Abstract
Light is a major environmental stimulus that has a broad effect on organisms, triggering a cellular response that results in an optimal adaptation enhancing fitness and survival. In fungi, light affects growth, and causes diverse morphological changes such as those leading to reproduction. Light can also affect fungal metabolism, including the biosynthesis of natural products. In this study we show that in Aspergillus nidulans the effect of light on the production of the sterigmatocystin (ST) toxin depends on the glucose concentration. In cultures grown with 1% glucose and exposed to light, ST production was lower than when grown in the dark. This lower ST production coincided with an elevated rate of cellular damage with partial loss of nuclear integrity and vacuolated cytoplasm. However, in cultures grown with 2% glucose these effects were reversed and light enhanced ST production. Glucose abundance also affected the light-dependent subcellular localization of the VeA (velvet) protein, a key regulator necessary for normal light-dependent morphogenesis and secondary metabolism in Aspergilli and other fungal genera. The role of other VeA-associated proteins, particularly the blue light-sensing proteins LreA and LreB (WC-1 and WC-2 orthologs), on conidiation could also be modified by the abundance of glucose. We also show that LreA and LreB, as well as the phytochrome FphA, modulate not only the synthesis of sterigmatocystin, but also the production of the antibiotic penicillin.
1. Introduction
Light has a broad effect on living organisms from humans to fungi, triggering chemical and morphological responses as environmental adaptations. In fungi, light can cause diverse changes in fungal metabolism, growth, and the balance between sexual and asexual differentiation (Gyula et al., 2003; Purschwitz et al., 2006; 2008; Calvo, 2008; Corrochano, 2007; Blumenstein et al., 2005). Fungi are responsive to a broad spectral range of light, where blue and red light display prominent effects on fungal growth and morphogenesis (Mooney and Yager, 1990; Mooney et al., 1990; Kues, 2000; Cerda-Olmedo, 2001; Liu et al., 2003; Purschwitz et al., 2006; 2008).
Chemical and morphological development in the model fungus Aspergillus nidulans is regulated through interplay of two light-sensing systems, the red and the blue light responsive systems, that interact with the VeA protein in the nucleus. Red light is sensed through a phytochrome-like receptor, FphA. This protein interacts directly with the VeA protein (Purschwitz et al., 2008). In addition, FphA interacts with the blue light-sensing protein apparatus that includes LreA and LreB, orthologs to the respective Neurospora crassa blue light-responsive WC-1 and WC-2. FphA interacts with LreB, which was found to interact with LreA (Purschwitz et al., 2008). VeA also interacts with LaeA, a putative methyl transferase that regulates the expression of secondary metabolism gene clusters, and other proteins involved in the regulation of development (Bok and Keller, 2004; Bayram et al., 2008; Calvo, 2008).
In our previous studies we have demonstrated that VeA is necessary for the production of numerous secondary metabolites, including mycotoxins, not only in the genus Aspergillus (Kato et al., 2003; Calvo et al., 2004; Duran et al., 2007; 2009) but also in other fungal genera (Myung et al., 2009). In addition to VeA, other VeA-interacting proteins are also influential in regulating the biosynthesis of natural products, such as the production of the sterigmatocystin (ST) toxin in A. nidulans. This includes LaeA, VelB (Bayram et al., 2008), as well as FphA, LreA and LreB (Purschwitz et al., 2008). We showed that while LreA and LreB promote the formation of ST, FphA acts as a repressor of the synthesis of this mycotoxin (Purschwitz et al., 2008). The possible effect of these light receptors on the biosynthesis of other secondary metabolites has not yet been determined.
It is likely that the light response is also integrated in a cross-talk with those responses derived from additional external stimuli. Several environmental factors that influence secondary metabolism have been identified. For example, in Aspergillus flavus simple sugars such as glucose, fructose, sucrose and sorbitol when used as sole carbon sources strongly support fungal growth, increasing sporulation and aflatoxin production (Abdollahi and Buchanan, 1981; Buchanan et al., 1984; Bu’Lock, 1961; Kacholz and Demain, 1983; Liu and Chu, 1998; Luchese and Harrigan, 1993). In contrast, peptone (Abdollahi and Buchanan, 1981; Bu’Lock, 1961; Feng and Leonard, 1998; Liu and Chu, 1998), galactose, xylose, mannitol and lactose (Kacholz and Demain, 1983) do not support aflatoxin production well. Our previous studies indicated that the effect of light on the production of ST by A. nidulans is medium-dependent (Kato et al., 2003). While production of ST in glucose minimal medium (GMM) was higher in the absence of light, light enhanced accumulation of ST in rich medium such as YGT (Kato et al., 2003). In A. nidulans extensive research has been done in deciphering the mechanisms of carbon catabolite repression, where the transcription factor CreA plays a key role as negative-acting regulator (Arst and MacDonald, 1975; Bailey and Arst, 1975; Dowzer and Kelly, 1991; Shroff et al, 1997). Our previous work on penicillin biosynthesis in A. nidulans has shown this pathway to be under carbon regulation, but CreA-independent, (Espeso and Peñalva, 1992; Espeso et al., 1993). The nature of this carbon regulatory system remains undetermined, however in the case of penicillin production, carbon regulation depends on the PacC-mediated pH regulatory system (Espeso et al, 1993); an example of a link between two regulatory signaling pathways responsive to different external cues.
In addition to the role of VeA in regulating secondary metabolism, it also regulates fungal morphogenesis. A. nidulans strains carrying a veA wild-type allele develop mainly asexually producing conidiophores, while in the dark fruiting bodies called cleistothecia are formed predominantly (Yager, 1992). FphA is involved in the repression of sexual fruiting body formation and induction of conidiation (Blumenstein et al., 2005), while LreA and LreB have antagonistic roles. Similar to the WC-orthologs, VeA represses conidiation and promotes sexual development.
Previous studies have shown that glucose concentration also affects the morphogenesis balance between sexual and asexual development. For example, in A. nidulans high concentrations of glucose promote sexual development and simultaneously inhibit asexual development, while low glucose concentrations have the opposite effect (Han et al., 1990, Jeong et al., 2000; Han et al., 2001).
In the present study we show a relationship between the effect of light and glucose as carbon source in the production of the mycotoxin ST and morphogenesis in the fungus A. nidulans. We also investigated the effect of blue and red light-sensing proteins interacting with VeA on the biosynthesis of other secondary metabolites. Our results indicate that the perception of external stimuli and the morphological and metabolic responses are complex and integrated in order to achieve optimal adaptation to a plurality of environmental cues. This study also indicates that part of this integration of sensing mechanisms and cellular responses is mediated by VeA.
2. Material and Methods
2.1 Fungal strains and growth conditions
A. nidulans strains used in this study are listed in Table 1. These strains were cultured on glucose minimum medium (GMM, in this study the percentage of glucose is indicated in each case) plus the appropriate supplements for the corresponding auxotrophic markers (Kafer, 1977). Agar (15 g/liter) was added in the case of solid medium. Strains were stored as 30% glycerol stocks at −80°C.
Table 1.
A. nidulans strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| FGSCA4 | wild-type | Fungal Genetics Stock Center, Kansas City, Missouri |
| LAV+ | biA1; ΔlreA::argB; veA+ | Purschwitz et al., 2008 |
| LBV+ | biA1; ΔlreB::argB; pyroA4;veA+ | Purschwitz et al., 2008 |
| SJP1 |
pyrG89; ΔargB::trpC ΔB;pyroA4; ΔfphA::argB;veA+ |
Purschwitz et al., 2008 |
| SJP13.3 | cross between LBV+ and SJP1—biA1; ΔlreB::argB; ΔargB:trpCΔB; pyroA4; ΔfphA::argB; veA+ |
Purschwitz et al., 2008 |
| SJP15G | cross between LAV+ and SJP1—pyrG89; ΔargB:trpC ΔB; pyroA4; ΔlreA::argB, ΔfphA::argB; veA+ |
Purschwitz et al., 2008 |
| SJP21.3 | cross between SJP15G and SJP13.3—pyrG89; ΔlreB::argB; ΔargB:trpCΔB; pyroA4; ΔlreA::argB, ΔfphA::argB; veA+ |
Purschwitz et al., 2008 |
| TRMD3.4.17 | pyrG89; pyroA4; veA::gfp::pyrGA.fumigatus | Stinnett et al., 2007 |
| MAD3140 |
pyrG89; pyroA4; veA::gfp::pyrGA. fumigatus, hhoA::mCh::pyroAA. fumigatus , inoB2 |
This study |
2.2 Mycotoxin analysis
Solid GMM containing different glucose concentrations (indicated in each case) was inoculated with FGSCA4 wild type (106spores/plate) and cultured in white light (28 microEinsteins/m2/s) or dark conditions. Experimental time points are indicated in each case. Four cores (16 mm diameter) from each replicate plate were collected in a 50 ml Falcon tube, and ST was extracted by adding 5 ml of CHCl3 three consecutive times. The extracts were allowed to dry and then resuspended in 500 µl of CHCl3 before 15 µl of each extract was fractionated on a silica gel thin-layer chromatography (TLC) plate using a toluene-ethyl acetate-acetic acid [80:10:10 (v/v/v)] solvent system. Alternatively, a solvent system consisting of benzene-acetic acid [95:5 (v/v)] was used. The TLC plates were sprayed with aluminum chloride (15% in ethanol) and baked for 10 min at 80°C to intensify ST fluorescence upon exposure to long wave (365 nm) UV light . ST purchased from Sigma was used as a standard.
2.3 Effect of cAMP on ST production
Solid GMM containing 1% or 2% glucose was supplemented with 400µM of dibutyryl cyclic adenosine monophosphate (db-cAMP) or 8-bromo-cAMP. The plates were inoculated with FGSCA4 wild type (106spores/plate). After 3 days of cultivation in light or dark conditions ST was extracted and analyzed according to the method described in section 2.2.
Also as a separate experiment, internal cAMP levels were measured in the FGSCA4 strain when growing in liquid GMM cultures with different glucose concentrations that included a range from 0.3 to 6%. Spores (106 spores/ml) were inoculated in 150 ml of GMM with different glucose concentrations. After 24 h, 36 h and 40 h of incubation the medium was refreshed, maintaining the glucose concentrations as constant as feasible. Mycelia were harvested, lyophilized and ground. Fifty mg were extracted with 1.5 ml 5% TCA and glass beads in a bead mill for 7.5 min. The extracts were analyzed using the Cyclic AMP EIA Kit from Biomedical Technologies Inc. following the manufacturer instructions.
2.4 mRNA analysis
Total RNA was isolated from mycelia at the specified time points by using Trizol (Invitrogen) as described by the supplier. Four micrograms of total RNA were treated with DNaseI RQI (Promega) and reverse transcribed with MMLV reverse transcriptase (Promega). qRT-PCR was performed in a Mx3000P thermocycler (Stratagene) using SYBR Green JumpStartTM Tag Ready Mix (Sigma). Primers pairs used for qRT-PCR are listed in Table 2.
Table 2.
Primers used in this study.
| Primer name | Sequence |
|---|---|
| actinF | 5’-ATGGAAGAGGAAGTTGCTGCTCTCGTTATCGACAATGGTTC-3’ |
| actinR | 5’-CAATGGAGGGGAAGACGGCACGGG-3’ |
| stcU-F | 5’-CATGTCAAGGACGTTACGCCAGATGAATTCGACCGAGTATTTCGGGTC-3’ |
| stcU-R | 5’-GCGGCACACTCATCCACCTGCTCATC-3’ |
| aflR-F | 5’-ATGGAGCCCCCAGCGATCAGCCAG-3’ |
| aflR-R | 5’-TTGGTGATGGTGCTGTCTTTGGCTGCTCAAC-3’ |
2.5 Microscopy analysis
Strains TRMD3.4.17 (Stinnett et al., 2007) and MAD3140 (Table 1) were used for microscopy studies to follow the localization of VeA wild-type protein in the cell as VeA::GFP fusion. MAD3140 was obtained by meiotic recombination between TRMD3.4.17 and MAD2446, a strain carrying hhoA::mCherry chimera (Etxebeste et al., 2009). Conidia were inoculated in liquid minimum medium with different glucose concentrations (as indicated) and cultured essentially as described in (Stinnett et al., 2007). Briefly, conidia were germinated onto the surface of a cover slip immersed in minimum medium and further incubated for 16 h with illumination or in the dark. Alternatively, conidia were point inoculated onto GMM containing 1%, 1.5% or 2% glucose. Cultures were incubated in light and dark conditions. Colonies grew under light, at 25 cm distance of the light source. Dark conditions were obtained by wrapping the plates with foil. The active growth front edge was labeled on the plate every 24 hours. Detailed microscopic examination was performed on mycelium after 72 h. The agar piece obtained was washed with water and then smashed onto the microscope slide with the help of the coverslide and a drop (20 µl) of 50% glycerol acting as mounting liquid. Samples were observed with an upright Nikon Eclipse 80i microscope equipped with Nomarski optics, a 63× oil objective 1.4 N.A. and Semrok filters adequate for GFP and mRFP fluorescence detection. Images were recorded with an Orca-ER camera (Hammamatsu Photonics). DIC images were taken with an exposure of 30 ms, mRFP with 150 ms and GFP with 750ms. Images were processed minimally using Wasabi (Hammamatsu Photonics) and ImageJ 1.37v software package (http://rsb.info.nih.gov/ij/).
2.6 Morphological studies
Asexual and sexual developmental studies were performed using the A. nidulans strains FGSCA4, LAV+, LBV+, SJP1, SJP13.3, SJP15G, SJP21.3 (Table 1). Plates containing 25 ml of solid GMM (1% and 2% glucose) plus the appropriate supplements were spread-inoculated with 100 µl of water containing 106 spores. The cultures were incubated at 37°C in the dark or in the light. After 7 days, a 16-mm-diameter core was removed from each spread plate culture and homogenized in water to release the spores. Conidia were counted using a hemacytometer. Identical cores were taken to examine cleistothecial production under a dissecting microscope. To increase visualization of cleistothecia the cores were spread with 70% ethanol to remove conidiphores.
2.7 Penicillin production
The penicillin (PN) bioassay was performed essentially as described by (Brakhage et al., 1992) by using Geobacillus stearothermophylus as a test organism. Bacillus cereus penicillase controls were included in each case (5 units per sample, purchased from Sigma) to evaluate whether the antibacterial activity observed was due to the presence of PN. This allowed us to distinguish the antibiotic activity of PN from those of other compounds that could have been produced.
3. Results
3.1 Effect of glucose and light on ST production in A. nidulans
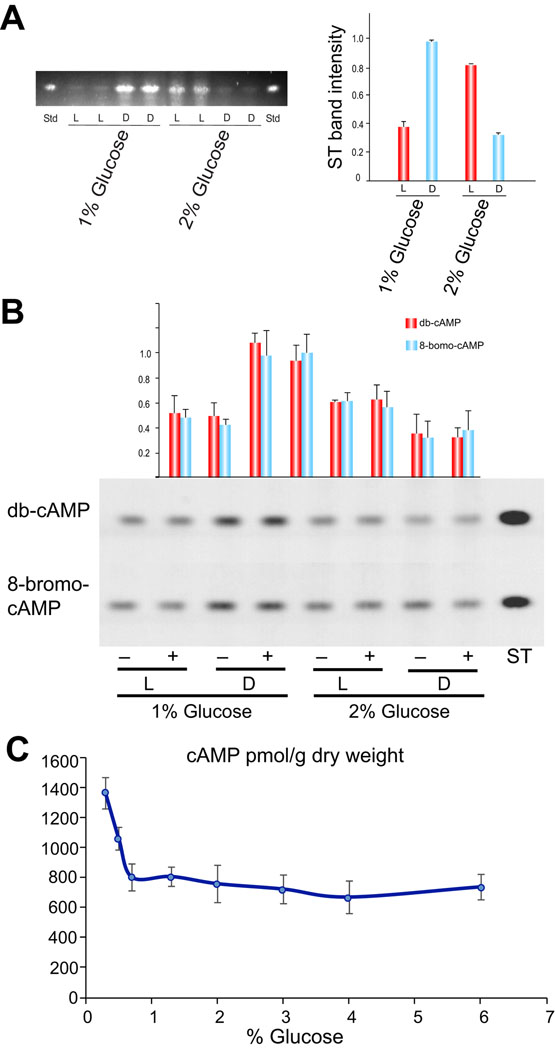
Our previous studies indicated that the effect of light on the production of ST is medium-dependent (Kato et al., 2003). While production of ST in GMM was greater in the dark, accumulation of ST was greater in light in rich medium such as YGT (yeast extract, glucose and trace elements). Other authors have also described a light stimulating effect in ST production in rich medium (Guzman-de-Pena et al., 1998). Our present study revealed a cross-talk between the response to a single nutritional component, namely glucose, and light response in controlling the production of the mycotoxin ST. We found greater ST accumulation in the dark than in the light when the fungus was growing on GMM with 1% glucose, and surprisingly, the opposite was true when GMM with 2% glucose was used (Fig. 1A).
Figure 1. Effect of light and glucose on ST production in A. nidulans and study of possible connections with cAMP levels.
A) Thin-layer chromatography analysis of ST produced by the wild-type A. nidulans FGSCA4 strain on GMM with 1% or 2% glucose cultured in light and dark conditions after 5 days of incubation. ST, standard. The densitometry was carried out with Scion Image Beta 4.03 software. The ST band intensity values were normalized with respect to the highest intensity considered as 1. B) Effect of db-cAMP and 8-bromo-cAMP supplementation on ST production. Thin-layer chromatography analysis of ST produced by the wild-type A. nidulans FGSCA4 strain. Four- hundred µM of db-c-AMP or 8-bromo-cAMP was added to the medium before inoculation. ST was extracted after 3 days of cultivation. C) Internal cAMP levels measured in strain FGSCA4 when growing in GMM cultures with different glucose concentrations. The error bars in graphs from panel A indicate standard error.
3.2 Effects of cAMP on ST production in A. nidulans
High cAMP level is an indicator of starvation (Lafon et al., 2006) that could cause a negative dominant effect resulting in possible alterations of the light effect on ST production. We investigated whether the addition of cAMP on 1% and 2% GMM could alter the effect on the ST biosynthesis stimulation by glucose under light in the wild-type strain A. nidulans FGSCA4. When 400 µM of the cAMP analog db-c-AMP or 8-bromo-cAMP were added to the medium before inoculation, the ST biosynthesis pattern did not change in the light or dark conditions (Fig. 1B).
In another experiment we examined the internal levels of cAMP in cultures containing different glucose concentrations. Our results revealed that endogenous cAMP levels remain fairly constant when a range of 0.7 to 6% glucose concentration was used in the medium (Fig. 1C). cAMP levels increased only at glucose levels below 0.7%. The experiment was repeated several times with similar results in light or dark conditions.
3.3 Glucose concentration affects light-dependent subcellular localization of the VeA protein
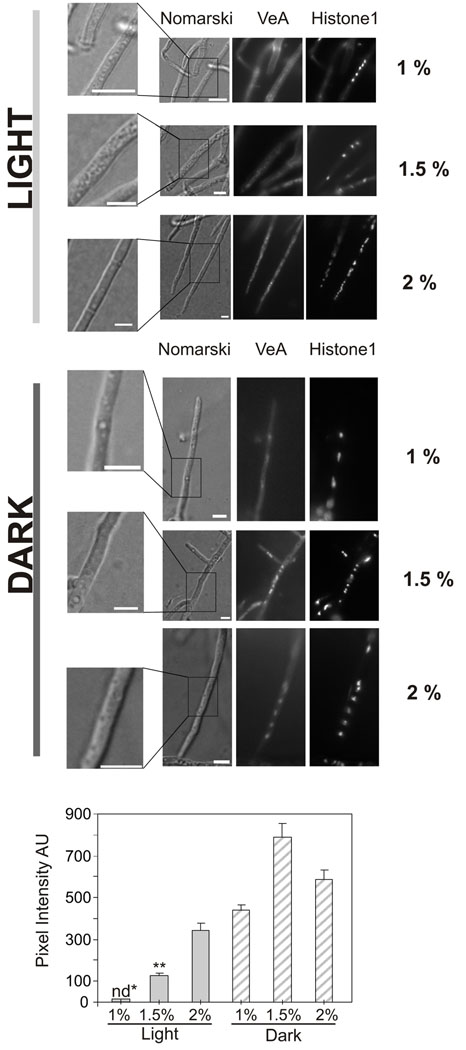
An increase in ST production occurs as the glucose concentration in the minimal medium is increased, obtaining the highest accumulation of ST in the range close to 1.5% in the presence of light (Fig. 2A). The glucose concentrations at which ST production is maximized are different in the dark, where high accumulation of ST occurs at lower concentrations of glucose. Because VeA is a central component in light sensing, the effect of the glucose concentration on VeA localization was analyzed. We investigated the nuclear accumulation of a VeA::GFP fusion expressed by strain TRMD3.4.17 and MAD3140 (Table 1). Both strains display a veA wild-type phenotype (Stinnet et al, 2007, and data not shown). VeA::GFP notably accumulated in nuclei in the dark at all glucose concentrations tested (Fig. 2B). Nuclear fluorescence accumulation, as described before (Stinnet et al, 2007; Araújo-Bazán et al, 2009), was visible in light although at a lower level (Fig. 2B). In the dark, a slight tendency of increased nuclear accumulation with larger amounts of glucose was detected, but significant differences were not found. ST production was increased in a narrow range of glucose concentrations (intermediate concentrations between 1% and 2%), particularly in light culture conditions (Fig. 2A). Coinciding with this increased production of mycotoxin, incubation with 1.5% glucose gave a notable accumulation of VeA fluorescent protein under light, with levels of fluorescence detected in nuclei similar to those found in cultures under dark growth conditions (Fig 2B). When medium with glucose concentrations higher than 2% was used, the levels of ST production remained low, particularly in the dark (Fig. 2A).
Figure 2. Dependence of VeA nuclear localization on glucose concentration and its relationship with ST biosynthesis.
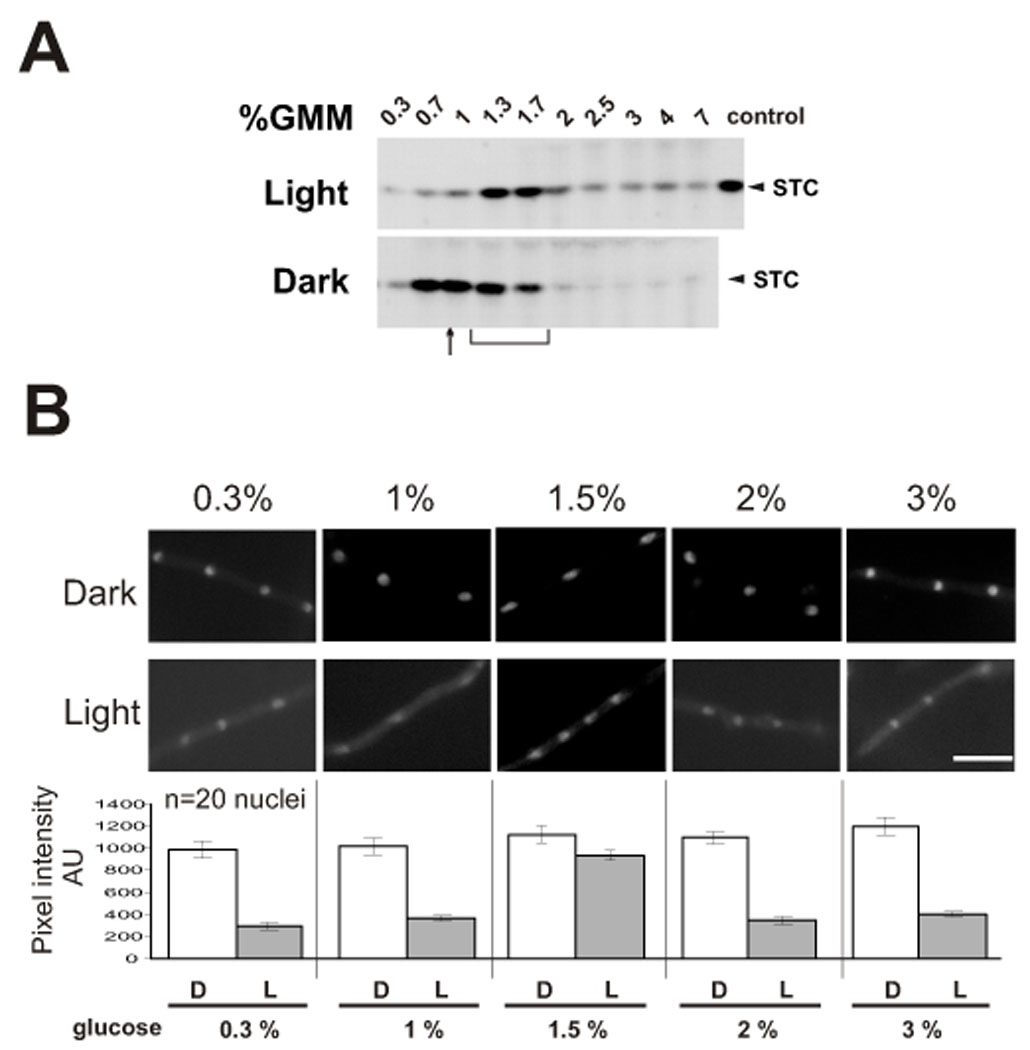
A) Thin-layer chromatography analysis of ST produced by the wild-type A. nidulans FGSCA4 strain on GMM for 72 h with a range of different glucose concentrations from 0.3% to 7%. B) Images of TRMD3.4.17 grown in minimum medium with glucose concentrations as indicated on the top, and with or without illumination. A quantification of the nuclear fluorescence, in arbitrary units (for 20 nuclei of different hyphae) is shown at the bottom of each condition. For each condition 20 nuclei were measured. The error bars in graphs from panel A indicate standard error. Each experiment was repeated at least three times.
In older cultures, 72 h after inoculation on solid medium, we observed that cellular integrity was partially compromised in those cultures exposed to light where medium with low levels of glucose concentration (1%) was used. In approximately 80% of vegetative cells the cytoplasm appeared vacuolated and the nuclear structures were not well defined (Fig. 3). However, in the medium containing higher glucose levels this detrimental effect of light was minimized and most of the cells presented defined nuclei in healthy cells. This effect on cell integrity in low glucose medium was not observed in cultures growing in the dark.
Figure 3. Localization of VeA in mycelia grown on solid media.
Images of hyphae from strain MAD3140 grown for 72 h on supplemented minimal media with the indicated percentage of glucose (on the right) as main carbon source. Localization of VeA::GFP was observed in the dark or in the light under the same conditions as in figure 2. Number, position and integrity of nuclei were in these experiments followed by using histone 1 tagged with mCherry as nuclear marker. On the left side, 2× magnifications of hyphae interferential images (Nomarski) show the presence or abundance of vesicles and vacuoles. Bars indicate 5 µm. The graph bar shows the quantification of nuclear VeA::GFP fluorescence by measuring pixel intensities. Grey and striped bars show measurements in the light and in the dark, respectively. Error bars indicate S.D. nd* and ** indicate that those VeA::GFP fluorescent measurements were taken in cells displaying intense nuclear H1-mCherry fluorescence.
3.4 Effect of light and glucose on A. nidulans ST gene expression
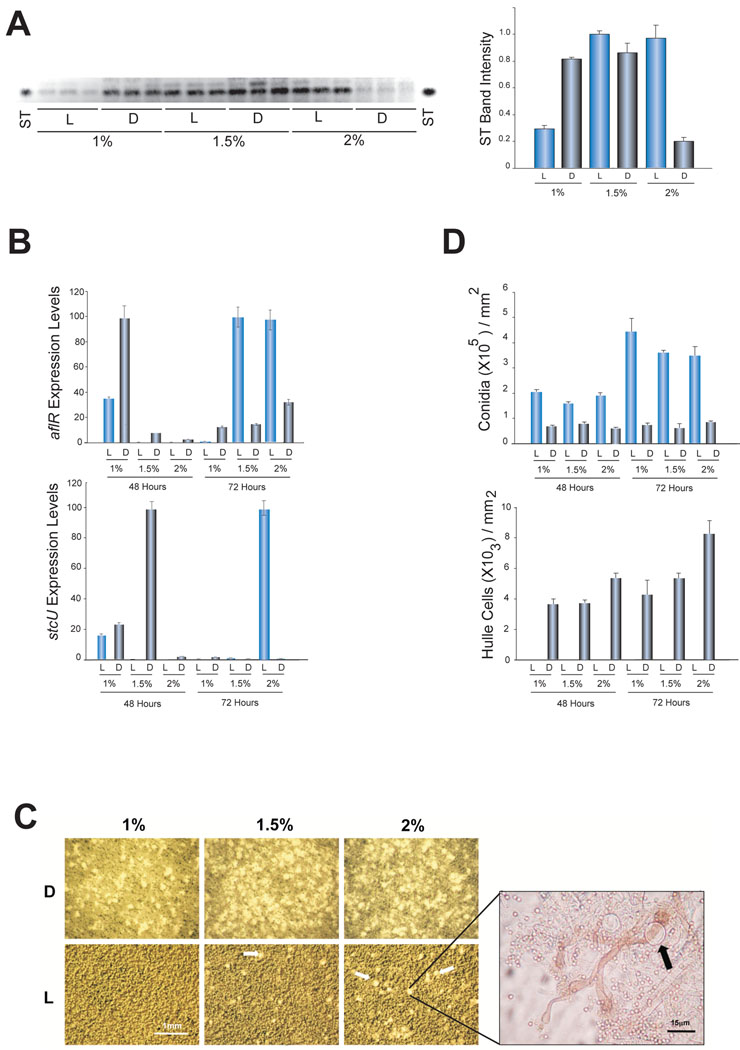
We analyzed ST production and the expression levels of ST genes in A. nidulans FGSCA4 wild-type strain growing on solid GMM with 1%, 1.5% and 2% glucose in the light and in the dark (Fig. 4A and B). The ST TLC analysis results were consistent with other results shown in this work (Figs. 1, 2, and Fig 4A). We examined aflR and stcU transcription levels. aflR encodes a ST-specific transcription factor necessary for the activation of the ST gene cluster (Yu et al., 1996; Fernandes et al., 1998). The expression of the structural gene stcU is often used as an indicator of ST cluster activation (i.e. Kato et al., 2003).
Figure 4. Effect of light and glucose on wild-type ST gene expression, ST accumulation and morphogenesis.
A) TLC analysis of ST from strain FGSCA4 grown for 96 hours on supplemented minimal media with the indicated percentage of glucose as main carbon source. On the right, densitometry showing the intensity of the ST bands. The densitometry was carried out using the Scion Image 4.03 software. B) qRT-PCR results showing expression levels of aflR and stcU genes. The relative expression levels were calculated using the method described by Kenneth and Schmittgen (2001), and all values were normalized to the expression of the A. nidulans tubulin gene. C and D) Developmental studies performed under the same experimental conditions. In C, micrographs of conidiophores and cleistothecial primordia (white arrows) with a magnification showing Hülle cells (black arrow) surrounding the primordia. In D, quantification of conidia and Hülle cells. The error bars indicate the ranges for three replicates.
Our qRT-PCR analysis shows that at 48 h aflR expression was greater in 1% glucose cultures, particularly in those growing in the dark. At this same time point, aflR levels were very low in light cultures with 1.5% and 2% glucose. However, over time aflR expression was greater when those same glucose concentrations (1.5% and 2%) were used in cultures growing in the light (Fig 4B), while the aflR transcript levels decrease in 1% glucose cultures.
In the case of stcU, at 48 h transcripts were detected in 1% and 1.5% glucose cultures (particularly in the latter) in the dark, while only low transcript levels were observed in 2% glucose cultures (Fig. 4B). Similarly to aflR, stcU transcripts increased at 72 h in 2% cultures exposed to light, while transcript levels notably decreased in cultures in which lower glucose concentrations were used.
3.5 Effect of light and glucose on wild-type A. nidulans development
In A. nidulans the presence or absence of illumination governs the developmental decision towards asexual (light) or sexual (dark) differentiation (Yager, 1992). In addition to the effect of light, other factors, such as nutrient presence or abundance, also influence fungal morphogenesis. For example, it has been previously shown that high levels of glucose promote sexual development, while repressing conidiation (Han et al., 1990, Jeong et al., 2000; Han et al., 2001). We investigated the possible integrative effect of illumination and glucose concentration in the model fungus A. nidulans.
As expected conidiation was higher at 48 h in the light than in the dark, and increased over time (72h) at all concentrations tested, particularly when low glucose concentrations were used (Fig. 4C and D). At those same time points Hülle cells were also counted as an indicator of the initiation of sexual development (Yager, 1992). Hülle cell numbers increased in dark cultures with higher glucose concentration and this increase continued over time. Few Hülle cells were observed in 1.5% and 2% glucose cultures exposed to light (Fig. 4C).
3.6 Role of A. nidulans FphA, LreA and LreB in morphogenesis in response to different glucose concentrations under light or dark conditions
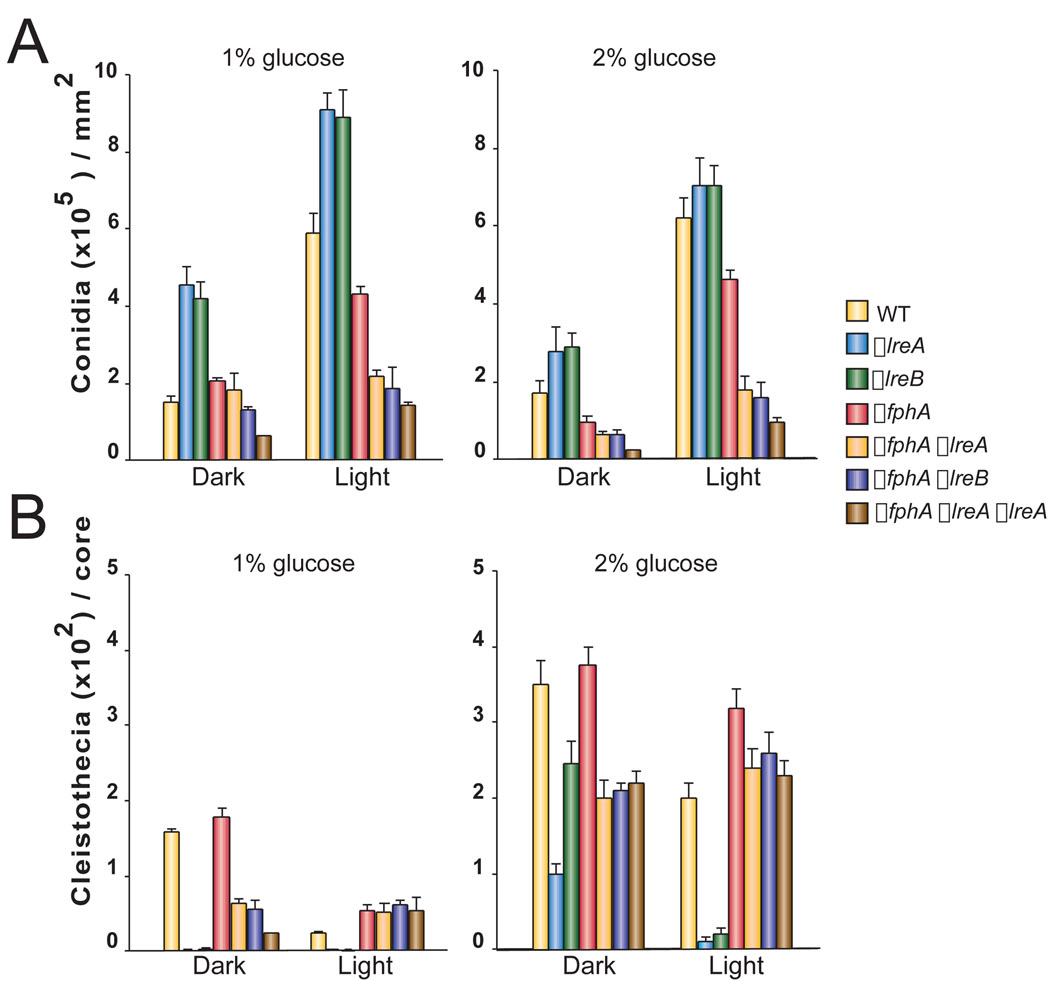
In our present study we investigated the role of light-sensing proteins in morphogenesis under light/dark conditions at different glucose concentrations. Previously we showed that the blue-light sensing proteins, LreA and LreB, have a repressing function on conidiospore production while the red-light sensing phytochrome-like protein, FphA, activates asexual development when growing on 2% glucose GMM (Purschwitz et al., 2008). A decrease in glucose (1%) did not alter the FphA/LreA/LreB-regulatory pattern of conidiation with respect to that previously described at a higher glucose concentration (2%) (Purschwitz et al., 2008, Fig. 5A and Suppl. Fig. 1). However, our current experiments show that de-repressed conidiation in lreA and lreB deletion mutants was enhanced at lower glucose concentration (1%) in both light and dark growing conditions. Conidiation of strains carrying fphA single null or in combination with either lreA or lreB null alleles or in the triple mutant combination was increased in the dark when 1% instead of 2% glucose was used in the medium (Fig. 5A, Suppl. Fig. 1).
Figure 5. Role of light-sensing components in the effect of light and glucose on A. nidulans morphogenesis.
A) Asexual development in A. nidulans FGSCA4 (WT), ΔfphA, ΔlreA, ΔlreB single mutants, ΔlreA, ΔfphA and ΔlreB, ΔfphA double mutants and triple mutant growing on 2% or 1% GMM in the dark or under white light for 7 days. B) Sexual development in the A. nidulans strains mentioned above under the same experimental conditions.
Regarding cleistothecial formation, whether the fungus was growing on 1% or 2% glucose media, FphA repressed the formation of these structures, while LreA and LreB are activators of the sexual cycle (Fig 5B, Suppl. Fig. 1). However, we observed a clear reduction of cleistothecial production in all strains tested in 1% glucose medium when compared with 2% glucose grown cultures (Fig. 5B). Production of cleistothecia in the lreA and lreB deletion mutants in the dark was particularly reduced when growing on 1% glucose. The increase in cleistothecial formation on 2% glucose cultures under light was limited in the lreA and lreB deletion mutants (Fig. 5B).
3.7 FphA, LreA and LreB regulate penicillin production
Our previous work revealed that A. nidulans VeA regulates the production of a variety of secondary metabolites, including mycotoxins, such as ST, and antibiotics, such as penicillin (PN) (Kato et al., 2003). Here we investigated whether other VeA-interacting and light-sensing components also participate in the regulation of PN biosynthesis.
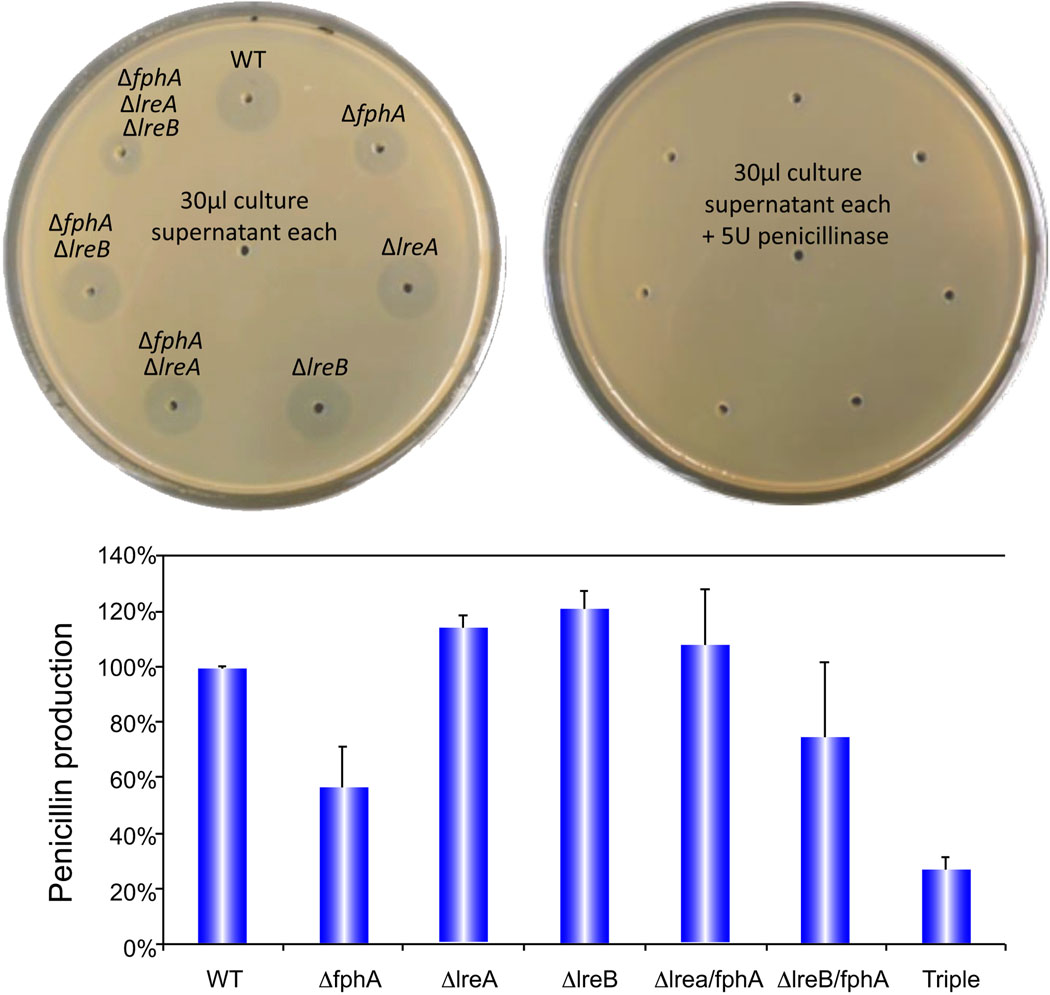
Our results in PN-inducing medium (Fig. 6) show that the light sensors FphA, LreA and LreB, play different roles in PN production. While PN biosynthesis was reduced in the fphA deletion mutant, lreA and lreB mutations resulted in a slight increase of PN production. Interestingly, the pattern observed (Fig. 6) was opposite with respect to that observed for ST production (Purschwitz et al., 2008). Double and triple mutants also resulted in a regulatory role opposite to that described for ST production. The PN-inducing medium (as in Brakhage et al., 1992) is opaque; therefore the mycelium was grown in the absence of light. As shown in Fig. 6, the antibacterial activity detected was exclusively due to the presence of PN.
Figure 6. Penicillin production is regulated by light-sensing proteins.
Bacillus stearothermophylus was used as testing organism against fungal culture supernatants. Amounts of penicillin are indicated as a percentage with respect to the amount produced by the wild-type strain. WT, wild type. Error bars indicate standard error.
4. Discussion
Metabolic changes, as well as changes in growth and sexual and asexual development, are part of the adaptative response to illumination (Purschwitz et al., 2006; Herrera-Estrella and Horwitz, 2007). Light affects the biosynthesis of certain metabolites, for example the production of carotenoids in N. crassa (Rau and Mitzka-Schnabel, 1985) or Fusarium oxysporum (Ruiz-Roldan et al., 2008). Light also influences polysaccharide and carbohydrate metabolism, nucleotide and nucleoside metabolism, fatty acid metabolism (Schuster et al., 2007; Tisch and Schmoll, 2010) and secondary metabolism (Tisch and Schmoll, 2010; Calvo 2008). We have previously shown that VeA is necessary for the synthesis of numerous natural products (Kato et al., 2003; Calvo et al., 2004; Duran et al., 2007, 2009; Myung et al., 2009; Calvo, 2008), and that FphA, LreA and LreB also have a role in regulating the production of the mycotoxin ST in A. nidulans (Purschwitz et al., 2008). In our present study we demonstrated that the effect of light-sensing proteins in metabolism is broader, also affecting the formation of the antibiotic PN (Fig. 6). Interestingly, the role of FphA, LreA, LreB on PN production is opposite to that described for ST regulation (Purschwitz et al., 2008). In PN-inducing medium, the production of this antibiotic decreased in the fphA deletion mutant, indicating that FphA is an activator of PN biosynthesis. On the other hand, lreA and lreB mutation resulted in a slight increase of PN production. A differential expression of fungal secondary metabolites, specifically ST and PN in A. nidulans, was also described for FadA, an alpha-subunit of a heterotrimeric G-protein (Tag et al., 2000). Future research will include the study of light-sensing proteins on the synthesis of additional natural products as well as exploring whether a possible connection with the FadA signaling pathway exists.
Previous studies showed some conflicting findings regarding the effect of light on secondary metabolism. For example, production of aflatoxin in A. flavus has been described as being negatively regulated by light (Joffe and Lisker, 1969). While Aziz and Moussa report that aflatoxin production by A. flavus is enhanced upon cultivation in light (Aziz and Moussa, 1997). Ochratoxin production in A. ochraceus appears to increase in cultures growing in the light (Aziz and Moussa, 1997). However, the photoperiod did not show an effect on ochratoxin production under field conditions (Belli et al., 2006). These differences in the outcomes of light regulation may be explained by different cultivation conditions, including abundance of nutrients. Our previous work indicates that the effect of light on the production of ST toxin in A. nidulans is medium-dependent (Kato et al., 2003). While production of ST in GMM was greater in the dark, accumulation of this toxin was greater in light in a rich medium such as YGT. The stimulating effect of light on ST production in rich medium is also supported by other studies (Guzman-de-Pena et al., 1998). One of the most significant findings in our current study is the interaction between the responses to different external stimuli that results in an integrated regulation of secondary metabolism. Specifically we found a relationship between the effect of glucose abundance and light in the control of ST biosynthesis, in which glucose concentration changes the effects of light on ST production (Fig. 1, 2A and Fig. 4A). In the dark, it is clear that increasing glucose concentrations exerted a repressive action on ST production, while in the light increasing amounts of glucose induced mycotoxin production.
In order to better understand the effect of glucose on the light regulatory role in ST biosynthesis we investigated whether variations in cAMP play a role in the interaction between these two regulatory systems. cAMP levels vary depending on glucose availability, increasing under glucose starvation (see review by Lafon et al., 2006 and references therein, and Lafon et al, 2005). cAMP is known to activate cAMP-dependent protein kinase A (PKA) (Neves et al., 2002; Pawson and Scott, 2005; Pierce et al., 2002). In addition, light has been shown to cause alterations in the internal cAMP level (Gresik et al., 1988; Farkas et al., 1990). cAMP/PKA signaling is known to govern morphogenesis of a number of fungi (Lee et al., 2003), including A. nidulans (Shimizu and Keller, 2000). This signaling pathway also has inhibitory effects on the expression of the aflR gene, encoding the ST gene cluster-specific transcription factor, and consequently inhibits ST production by A. nidulans (Shimizu and Keller, 2001). Our results suggest that the observed effect of glucose on light-dependent ST biosynthesis regulation was not mediated by cAMP under the experimental conditions tested.
We previously showed that light regulates the subcellular localization of VeA, and therefore, the VeA nuclear protein interactions that include the light sensing protein apparatus and other proteins including LaeA, a putative methyl transferase that regulates the expression of secondary metabolism gene clusters, including the ST gene cluster (Bok et al., 2004; Bayram et al., 2008; Calvo, 2008). Here we tested whether differences in glucose concentration could affect the VeA subcellular localization pattern determined by light. Essentially VeA accumulates in the nuclei in the dark under all conditions tested, where it has been shown to maintain interactions with photoreceptors and transcription factors (Calvo, 2008; Bayram et al., 2008). A key question here is whether VeA in different glucose conditions may alter its range of possible interactors/effectors. Interestingly, we have observed light independent nuclear accumulation when 1.5% glucose was added. Although on 1% and 1.5% solid medium at 72 h VeA visualization was prevented due to partial cellular damage caused by light, in the dark we also observed higher nuclear accumulation of VeA in cultures with 1.5% glucose. The narrow range of glucose effect on VeA nuclear accumulation might reflect a highly specific regulatory system depending on the carbon source. In this context it is possible that two ways of VeA cellular regulation could exist depending on either activating nuclear transport via the NLS recognized by the importin a KapA or altering VeA protein turn over, since there is no evidence of a nuclear export pathway involved (see Araújo-Bazán et al., 2009). Additional protein-protein interactions with carbon-regulators or effectors might account for the latter, although experiments done by us and others gave no evidence for such interactions however the conditions used differ from those now analyzed in this work.
Our microscopy studies showed that at low glucose concentration (1%), light had a particularly harmful effect on fungal cells over time. This was evidenced by the loss of nuclear integrity in most of the hyphal compartments and the vacuolated cytoplasm at 72 h after inoculation onto solid medium (Fig. 3). It is possible that light might have caused a more rapid consumption of glucose and that glucose starvation resulted in partial cellular damage. This damage was accompanied by, and might have contributed to, a decrease in the ability of A. nidulans to produce ST toxin over time under these conditions. At the same time, under illumination the fungus produces abundant air-born conidia, slightly more at low glucose concentrations, that allow dissemination to a more suitable environment. Higher glucose concentrations (1.5% and 2%) remediate the detrimental effects of light on the cells, allowing more ST accumulation. In addition, our results revealed that while ST gene expression levels were low in 48 h light cultures, these levels increased in older 2% glucose cultures, which could contribute to the accumulation of ST in the 2% glucose light cultures.
In the dark grown cultures, cellular integrity was not compromised at any glucose concentration studied over time, and ST production was abundant at 1%, where ST gene transcription was higher than in light cultures as observed after 48 h of incubation. At glucose concentrations greater than 2%, ST levels remain low, particularly in the dark. It is possible that under those conditions ST production could be repressed by carbon-regulators perhaps interacting with VeA, and/or by a VeA independent mechanism. Overall, our results suggest that carbon metabolism conditions the light effect on toxin production.
As expected, in the absence of light the development of cleisthothecial primordia was much more extensive than in the light. The increase in Hülle cells occurred more rapidly at higher glucose levels. Fungal morphogenesis is also regulated by VeA. Deletion of the veA gene results in a complete blockage in cleistothecial formation and increased conidiation (Kim et al., 2002, Kato et al., 2003; Calvo, 2008), indicating that VeA is indispensable for sexual development while repressing asexual development. FphA represses sexual development and promotes conidiation while LreA and LreB have antagonistic effects with respect to the role of FphA (Purschwitz et al., 2008). In the present study we show that induction of A. nidulans conidiation at low glucose concentration is particularly notable in the absence of LreA and LreB, in both light and dark conditions (Fig. 4 and Suppl. Fig. 1). This suggests an additional role for LreA and LreB in negatively modulating the response to a decrease in carbon source availability that leads to conidiation in this fungus. In Trichoderma atroviridae, a link between blue light sensing proteins BLR1 and BLR2 (also orthologs of N. crassa WC-1 and WC-2) and carbon starvation-induced conidiation has been shown (Casas-Flores et al., 2006). However, this was not the case in N. crassa (Arpaira et al., 1995; Sokolovsky et al., 1992), indicating that the relationship between the responses to light and carbon source abundance that conditions conidiation could vary in different fungi. With respect to sexual reproduction, while cleistothecial production was drastically enhanced in most of the strains tested when growing on high glucose medium, that increase was modest in the absence of LreA and LreB in cultures exposed to light, suggesting a possible interaction among light stimulus, LreA/B presence and glucose abundance. Our studies, together with those in T. atroviridae, further support a multitasking role for WC-1 and WC-2 orthologs in cross-talks with other signaling pathways responsive to external stimuli regulating fungal morphogenesis.
In conclusion, our study revealed that changes in light and carbon source concentration are important environmental cues, and that the sensing of these simultaneous stimuli and the concomitant cellular response does not occur independently but in an interdependent manner and in part mediated by VeA and VeA-interacting proteins, resulting in metabolic and morphological changes that could differ from those individually induced by variations in light or glucose concentration. Indeed, in nature fungi sense and react to a complexity of stimuli occurring simultaneously. Thus, it is predictable that future studies will reveal additional interactions linking diverse and interactive sensing and signaling mechanisms that fungi utilize to reach an optimal adaptative response to a complex environment.
Supplementary Material
Micrographs of the colony surface of A. nidulans FGSCA4 (WT), ΔfphA, ΔlreA, ΔlreB single mutants, ΔlreA, ΔfphA and ΔlreB, ΔfphA double mutants and triple mutant growing on 1% or 2% GMM in the dark or under white light. Images were captured with a Spot Insight Color camera on a Leica MZ75 dissecting microscope with a 50 × magnification.
Acknowledgements
This study was funded by Northern Illinois University and by NIH 1R15 A1081232-01. EAE wishes to thank the Ministerio de Ciencia e Innovación for grants BFU2006-04185 and BFU2009-08701(BMC). The work of the german group was funded by the DFG and the Landesstiftung Baden Württemberg. A.M. Calvo thanks Barbara Ball and Scott Grayburn for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdollahi A, Buchanan RL. Regulation of aflatoxin biosynthesis: characterization of glucose as an apparent inducer of aflatoxin production. J. Food Sci. 1981;46:143–146. [Google Scholar]
- Arpaia G, Cerri F, Baima S, Macino G. Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol. Gen. Genet. 1999;262:314–322. doi: 10.1007/s004380051089. [DOI] [PubMed] [Google Scholar]
- Araújo-Bazán L, Dhingra S, Chu J, Fernández-Martínez J, Calvo AM, Espeso EA. Importin alpha is an essential nuclear import carrier adaptor required for proper sexual and asexual development and secondary metabolism in Aspergillus nidulans. Fungal Genet Biol. 2009;46:506–515. doi: 10.1016/j.fgb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Arst HN, Jr, MacDonald DW. A gene cluster in Aspergillus nidulans with an internally located cis-acting regulatory region. Nature. 1975;254:26–31. doi: 10.1038/254026a0. [DOI] [PubMed] [Google Scholar]
- Aziz NH, Moussa LA. Influence of white light, near-UV irradiation and other environmental conditions on production of aflatoxin B1 by Aspergillus flavus and ochratoxin A by Aspergillus ochraceus. Nahrung. 1997;41:150–154. doi: 10.1002/food.19970410307. [DOI] [PubMed] [Google Scholar]
- Bailey C, Arst HN., Jr Carbon catabolite repression in Aspergillus nidulans. Eur. J. Biochem. 1975;51:573–577. doi: 10.1111/j.1432-1033.1975.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- Belli N, Ramos AJ, Sanchis V, Marin S. Effect of photoperiod and day-night temperatures simulating field conditions on growth and ochratoxin A production of Aspergillus carbonarius strains isolated from grapes. Food Microbiol. 2006;23:622–627. doi: 10.1016/j.fm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg- Dinkel N, Fischer R. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 2005;15:1833–1838. doi: 10.1016/j.cub.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage AA, Browne P, Turner G. Regulation of Aspergillus nidulans penicillin biosynthesis and penicillin biosynthesis genes acvA and ipnA by glucose. J. Bacteriol. 1992;174:3789–3799. doi: 10.1128/jb.174.11.3789-3799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RL, Stahl HG. Ability of various carbon sources to induce and support aflatoxin biosynthesis by Aspergillus parasiticus. J. Food Safety. 1984;6:271–279. [Google Scholar]
- Bu’Lock JD. Intermediary metabolism and antibiotic synthesis. Adv. Appl. Microbiol. 1961;3:293–342. doi: 10.1016/s0065-2164(08)70514-8. [DOI] [PubMed] [Google Scholar]
- Casas-Flores S, Rios-Momberg M, Rosales-Saavedra T, Martinez-Hernandez P, Olmedo-Monfil V, Herrera-Estrella A. Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway. Eukaryot Cell. 2006;5:499–506. doi: 10.1128/EC.5.3.499-506.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda-Olmedo E. Phycomyces and the biology of light and color. FEMS Microbiol. Rev. 2001;25:503–512. doi: 10.1111/j.1574-6976.2001.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Calvo AM, Bok J, Brooks W, Keller NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Corrochano LM. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 2007;6:725–736. doi: 10.1039/b702155k. [DOI] [PubMed] [Google Scholar]
- Dowzer CE, Kelly JM. Analysis of the creA gene, a regulator of catabolite repression in Aspergillus nidulans. Mol. Cell Biol. 1991;11:5701–5709. doi: 10.1128/mcb.11.11.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RM, Cary JW, Calvo AM. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- Duran RM, Cary JW, Calvo AM. The Role of veA in Aspergillus flavus infection of Peanut, Corn and Cotton. Open Mycology Journal. 2009;3:27–36. [Google Scholar]
- Espeso EA, Peñalva MA. Carbon catabolite repression can account for the temporal pattern of expression of a penicillin biosynthetic gene in Aspergillus nidulans. Mol Microbiol. 1992;16:1457–1465. doi: 10.1111/j.1365-2958.1992.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Espeso EA, Tilburn J, Arst HN, Jr, Peñalva MA. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993;12:3947–3956. doi: 10.1002/j.1460-2075.1993.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxebeste O, Markina-Iñarrairaegui A, Garzia A, Herrero-García E, Ugalde U, Espeso EA. KapI, a non-essential member of the Pse1p/Imp5 karyopherin family, controls colonial and asexual development in Aspergillus nidulans. Microbiology. 2009;155:3934–3945. doi: 10.1099/mic.0.032615-0. [DOI] [PubMed] [Google Scholar]
- Farkas V, Gresik M, Kolarova N, Sulova Z, Sestak S. Biochemical and physiological changes during photoinduced conidiation and derepression of cellulase synthesis in Trichoderma. In: Kubicek CP, Eveleigh DE, Esterbauer H, Steiner W, Kubicek-Pranz EM, editors. Trichoderma reesei cellulase: biochemistry, genetics, physiology and application. Cambridge: Graham; 1990. pp. 139–155. [Google Scholar]
- Feng GH, Leonard TJ. Culture conditions control expression of the genes for aflatoxin and sterigmatocystin biosynthesis in Aspergillus parasiticus and A. nidulans. Appl. Environ. Microbiol. 1998;64:2275–2277. doi: 10.1128/aem.64.6.2275-2277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M, Keller NP, Adams TH. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 1998;28:1355–1365. doi: 10.1046/j.1365-2958.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- Gresik M, Kolarova N, Farkas V. Membrane potential, ATP, and cyclic AMP changes induced by light in Trichoderma viride. Exp. Mycol. 1988;12:295–301. [Google Scholar]
- Guzman-de-Pena D, Aguirre J, Ruiz-Herrera J. Correlation between the regulation of sterigmatocystin biosynthesis and asexual and sexual sporulation in Emericella nidulans. Antonie Van Leeuwenhoek. 1998;73:199–205. doi: 10.1023/a:1000820221945. [DOI] [PubMed] [Google Scholar]
- Gyula P, Schafer E, Nagy F. Light perception and signalling in higher plants. Curr. Opin. Plant Biol. 2003;6:446–452. doi: 10.1016/s1369-5266(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Joffe AZ, Lisker N. Effects of light, temperature, and pH value on aflatoxin production in vitro. Appl. Microbiol. 1969;18:517–518. doi: 10.1128/am.18.3.517-518.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DM, Han YJ, Lee YH, Jahng K-Y, Jahng SH, Chae KS. Inhibitory conditions of asexual development and their application for the screening of mutants defective in sexual development. Korean J. Mycol. 1990;18:225–232. [Google Scholar]
- Han K-H, Han K-Y, Yu J-H, Chae K-S, Jahng K-Y, Han D-M. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 2001;41:299–309. doi: 10.1046/j.1365-2958.2001.02472.x. [DOI] [PubMed] [Google Scholar]
- He Q, Cheng P, Yang Y, Wang L, Gardner KH, Liu Y. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella A, Horwitz BA. Looking through the eyes of fungi: molecular genetics of photoreception. Mol. Microbiol. 2007;64:5–15. doi: 10.1111/j.1365-2958.2007.05632.x. [DOI] [PubMed] [Google Scholar]
- Jeong H-Y, Han DM, Jahng K-Y, Chae K-S. The rpl16a gene for ribosomal protein L16A identified from expressed sequence tags is differentially expressed during sexual development of Aspergillus nidulans. Fung. Genet. Biol. 2000;31:69–78. doi: 10.1006/fgbi.2000.1233. [DOI] [PubMed] [Google Scholar]
- Kacholz T, Demain AL. Nitrate repression of averufin and aflatoxin biosynthesis. J. Nat. Prod. 1983;46:499–506. [Google Scholar]
- Kafer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth JL, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Kim H, Han K, Kim K, Han D, Jahng K, Chae K. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:72–80. doi: 10.1016/s1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- Kues U. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. Mol. Biol. Rev. 2000;64:316–353. doi: 10.1128/mmbr.64.2.316-353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon A, Han KH, Seo JA, Yu J-H, d'Enfert C. G-protein and cAMP-mediated signaling in aspergilli: a genomic perspective. Fungal Genet. Biol. 2006;43:490–502. doi: 10.1016/j.fgb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Lafon A, Seo JA, Han KH, Yu J-H, d'Enfert C. The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans. Genetics. 2005;171:71–80. doi: 10.1534/genetics.105.040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, D’Souza CA, Kronstad JW. Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu. Rev. Phytopathol. 2003;41:399–427. doi: 10.1146/annurev.phyto.41.052002.095728. [DOI] [PubMed] [Google Scholar]
- Liu Y, He Q, Cheng P. Photoreception in Neurospora: a tale of two White Collar proteins. Cell. Mol. Life Sci. 2003;60:2131–2138. doi: 10.1007/s00018-003-3109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B-H, Chu FS. Regulation of aflR and its product, AflR, associated with aflatoxin biosynthesis. Appl. Environ Microbiol. 1998;64:3718–3723. doi: 10.1128/aem.64.10.3718-3723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchese RH, Harrigan WF. Biosynthesis of aflatoxin—the role of nutritional factors. J. Appl. Bacteriol. 1993;74:5–14. doi: 10.1111/j.1365-2672.1993.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Mooney JL, Hassett DE, Yager LN. Genetic analysis of suppressors of the veA1 mutation in Aspergillus nidulans. Genetics. 1990;126:869–874. doi: 10.1093/genetics/126.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney JL, Yager LN. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- Myung K, Li S, Butchko RA, Busman M, Proctor RH, Abbas HK, Calvo AM. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J. Agric. Food. Chem. 2009;57:5089–5094. doi: 10.1021/jf900783u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Protein phosphorylation in signaling— 50 years and counting. Trends Biochem. Sci. 2005;30:286–290. doi: 10.1016/j.tibs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nature Rev. Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, Muller S, Kastner C, Fischer R. Seeing the rainbow: light sensing in fungi. Curr. Opin. Microbiol. 2006;9:566–571. doi: 10.1016/j.mib.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA, Atoui A, Calvo AM, Fischer R. Functional and physical interaction of blue and red-light sensors in Aspergillus nidulans. Curr. Biol. 2008;18:1–5. doi: 10.1016/j.cub.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Rau W, Mitzka-Schnabel U. Carotenoid synthesis in Neurospora crassa. Methods Enzymology. 1985;110:253–267. doi: 10.1016/s0076-6879(85)10082-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Roldan MC, Garre V, Guarro J, Marine M, Roncero MI. Role of the white collar 1 photoreceptor in carotenogenesis, UV resistance, hydrophobicity, and virulence of Fusarium oxysporum. Eukaryot. Cell. 2008;7:1227–1230. doi: 10.1128/EC.00072-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A, Kubicek CP, Friedl MA, Druzhinina IS, Schmoll M. Impact of light on Hypocrea jecorina and the multiple cellular roles of ENVOY in this process. BMC Genomics. 2007;8:449. doi: 10.1186/1471-2164-8-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Keller NP. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff RA, O'Connor SM, Hynes MJ, Lockington RA, Kelly JM. Null alleles of creA, the regulator of carbon catabolite repression in Aspergillus nidulans. Fungal Genet. Biol. 1997;22:28–38. doi: 10.1006/fgbi.1997.0989. [DOI] [PubMed] [Google Scholar]
- Sokolovsky VY, Lauter FR, Muller-rober B, Ricci M, Schmidhauser TJ, Russo VEA. Nitrogen regulation of blue light-inducible genes in Neurospora crassa. J. Gen. Microbiol. 1992;138:2045–2049. [Google Scholar]
- Stinnett SM, Espeso EA, Cobeño L, Araújo-Bazán L, Calvo AM. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- Tag A, Hicks J, Garifullina G, Beremand M, Keller N. G-protein signaling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 2000;38:658–665. doi: 10.1046/j.1365-2958.2000.02166.x. [DOI] [PubMed] [Google Scholar]
- Tisch D, Schmoll M. Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol. Appl. Microbiol. Biotechnol. 2010;85:1259–1277. doi: 10.1007/s00253-009-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LN. Early developmental events during asexual and sexual sporulation in Aspergillus nidulans. In: Bennett JW, Klich MA, editors. Aspergillus: Biology and Industrial Applications. Boston: Butterworth-Heinemann; 1992. pp. 19–41. [PubMed] [Google Scholar]
- Yu J-H, Butchko RAE, Fernandes M, Keller NP, Leonard TL. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Micrographs of the colony surface of A. nidulans FGSCA4 (WT), ΔfphA, ΔlreA, ΔlreB single mutants, ΔlreA, ΔfphA and ΔlreB, ΔfphA double mutants and triple mutant growing on 1% or 2% GMM in the dark or under white light. Images were captured with a Spot Insight Color camera on a Leica MZ75 dissecting microscope with a 50 × magnification.