sexually receptive female. This occurred even though nosepoke behavior was displayed in the absence of the female. Therefore, the exploratory action of the rat may have acquired a different connotation when the female was presented on the other compartment opposite to the nosepoke hole. It is interpreted that in the presence of stimuli arising from the receptive female, the nosepoke behavior constitutes an approach response towards sex or, at minimum, towards social interaction. It is unclear what specific female-derived sensory stimulation caused this change in prefrontal cell firing, but it is likely that odors and even vocalizations may have been important as sensory feedback to the male. It is important to note that the present preliminary results fall short of a true sexual motivation study due to the fact that the males were sexually naïve and had not yet experienced physical interactions with females. The responses are thus assumed to be innate in the male and not learned through experience. It will be interesting in future studies to take into account the males sexual experience, the females receptivity status, and whether appetitive and consummatory aspects of sexual behavior would modify prefrontal cortical cell firing patterns. There may also be temporal ordering effects in the sequence in which stimuli were presented. This should be taken into account in future experiments. Neurons that responded only after cocaine was administered could arguably be due to the second exposure and not the drug per se. However, cocaine did have two very noticeable effects on the animal’s behavior that did not arise from merely the temporal ordering of the study design. First, there was no nosepoking immediately after cocaine administration during a 10-minute baseline recording. Also, once the female was reintroduced the animals showed significant delay in their re-initiation of nosepoking. Third, the nosepoking remained stable throughout the second session whereas it dropped significantly towards the end of the first session. This latter observation may be due to loss of interest in the first session and a regain in the second session after cocaine was given to rats. The behavior observations, which were used to analyze cell firing, appear to suggest that the changes in neuronal activity were related to drug treatment and not the temporal ordering. A better design would clarify this.
There are a handful of experiments specifically directed at testing the role of the prefrontal cortex in male and female sexual behaviors, including preparatory and consummatory aspects. The available studies have used focal lesions of the mPFC and other surrounding frontal cortical areas (Agmo et al., 1995), c-fos expression following mounting, ejaculatory bouts and just distal exposure (Balfour et al., 2006), and recordings of multiunit field activity and electroencephalographic patterns (Hernandez-Gonzalez et al., 2005a; Hernandez-Gonzalez et al., 2005b; Hernandez-Gonzalez et al., 2007). There is consensus across these cited studies that the mPFC is involved in varying aspects of sexual behavior, from motivational components to consummatory behavioral aspects such as pelvic thrusts preceding ejaculation. The present study adds additional information by showing a direct relationship between approach responses towards a sexually receptive female and real-time activity in single cells of the mPFC. There are two important findings of the present study that deserve future attention. The first is that groups of neurons that had not changed their firing patterns before exposure to the female, during the first baseline, did so during the second baseline. This might suggest that plasticity related to the social stimulus may have occurred. This remains to be further examined. Furthermore, cocaine had a dramatic effect on cell firing in the mPFC. Neurons that did not fire in the presence of the sexually receptive female, during the first encounter, displayed increased activity following a single injection of cocaine. It is likely that local glutamatergic (regular spiking) neurons receiving mesencephalic dopaminergic inputs are mediating this ‘switch’ to firing in the presence of the socially relevant stimulus.
The medial prefrontal cortex receives prominent mesencephalic monoaminergic inputs that are associated with reward seeking, goal-oriented behavior, working-memory and emotional learning (Laviolette et al., 2005; Phillips et al., 2004; Rossetti and Carboni, 2005). Thus, motivation and cognitive functions are controlled by dopamine (DA), norepinephrine (NE) and serotonin (5-HT) receptors within frontal cortical regions. Although partly speculative, the present results seem to support the idea that mPFC 5-HT, NE and/or DA inputs that are also modulated by cocaine and other drugs of abuse might also control sexual motivation. Furthermore, the results provide partial evidence that cocaine might alter how neurons in the mPFC respond to a sexually-motivating stimulus. If true, this may have interesting implications on the influence of drugs of abuse on sexual motivation and it would remain to be tested whether a similar mechanism would be observed in frontal cortical areas of primates (Watanabe, 1996). There is, however, evidence for increased sexual desire in humans administered the psychostimulant methylphenidate (Volkow et al., 2007). These results also suggest that the mPFC may be a site for the interaction between competing hedonic stimuli (Mattson and Morrell, 2005). Indeed, amphetamine has recently been shown to enhance female and male sexual motivation and copulatory performance, respectively (Afonso et al., 2009; Fiorino et al., 1999). What is important in the context of the present evidence is that the interactions between two hedonic stimuli occur within the mPFC. Although some cells fire in response to the presence of a receptive female and other studies have shown that cues predicting rewards also increase firing, here we find a subset of neurons that only respond with the combination of receptive female and cocaine. Interestingly, the firing occurs prior to the nosepoke exploratory behavior, perhaps suggesting that anticipation may play a role in the processing of neuronal activity in mPFC.
Acknowledgments
Support provided by NIH grant DA019946 and Northeastern University seed funds.
Cited References
- Afonso VM, Mueller D, Stewart J, Pfaus JG. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology. 2009;205(1):35–43. doi: 10.1007/s00213-009-1511-x. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behavioral neuroscience. 2007;121(3):515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Agmo A, Villalpando A. Central nervous stimulants facilitate sexual behavior in male rats with medial prefrontal cortex lesions. Brain research. 1995;696(1–2):187–193. doi: 10.1016/0006-8993(95)00853-i. [DOI] [PubMed] [Google Scholar]
- Agmo A, Villalpando A, Picker Z, Fernandez H. Lesions of the medial prefrontal cortex and sexual behavior in the male rat. Brain research. 1995;696(1–2):177–186. doi: 10.1016/0006-8993(95)00852-h. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Brown JL, Yu L, Coolen LM. Potential contributions of efferents from medial prefrontal cortex to neural activation following sexual behavior in the male rat. Neuroscience. 2006;137(4):1259–1276. doi: 10.1016/j.neuroscience.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18(8):3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98(1):303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neuroscience and biobehavioral reviews. 1990;14(2):217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem. 2003;86(2):405–412. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19(1):456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005a;70(2):132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Prieto-Beracoechea C, Navarro-Meza M, Ramos-Guevara JP, Reyes-Cortes R, Guevara MA. Prefrontal and tegmental electrical activity during olfactory stimulation in virgin and lactating rats. Physiology & behavior. 2005b;83(5):749–758. doi: 10.1016/j.physbeh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Prieto-Beracoechea CA, Arteaga-Silva M, Guevara MA. Different functionality of the medial and orbital prefrontal cortex during a sexually motivated task in rats. Physiology & behavior. 2007;90(2–3):450–458. doi: 10.1016/j.physbeh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 2005;102(12):4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25(26):6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135(2):315–328. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18(4):529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56(2):74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24(2):547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J Neurosci. 2005;25(9):2322–2329. doi: 10.1523/JNEUROSCI.3038-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. The European journal of neuroscience. 1999;11(11):4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Jayne M, Wong C. Stimulant-induced enhanced sexual desire as a potential contributing factor in HIV transmission. Am J Psychiatry. 2007;164(1):157–160. doi: 10.1176/ajp.2007.164.1.157. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382(6592):629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Effects of repeated cocaine on the release and clearance of dopamine within the rat medial prefrontal cortex. Synapse. 2005;55(2):98–109. doi: 10.1002/syn.20093. [DOI] [PubMed] [Google Scholar]