Abstract
Rationale
Moderate doses of alcohol impair response inhibition and slow response activation, and some recent work has shown that during a single dose, response inhibition recovers from the impairing effects of alcohol more slowly than response activation. Evidence for a possible lag in tolerance development to inhibitory versus activational mechanisms suggests that as blood alcohol declines, drinkers' response inhibition might continue to be impaired despite having an unimpaired ability to activate responses; however, this effect has not been studied across repeated doses.
Objective
The present study examined how cross-session tolerance to the impairing effects of alcohol develops differentially between response activation and inhibition.
Methods
Thirty-two healthy adults performed a cued go/no-go task that measured response activation and inhibition. The study tested the degree to which response activation and inhibition developed acute and cross-session tolerances to a moderate dose of alcohol (0.65 g/kg) administered twice on separate days.
Results
Alcohol slowed response activation and decreased response inhibition during both administrations. Response activation displayed acute tolerance to alcohol impairment during both administrations and cross-session tolerance from the first to second administration. By contrast, response inhibition showed no acute or cross-session tolerance.
Conclusion
Biased recovery of response activation over inhibition during a single dose and as doses are repeated could contribute to some of the impulsive behavior commonly observed under alcohol.
Keywords: Alcohol, Inhibition, Activation, Tolerance, Response Conflict, Go/no-go task
Laboratory studies have provided considerable support for the notion that alcohol can promote impulsive action by impairing basic inhibitory mechanisms that normally serve to suppress inappropriate behavior. Studies using cued go/no-go and stop-signal tasks find that alcohol reduces the ability to inhibit pre-potent (i.e., instigated) actions, even at moderate blood alcohol concentrations (BACs) below 100 mg/100 ml (e.g., de Wit et al. 2000; Marczinski and Fillmore 2003; Mulvihill et al. 1997). Studies also suggest that the disinhibiting effects of alcohol are most pronounced when the inhibition of a response is in conflict with a strong instigation to display the response (Conger 1956; Curtin and Fairchild 2003; Fillmore and Vogel-Sprott 2000). Such conflict is present in situations where there are equal reinforcers or punishments for the expression and inhibition of a behavior. Together, these findings are important because they identify a basic inhibitory mechanism that is impaired by alcohol which could contribute to the display of impulsive, aggressive, and other socially inappropriate behaviors when under the influence of drug (Fillmore 2003, 2007; Jentsch and Taylor 1999). Moreover, some recent studies suggest that alcohol-induced impairment of inhibitory control might also contribute to alcohol abuse by promoting excessive or binge drinking (e.g., Marczinski et al. 2007; Weafer and Fillmore 2008). As such, it is important to understand factors that might alter the degree to which inhibitory control is impaired by alcohol. One such factor that has received little attention is alcohol tolerance.
Alcohol tolerance refers to a diminished response to the drug as a function of repeated exposures. Tolerance to the behaviorally impairing effects of alcohol has been well documented for some time (e.g., Goldberg 1943; Newman and Card 1937). Much of the work has focused on impaired motor coordination and ataxia. Studies of laboratory animals and humans have shown that performing motor tasks while intoxicated promotes rapid development of tolerance to the motor-impairing effects of the drug (e.g., Chen 1968; LeBlanc et al. 1973; Mann and Vogel-Sprott 1981). Such “functional” or “learned” tolerance is often task-specific and is assumed to result from learning to overcome the impairing effects of alcohol during intoxicated task practice (Kalant 1987). Alcohol tolerance also has been observed over the time course of a single dose. Early last century, Mellanby (1919) compared the intensity of alcohol impairment at a given BAC during ascending versus descending limbs of the blood alcohol curve (Mellanby 1919). He observed that alcohol-induced ataxia in dogs was less intense at a given BAC during the descending versus the ascending limb of the curve. This reduced impairment during the descending limb is commonly referred to as “acute tolerance”. Since that time, the finding has been replicated using a wide range of behaviors in a variety of subjects, including humans (e.g., Hurst and Bagley 1972; Haubenreisser and Vogel-Sprott 1987; Post et al. 1998).
Although much is known about tolerance to motor-impairing effects of alcohol, little is known about tolerance to the impairing effects of alcohol on inhibitory control. A recent study used the cued go/no-go model to examine acute tolerance to alcohol impairment of response inhibition and response activation (Fillmore et al. 2005). Subjects received a moderate dose of alcohol (0.65 g/kg) and performed the cued go/no-go task at the same BAC during the ascending and descending limb. Alcohol impaired response inhibition and response activation during the ascending limb as evident by increased failures to inhibit responses to no-go targets and slower reaction times to go targets. Impaired response activation showed acute tolerance as response speed increased during the descending limb. By contrast, impaired response inhibition showed no acute tolerance, as subjects continued to display increased inhibitory failures on the descending limb.
Taken together, these findings indicate that during a single dose, response activation recovers from the impairing effects of alcohol more swiftly than response inhibition. Evidence for a lag in tolerance development to inhibitory versus activational mechanisms suggests that as blood alcohol declines, drinkers' response inhibition might continue to be impaired, despite having an unimpaired ability to activate responses (see also Pihl et al. 2003; Schweizer et al. 2004). Evidence that acute tolerance results in such an “activational bias” of behavior would have important implications for understanding some of the behaviorally disruptive effects of the drug. An activational bias after drinking could increase the likelihood of disinhibited or impulsive behaviors. As such, it is important to understand how inhibitory control is altered as alcohol tolerance develops.
To date, tolerance to alcohol impairment of inhibitory and activational mechanisms of behavioral control has only been studied over the time course of a single dose, and no studies have examined tolerance when the dose is repeated. Furthermore, nothing is known about how tolerance might develop in the presence of a heightened motivational conflict. Therefore, this study used the cued go/no-go model to examine tolerance to alcohol-induced impairment of activational and inhibitory mechanisms in a motivated conflict situation. Tolerance was examined over two separate sessions in which subjects received a moderate dose of alcohol (0.65 g/kg). The cued go/no-go task assessed response inhibition and activation twice during each administration: once as BAC ascended and again at a comparable BAC on the descending limb. Participants were tested under motivated conflict in which they were monetarily rewarded for fast responding to go targets and punished by an equal monetary loss for failures to inhibit responses to no-go targets. In accord with previous research (Fillmore et al. 2005), it was hypothesized that response activation would display greater acute tolerance to the impairing effects of alcohol than response inhibition. With regard to tolerance across dose administrations, some theories suggest that acute tolerance contributes to tolerance that is observed as doses are repeated (e.g., Beirness and Vogel-Sprott 1984; Kalant et al. 1971). In accordance, it was predicted that response activation would also display greater tolerance across dose administrations compared with response inhibition.
Methods
Participants
Thirty-two healthy adult volunteers (16 men and 16 women) were recruited through advertisements in the local community. Volunteers were between the ages of 21 and 36, and were non-dependent social drinkers. Respondents were required to be in good health and report occasional alcohol use (e.g., at least one occasion per week and at least three drinks on one occasion within the past 30 days). Potential volunteers were excluded if they had histories of serious physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma, CNS tumors, past histories of psychiatric disorder (i.e., Axis I, DSM IV), substance abuse disorder, medical contraindications to alcohol, use of prescription medications, and current pregnancy or were breast-feeding. Additionally, those reporting a score of 5 or higher on the Short-Michigan Alcoholism Screening Test (S-MAST) (Seltzer et al. 1975) were excluded from participation. Volunteers were required to have a minimum of grade 8 education, a demonstrated reading ability, and no uncorrected vision or auditory problems. The study was approved by the University of Kentucky Medical Institutional Review Board, and all subjects provided written informed consent. Subjects earned a minimum of $70 (and a maximum of $95) for participating in the study.
Apparatus and materials
Cued go/no-go task
Response activation and inhibition were measured by a cued go/no-go reaction time task used in other research (e.g., Fillmore et al. 2005; Marczinski and Fillmore 2003). The task was operated using E-Prime software (Schneider et al. 2002). A trial involved the following sequence of events: a cue (a horizontal or vertical white rectangle) displayed for one of five stimulus onset asynchronies (SOAs: 100, 200, 300, 400, and 500 ms), a go or no-go target (green or blue rectangle) that remained visible until the participant made a response or 1,000 ms had elapsed, and an inter-trial interval of 700 ms.
The orientation of the cue (horizontal or vertical) correctly signaled the target 80% of the time. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to inhibit this response if a no-go (blue) target appeared. Inhibitory and activational tendencies show rapid development of cue-dependence as cues come to elicit preparatory processes for the inhibition or execution of behavior (e.g., Miller et al. 1991; Posner 1980). The go cue condition was of particular interest with respect to response inhibition as it generates response prepotency; however, subjects must overcome this response prepotency in order to inhibit the response when a no-go target is displayed. Failures to inhibit responses to no-go targets are more frequent following go cues compared with no-go cues (Miller et al. 1991). Similarly, the no-go cue condition was of particular interest regarding response activation because alcohol's slowing effect on reaction time is most evident in this condition. A test consisted of 250 trials that presented the four possible cue-target combinations.
Response conflict was motivated by monetarily rewarding performance. Participants received 5 cents for each fast response to go targets (255 ms or less) and lost 5 cents for each failure to inhibit a response to a no-go target (incorrect response). Participants were informed of how much money was earned immediately upon completion of each test. A test required approximately 15 min to complete.
Additional measures
A grooved pegboard task (Lafayette Instruments) measured subjects' motor coordination. Participants picked up pegs one at a time to fill the 25 holes on the board one row at a time from left to right. Time to complete the trial was measured in seconds. A test consisted of four trials, and the average completion time was the measure of motor coordination. Motor coordination is impaired by alcohol at this dose and typically shows acute tolerance (Beirness and Vogel-Sprott 1984). The pegboard task was included to verify acute tolerance to motor coordination in the present study.
Subjective intoxication was also measured. Participants rated their subjective intoxication from alcohol on a 100-mm visual analog scale (VAS) with the left side (0 mm) indicating “not at all” and the right side (100 mm) indicating “very much”. The personal drinking habits questionnaire (Vogel-Sprott 1992) provided three measures of a participant's typical drinking habits: weekly frequency of drinking, number of drinks typically consumed per occasion, and hourly duration of a typical occasion.
Procedure
Design overview
Half of the men and women were randomly assigned to an alcohol condition (ALC) or to a placebo condition (PLA). The initial sober baseline performance level of both groups was assessed by having subjects perform the tests after receiving a placebo drink that contained no alcohol. Following baseline, subjects attended two separate dose testing sessions on different days. During these sessions the ALC group had their performance measured twice following 0.65 g/kg of alcohol: once as BAC ascended and again as BAC descended. The PLA group was treated identically but instead received a placebo to control for any changes in performance due to repeated testing over the sessions. The following sections describe the procedures in detail.
Intake assessment and familiarization
Participants provided informed consent and completed medical history, alcohol/drug-use, and demographic questionnaires. Participants were familiarized with the pegboard and cued go/no-go task. Task familiarization was sufficient to prevent practice effects during subsequent sessions.
Pre-session checks
Subjects were required to fast and to abstain from caffeine for 4 h prior to each session. At the beginning of each dose session, volunteers completed a pre-session questionnaire that collected information about alcohol consumption, recent medication use, food consumption, and caffeine consumption. Subjects also performed a standard field sobriety test of motor coordination, and provided a breath sample using a breath analyzer to verify a zero blood alcohol concentration (BAC) (Alco-Sensor III, Intoximeters, Inc., St Louis, MO). A urine sample was then obtained to test for the presence of cocaine/benzoylecgonine, benzodiazepines, barbiturates, tetrahydrocannabinol (THC), d-amphetamine, and opiates (On Trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN). Females were also tested for pregnancy via this urine analysis. No smoking was allowed during any session.
Baseline session
Participants received a placebo consisting of carbonated mix with 3 ml of alcohol floated on the surface to provide a strong alcoholic scent as the beverage was consumed. Participants had 6 min to consume the drink. Ten minutes later, subjects performed the test battery that consisted of the cued go/no-go task, the pegboard task, and subjective intoxication rating. The battery required approximately 20 min. Baseline performance was tested after a placebo in order to hold constant the expectancy of alcohol during all test sessions in the experiment.
Dose administration sessions
During the two dose administration sessions, those in the ALC group were tested in response to 0.65 g/kg of alcohol, and those in the PLA group were tested following a placebo. All drinks were consumed in 6 min. For ALC, doses were calculated based on body weight and administered as absolute alcohol mixed with three parts carbonated soda. The dose produces an average peak BAC of 80 mg/100 ml at approximately 60 min and begins to decline at about 70 min (Fillmore and Blackburn 2002; Fillmore et al. 2005). Participants performed the test battery while BAC ascended (40 min post-administration) and while it descended (100 min post-administration). Based on previous studies, these testing times were expected to occur at comparable BACs on each limb (approximately 70 mg/100 ml) (Fillmore et al. 2005). Subjects' BACs were measured just prior to each test battery (40 and 100 min post-administration). After the second test battery, subjects watched television and relaxed until BACs fell below 20 mg/100 ml, after which, they were released following a field sobriety test. The two dose sessions were conducted on different days, with a minimum inter-session interval of 24 h and a maximum interval of 2 weeks. The PLA group was treated identically, except that they received a placebo instead of alcohol. The placebo was identical to the placebo used in the baseline session. All participants were paid and debriefed following the completion of the final session.
Criterion measures of performance and data analyses
Cued go/no-go performance
Response activation was measured by subjects' mean reaction time (RT) to go targets in the no-go cue condition for each test. Dose effects during the test sessions were measured as the change from baseline. A subject's baseline RT score was subtracted from the subject's RT score on each test so that positive change scores indicated impaired (i.e., slower) response activation on the test. Acute and cross-session tolerances were examined by a 2-group (ALC versus PLA) × 2-test (ascending versus descending limb) ×2-session (first versus second) mixed-design analysis of variance (ANOVA) of these scores.
Failure of response inhibition was measured as the proportion (p) of no-go targets in the go cue condition for which a subject failed to inhibit a response (p-failure). Dose effects during test sessions were measured as change from baseline. An increase in p-failures indicated impaired response inhibition compared with baseline. Acute and cross-session tolerances were examined by a 2 (group) × 2 (test) × 2 (session) ANOVA of these scores.
Motor coordination and subjective effects
Dose effects on subjects' motor coordination and ratings of subjective intoxication were also measured as change from baseline and analyzed by 2 (group) × 2 (test) × 2 (session) ANOVAs.
Results
Sample characteristics
The groups did not differ significantly in age or weight (p values>.68). The average age of the sample was 22.9 years (SD=2.4), and the average weight of the sample was 68.9 kg (SD=11.7). The groups also did not differ in drinking habits as measured by weekly frequency, number of drinks typically consumed, and duration of typical drinking episode (p values>.27). The entire sample reported an average weekly frequency of 2.8 (SD = 1.9) drinking occasions, with a typical consumption of 4.7 (SD=2.2) drinks per occasion over an average duration of 3.8 h (SD= 1.6). The groups also did not differ in the inter-session interval between dose sessions (p>.41). The average inter-session interval for the sample was 5.4 days (SD=3.6).
There was no detectable BAC in the PLA. BACs of the ALC group were analyzed by a 2-gender × 2-test (ascending versus descending limb)×2-session (first versus second) ANOVA that revealed no significant main effects or interactions (p values>.13). The BACs were comparable between men and women across limbs and across the sessions. During dose session 1, the mean BACs for the ascending and descending limbs were 75 mg/100 ml (SD = 19) and 84 mg/100 ml (SD=18), respectively. During dose session 2, the mean BACs for the ascending and descending limbs were 81 mg/100 ml (SD=21) and 80 mg/100 ml (SD=16), respectively.
Cued go/no-go performance
Response activation
No significant group difference in baseline RT was obtained (p>.47). The mean baseline RT of the sample was 301.8 ms (SD=22.8). The 2 (group)×2 (test) × 2 (session) ANOVA of change in RT showed a significant main effect of Group, F(1, 30)= 10.43, p<.01, and a significant group×test interaction, F(1, 30)=6.19, p=.02. Figure 1 plots the mean change in RT for each group during the ascending and descending limbs on each dose session. The figure shows that the group effect was due to a slowing of RT in the ALC group compared with little change from baseline displayed in the PLA group. Thus, as expected, alcohol impaired response activation by slowing RT. Figure 1 also shows that the interaction was due to reduced impairment over limbs in the ALC group (i.e., acute tolerance) coupled with little change in RT across these tests in the PLA group. For the ALC group, planned comparison t tests confirmed significantly less impairment on tests during the descending versus the ascending limbs (p values<.03) and less impairment during the second versus first dose administration (p values<.04). By contrast, no significant changes in RT were observed in the PLA group (p values>.05).
Fig.1.
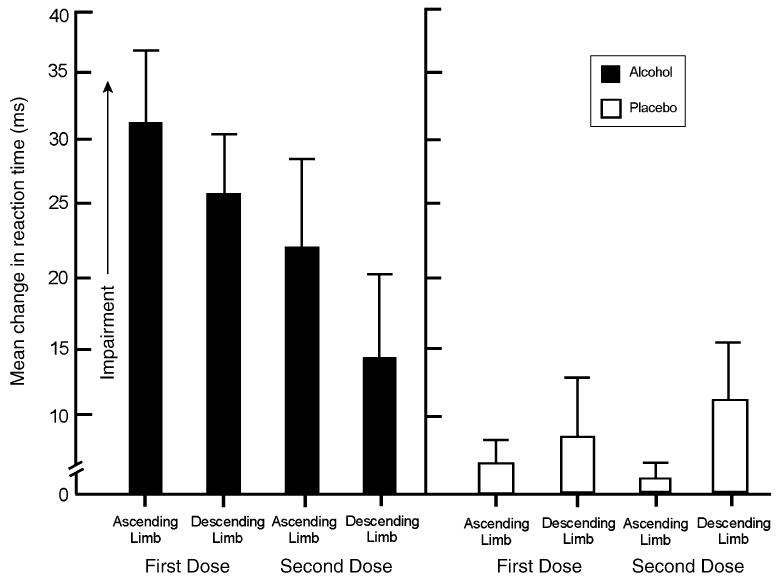
Mean change from baseline reaction time on the cued go/no-go task in response to 65 g/kg of alcohol (ALC) or placebo (PLA). Capped vertical lines show the standard error of the mean
Response inhibition
No significant group difference in baseline p-failures was obtained (p>.94). The mean p-failure score of the sample during baseline was 0.07 (SD= 0.1). The 2 (group) × 2 (test) × 2 (session) ANOVA showed a significant main effect of Group, F(1, 30)=2.99, p=.05. No other main effects or interactions were observed. Figure 2 plots the mean change in p-failures for each group during the ascending and descending limbs during each dose session. The figure shows that the group effect was due to increased inhibitory failures of the ALC group compared with the PLA group during both sessions.
Fig.2.
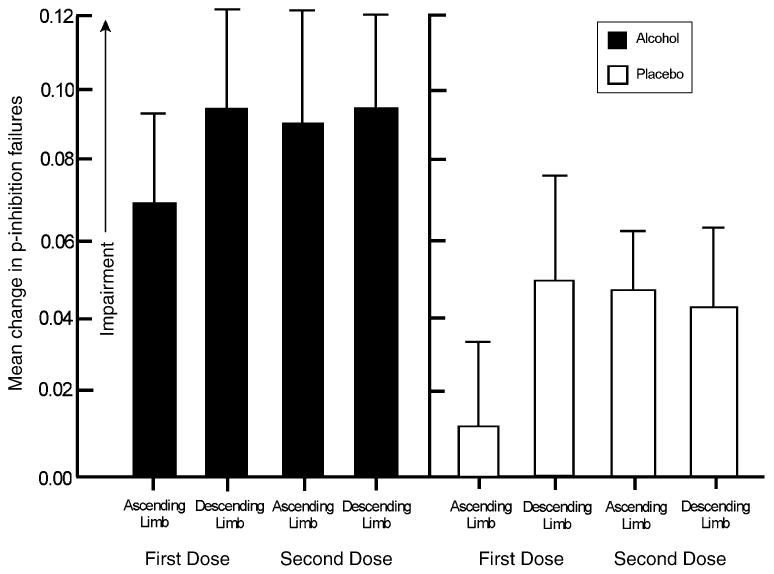
Mean change from baseline in inhibitory failures on the cued go/no-go task in response to 0.65 g/kg of alcohol (ALC) or placebo (PLA). Capped vertical lines show the standard error of the mean
Motor skill performance and subjective intoxication
No significant group difference in baseline motor skill on the pegboard task was obtained (p>.58). The mean baseline completion time of the sample was 54.0 s (SD=5.7). A 2 (group) × 2 (test) × 2 (session) ANOVA of change from baseline showed a significant main effect of session, F(1, 30)=8.41, p=.01, and a group×test interaction, F(1, 30)= 7.27, p=.01. Table 1 presents the change scores. Performance was impaired (i.e., slowed) following alcohol but not placebo. With respect to acute tolerance, planned comparisons confirmed significantly less impairment in the ALC group during the descending versus ascending limbs (p<.01). No significant difference in impairment was observed between dose sessions (p>.51). The PLA group showed little change in performance on the tests during each session. The table shows that the main effect of session was due to a slight speeding effect for both groups during the second versus first dose session.
Table 1. Change scores from baseline for grooved pegboard performance and subjective intoxication ratings.
| Dose session 1 | Dose session 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | ||||||
| Means | SD | Means | SD | Means | SD | Means | SD | ||
| Group | |||||||||
| Pegboard | ALC | 2.8 | 3.3 | 0.8 | 3.0 | 2.7 | 4.2 | −0.02 | 4.9 |
| PLA | 0.2 | 2.9 | 0.4 | 3.0 | −1.9 | 2.9 | −2.1 | 2.6 | |
| Intoxication | ALC | 48.3 | 23.7 | 35.4 | 27.2 | 37.4 | 24.2 | 19.3 | 23.0 |
| PLA | 4.5 | 14.1 | −10.0 | 10.3 | −8.4 | 9.9 | −11.8 | 13.6 | |
The numerical values presented are the means and standard deviations of change from baseline for groups ALC and PLA. Pegboard scores are presented in seconds and positive scores indicate slowed performance compared to baseline. Positive change scores of “intoxicated” indicate an increase in perceived level of intoxication
No significant group difference in baseline perceived intoxication was obtained (p values>.62). The mean rating of the sample during baseline assessment was 13.2 (SD= 12.5). Change from baseline on the test sessions is presented in Table 1. A 2 (group)×2 (test)×2 (session) ANOVA showed a significant main effect of group, F(1, 30)=63.11, p<.01, and a group×test×session interaction, F(1, 30)=6.86, p=.01. The ALC group reported increased subjective intoxication that diminished on the descending limb tests (i.e., acute tolerance). Simple effects comparisons confirmed that subjective intoxication decreased significantly from ascending to descending limbs in both sessions (p<.01). The ALC group's subjective intoxication ratings were significantly lower during the second versus first dose session (p<.01).
Supplemental analyses
Inter-session interval
Although the inter-session interval did not differ between groups, the possibility that the inter-session interval affected tolerance development across sessions was considered, and analyses were performed in order to test for this possibility. Measures were re-analyzed by a mixed-design 2 (group)× 2 (test)× 2 (session) analysis of covariance (ANCOVAs) with inter-session interval as a covariate. No main effects or interactions involving inter-session interval were observed. Therefore, inter-session interval had no effect on the degree of tolerance observed across sessions for any measure.
Gender differences
The effects were also examined for possible gender differences. Measures were analyzed by 2 (group)× 2 (test)× 2 (session)× 2 (gender) ANOVAs. Men and women did not differ in their response to alcohol on any measure, as evident by the lack of significant group×gender interactions (p values>.18).
Discussion
This study examined the differential development of tolerance to the impairing effects of alcohol on activational and inhibitory mechanisms of behavioral control. The study showed that response activation and inhibition were significantly impaired by alcohol during both sessions. However, impairment of response activation displayed evidence of acute tolerance as impairment was significantly less during the descending versus ascending limbs in both dose sessions. Alcohol-induced impairment of response activation also displayed evidence of cross-session tolerance as impairment was diminished during the second dose session. Alcohol also impaired response inhibition, and this impairment was evident by increased failures to inhibit responses following both doses of alcohol. In contrast to response activation, response inhibition showed no acute or cross-session tolerance to the impairing effects of alcohol. Indeed, the degree to which alcohol increased inhibitory failures remained fairly similar across limbs within a session and between the sessions. Finally, the study also demonstrated acute tolerance to the impairing effect of alcohol on psychomotor function, as measured by the pegboard task, and cross-session tolerance to perceived intoxication as measured by self-report.
This study is the first to examine the differential development of tolerance to impaired response activation and response inhibition across repeated alcohol administrations. The evidence was obtained from a within-subjects design, using a single task that provided concurrent assessment of activational and inhibitory aspects of behavioral control. The scheduling of tests was based on considerable prior research on the pharmacokinetic profile of the active dose. The average BACs during the testing periods on each limb of the blood alcohol curve were nearly identical, and thus the reduced impairment observed on the descending limb cannot be attributed to limb differences in BAC. Differences in the recovery of impaired activation and inhibition during the dose also cannot be attributed to any speed-versus-accuracy trade-off. In a speed–accuracy trade-off, any alcohol-induced slowing of RT should actually improve response inhibition by allowing more time to inhibit responses when necessary. Instead, the alcohol-induced slowing of response activation in the present research was accompanied by a reduction in the ability to inhibit responses.
The findings are consistent with those investigating the differential development of acute tolerance between response activation and response inhibition during a single-dose administration (e.g., Fillmore et al. 2005). That study found that during a single alcohol exposure, impaired response activation displayed acute tolerance while response inhibition remained impaired from ascending to descending limb tests; however, previous demonstrations of this “activational bias” in recovery during the declining limb did not involve a motivated conflict situation. The present study employed a motivational conflict situation because it has been argued to better represent conflicting response tendencies outside the laboratory. Outside of the laboratory, there are often consequences motivating competing response tendencies. Despite the addition of motivated conflict, the differences in tolerance development between activation and inhibition were remarkably similar to those observed without the motivating consequences (e.g., Fillmore et al. 2005). Moreover, as the current study obtained evidence of acute tolerance for response activation during two separate dose sessions, it provides a repeated demonstration of this effect in the same subjects. Taken together, these studies provide support for the argument that tolerance develops more rapidly to the impairing effects of alcohol for activational aspects compared with inhibitory aspects of behavioral control, and that this activational bias in recovery might be a robust effect, evident across a range of environmental circumstances.
Evidence for an “activational bias” in recovery of behavioral control during the declining limb might have important implications for understanding some of the impulsive behavior and poor decision making commonly observed under alcohol. Generally speaking, impulsivity reflects a biased tendency for action over inaction. Accordingly, the biased recovery of response activation over inhibition could contribute to the display of impulsive behavior while BAC falls. Moreover, we obtained evidence of acute tolerance to the impairing effect of alcohol on motor coordination and cross-session tolerance to the perceived level of intoxication. Taken together, these results indicate that the disinhibiting effects of alcohol are present even when the impairing effects of alcohol on other aspects of performance have diminished under the dose. This has implications for understanding risky behaviors during the descending limb of intoxication, for example, drinkers might decide to drive after drinking because they feel sober or detect no impairment of their motor coordination; however, they might be at risk for impulsive or reckless driving because their inhibitory control has not recovered from the impairing effects of alcohol. Such problems of self-appraisal also might become exacerbated upon repeated administrations of alcohol as tolerance develops to the perceived intoxicating effects of the drug.
The study also provides information on the relationship between acute tolerance during a single dose and cross-session tolerance as the dose is repeated. Some theories suggest that acute tolerance contributes to the development of chronic tolerance because they both share some common underlying adaptive processes (e.g., Kalant et al. 1971). Other investigators have suggested that tolerance over repeated doses might actually occur as a result of greater acute tolerance as doses are repeated (e.g., Beirness and Vogel-Sprott 1984). That is, tolerance develops over repeated doses because acute recovery during the declining limb increases each time the dose is administered. In the present study, speed of response activation and subjective intoxication both demonstrated acute tolerance during each dose and tolerance across the two doses; however, the increased tolerance observed during the second dose was not confined to the declining limb. Figure 1 shows marked reduction in impairment during the ascending limb of the second dose compared with either test during the first dose for response activation. In addition, Table 1 shows that subjective intoxication is lower on the ascending limb of dose session 2 versus dose session 1. Thus, the tolerance displayed during the second dose appeared on both limbs and was not simply due to greater recovery during the declining limb.
It is important to recognize that the evidence of cross-session tolerance in the current study likely reflects task-specific, functional tolerance, and not some general physiological tolerance to alcohol that occurs as a function of prolonged heavy consumption. Subjects were exposed to only two doses of alcohol administered in the laboratory. The average inter-session interval was brief (i.e., less than 1 week). The subjects were moderate drinkers with no history of alcohol abuse or heavy consumption. As such, it is unlikely that the tolerance to the impairing effect of alcohol on response activation observed across the two sessions could reflect some general physiological adaptation to the drug. Rather, it is likely that this reduced impairment across sessions was task-specific functional tolerance whereby intoxicated performance of the tasks allowed subjects to learn to compensate for the disruptive effects of the drug on the specific behavioral responses required by the tasks.
The study raises some important questions, some of which are due to limitations of the current study design. The findings of cross-session tolerance were limited to two administrations of the dose, and so it is not known whether or not impaired inhibitory control might eventually show tolerance as alcohol administrations continue. Second, the point at which inhibitory control does recover during the time course of a single dose is not known. Studies that include more repetitions of the dose coupled with prolonged testing of inhibitory control under each dose would provide this information. It might also be important to assess impairment in a double-blind manner in order to better minimize the potential for bias owing to experimenter-subject interactions. In conclusion, the results highlight the importance of considering behavioral requirements when testing for the development of tolerance under a dose of alcohol. By modeling behavioral control as the net effect of countervailing activational and inhibitory influences, the study suggests that fundamental mechanisms of control might not display uniform tolerance development.
Acknowledgments
This research was supported by grants R01 AA012895 and R01 AA018274 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Beirness D, Vogel-Sprott M. The development of alcohol tolerance: acute recovery as a predictor. Psychopharmacology. 1984;85:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Chen CS. A study of the alcohol-tolerance effect and an introduction of a new behavioral. Psychopharmacology. 1968;12:433. doi: 10.1007/BF00401349. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112(3):424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cog Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Acute alcohol–induced impairment of cognitive functions: past and present findings. Int J Dis Hum Dev. 2007;6(2):115–1125. [Google Scholar]
- Fillmore MT, Blackburn JS. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;33:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Goldberg L. Quantitative studies on alcohol tolerance in man: the influence of ethyl alcohol on sensory, motor, and psychological functions referred to blood alcohol in normal and habituated individuals. Acta Physiol Scand. 1943;5(16):1–128. [Google Scholar]
- Haubenreisser T, Vogel-Sprott M. Reinforcement reduces behavioural impairment under an acute dose of alcohol. Pharmocol Biochem Behav. 1987;26(1):29–33. doi: 10.1016/0091-3057(87)90528-4. [DOI] [PubMed] [Google Scholar]
- Hurst PM, Bagley SK. Acute adaptation to the effects of alcohol. Q J Stud Alcohol. 1972;33:358–378. [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implication for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalant H. Current trends in biomedical research on alcohol. Alcohol Alcohol. 1987;1:1–12. [PubMed] [Google Scholar]
- Kalant H, Leblanc AE, Gibbins RJ. Tolerance to, and dependence on, ethanol. In: Israel Y, Mardonze J, editors. Biological basis of alcoholism. Wiley/Interscience; New York: 1971. pp. 235–269. [Google Scholar]
- LeBlanc AE, Gibbins RJ, Kalant H. Behavioral augmentation of tolerance to ethanol in the rat. Psychopharmacology. 1973;30:117–122. doi: 10.1007/BF00421426. [DOI] [PubMed] [Google Scholar]
- Mann RE, Vogel-Sprott M. Control of alcohol tolerance by reinforcement in nonalcoholics. Psychopharmacology. 1981;75:315–320. doi: 10.1007/BF00432446. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21(3):346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: its absorption into and disappearance from the blood under different conditions (Special Report Series Monograph No 31) London: Medical Research Committee; 1919. [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychol. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Newman H, Card J. The nature of tolerance to ethyl alcohol. J Nerv Ment Dis. 1937;86:428–440. [Google Scholar]
- Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcohol Clin Exp Res. 2003;27:773–779. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Post RB, Tavano LA, Maddock RI. Role of feedback in formation of acute tolerance to alcohol. J Stud Alcohol. 1998;59:723–730. doi: 10.15288/jsa.1998.59.723. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user's guide. Psychology Software Tools; Pittsburgh: 2002. [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error prone, responses during acute alcohol intoxication: effects of stimulus-response mapping complexity. Alcohol Clin Exp Res. 2004;28:643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Seltzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. Alcohol tolerance and social drinking: learning the consequences. Guilford; New York: 1992. [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology. 2008;201(3):315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


