Abstract
This review focuses on six important parasitic diseases that adversely affect the health and lives of over one billion people worldwide. In light of the global human impact of these neglected tropical diseases (NTDs), several initiatives and campaigns have been mounted to eradicate these infections once and for all. Currently available therapeutics summarized herein are either ineffective and/or have severe and deleterious side effects. Resistant strains continue to emerge and there is an overall unmet and urgent need for new antiparasitic drugs. Marine-derived small molecules (MDSMs) from invertebrates comprise an extremely diverse and promising source of compounds from a wide variety of structural classes. New discoveries of marine natural product privileged structures and compound classes that are being made via natural product library screening using whole cell in vitro assays are highlighted. It is striking to note that for the first time in history the entire genomes of all six parasites have been sequenced and additional transcriptome and proteomic analyses are available. Furthermore, open and shared, publicly available databases of the genome sequences, compounds, screening assays, and druggable molecular targets are being used by the worldwide research community. A combined assessment of all of the above factors, especially of current discoveries in marine natural products, implies a brighter future with more effective, affordable, and benign antiparasitic therapeutics.
Introduction
Today over one billion people worldwide are at risk for contracting and battling tropical infectious diseases caused by parasitic organisms. Scores of countries, mostly in the Third World, are impacted and the World Health Organization now classifies many of these ailments as neglected tropical diseases (NTDs) (Neglected tropical diseases; URL: http://www.who.int/neglecteddiseases/). For decades the natural products community has engaged in the quest to identify small molecule leads to develop robust chemo-therapeutics against NTDs. However, most of these efforts are nascent and must now be invigorated because the humanitarian benefit from a breakthrough could be enormous. For example, as we begin a new decade, it is unthinkable that in the Third World one child dies every 30 s due to Malaria (10 Facts on malaria; URL: http://www.who.int/features/factfiles/malaria/). The goal of this brief account, which covers the period January 2008–August 2010, is to focus on six important parasitic diseases. These constitute notable targets for discovery and pipeline-building based on compounds emerging from the study of marine-derived small molecules (MDSMs) from invertebrates.
In a review published in 1993 [1] we discussed the existence of relevant parasitic diseases caused by helminth (19 examples) and protozoal (12 examples) parasites that could be targets for the discovery of MDSMs. In this focused update, we present trends and opportunities through an important subset of six diseases —Malaria, Schistosomiasis, Chagas Disease, River Blindness, Leishmaniasis, and Sleeping Sickness. An overview of the various terms associated with these diseases, their causative parasite, the transferring host organism, and estimated global human impact is summarized in Table 1. Protozoan parasites transferred to a human host through an insect bite are the cause of Malaria, Chagas Disease, Leishmaniasis, and Sleeping Sickness. Helminth diseases are the category for the other two entries, Schistosomiasis and River Blindness. Trematodes (blood flukes) of the genus Schistosoma flourish inside freshwater snails and enter the human host directly through the skin causing schistosomiasis. Lastly, River Blindness is the result of a common endosymbiotic relationship between a bacterium (Wolbachia pipientis) and a nematode (Onchocerca volvulus), which enter the human bloodstream through a blackfly bite. For a more detailed overview of life cycles of these parasites and disease symptoms the reader is directed to the World Health Organization (WHO Fact Sheets; URL: http://www.who.int/mediacentre/fact-sheets) and the Centers for Disease Control and Prevention (CDC Index of Parasitic Diseases; URL: http://www.cdc.gov/ncidod/dpd/parasites/).
Table 1.
Important parasitic diseases and their human impact
| Entry # | Disease name | Organism | Source/transmission | Estimated global relevance | |
|---|---|---|---|---|---|
| 1 | Malaria | Plasmodium falciparum | PSa | Mosquito | 247 milliond |
| P. vivax | Anopheles sp. | ||||
| P. malariae | |||||
| P. ovale | |||||
| P. knowlesi | |||||
| 2 | Schistosomiasis | Schistosoma mansoni | Hb | Freshwater snail | 207 milliond |
| S. haematobium | Biomphalaria sp. | ||||
| S. japonicum | |||||
| S. mekongi | |||||
| S. intercalatum | |||||
| 3 | Chagas Disease (American Trypanosomiasis) | Trypanosoma cruzi | PKc | Triatomine bug | 16–18 millione |
| Triatoma sp. | |||||
| Reduviidae sp. | |||||
| 4 | River Blindness (Onchercerciasis) | Onchocerca volvulus | H | Black fly | 17.7 millione |
| Wolbachia pipientis | Simulium sp. | ||||
| 5 | Leishmaniasis (Kala-Azar) | Leishmania sp. | PK | Sand fly | 12 millionf |
| Phlebotomus sp. | |||||
| 6 | Sleeping Sickness (Human African Trypanosomiasis) | Trypanosoma brucei | PK | Tsetse fly | 50–70 000e |
| T. brucei gambiense | Glossina sp. | ||||
| T. brucei rhodesiense |
PS: protozoan parasite, sporozoan subclass.
H: helminth parasite.
PK: protozoan parasite, kinetoplastid subclass.
Data from the World Health Organization; URL: http://www.who.int/mediacentre/factsheets/.
Data from the Centers for Disease Control and Prevention; URL: http://www.cdc.gov/ncidod/dpd/parasites/.
Data from Ref. [10].
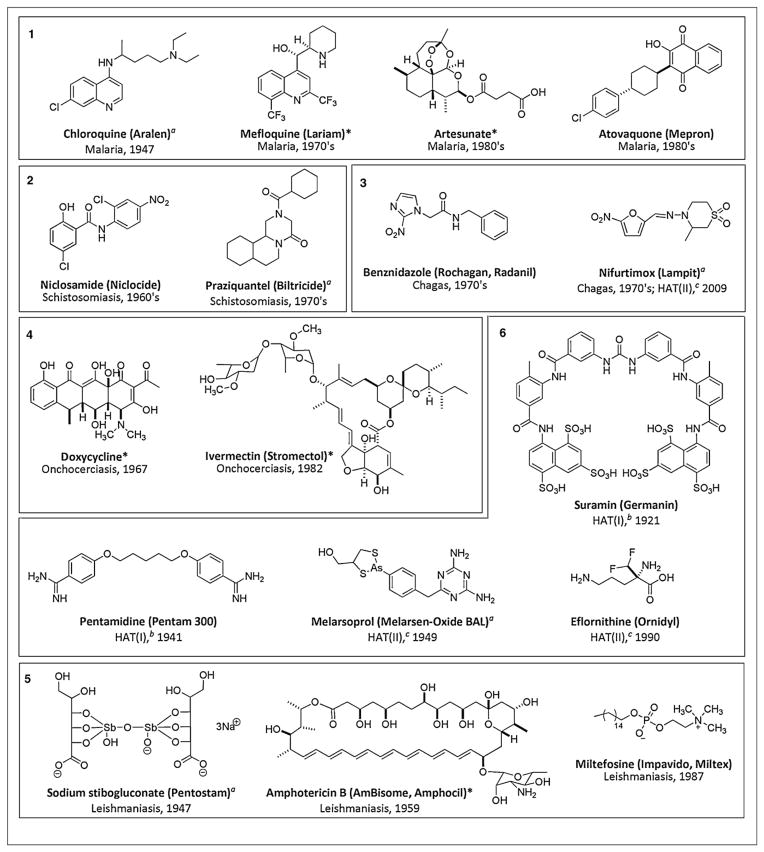
The answer to the obvious question — What current therapeutics exist for treating these diseases? — appears in the form of the 17 molecular structures shown in Figure 1. The compounds of this list represent ancient first generation drugs (see dates of their discovery — one can be traced back to 1921), many are somewhat toxic (see examples with As and Sb), several are difficult to administer, and almost all suffer from the continuing emergence of resistant parasite strains. Few among this list (only 31%) are based on a natural product, which is in contrast to the situation with anti-infectives where greater than 65% of clinical agents are of such origin [2].
Figure 1.
Examples of current therapeutics against important parasitic diseases with trade names, target disease (entries 1–6, Table 1), and year of discovery. *Natural product or based on a natural product scaffold. aAdministered as a mixture of stereoisomers. bHAT(I): First stage of Human African trypanosomiasis; cHAT(II): Second stage of Human African trypanosomiasis.
Initiatives are now rapidly emerging to overcome the well-known hurdles (development expense, distribution to low income and remote populations, target finding) for developing antiparasitics to combat the entries of Table 1. Assisting in the push forward to encourage natural products based-discoveries are a multitude of opportunities headed by US federally funded International Cooperative Biodiversity Groups (ICBG), The Bill & Melinda Gates Foundation, the Drugs for Neglected Diseases Initiative (DNDi), the Special Program for Research and Training in Tropical Diseases (TDR), the Medicines For Malaria Venture (MMV), the Anti-Wolbachia (A-WOL) Consortium, the Institute for One World Health, the Seattle Biomedical Research Institute, and the Sandler Center for Basic Research in Parasitic Diseases at UC San Francisco. These and other programs will lead to the next generation of discoveries and we believe MDSMs will play an ever-expanding role because of proof-of-concept discoveries that have emerged during the last decade.
An important preamble
Before proceeding, it is important to mention some trends as well as milestone discoveries that provide a platform for future developments. First, very few compounds on the list in Table 1 have dates post 1990. This indicates there may be low-hanging molecular fruit as templates for future research. Second, several antiparasitic drugs in Figure 1 are derived directly from natural sources (amphotericin B, ivermectin) or are based on natural product scaffolds (artesunate, mefloquine, and doxycy-cline), demonstrating the power of natural products in the antiparasitic drug discovery pipeline. Third, recently, the screening of natural product libraries using whole cell in vitro assays proved to be an effective paradigm for Novartis to uncover a new antimalarial lead compound [3]. Fourth, several academic groups are also engaged in the screening of MDSMs for antiparasitic leads and encouraging results have emerged in the past decade. Strikingly, sponges have been a significant source of antimalarial active scaffolds headed by compounds with diverse structures such as the manzamines, plakortins, isoaaptamines, axisonitriles, and homofascaplysins [4••,5••].
Despite the global relevance and lack of adequate therapeutics for Schistosomiasis and River Blindness (Table 1), screening of both plant and marine natural products has been focused only on P. falciparum, T. brucei sp., Leishmania sp. and T. cruzi and lead natural product scaffolds against these four protozoa have been outlined in a recent review [6]. Other reviews of interest include more specific reports covering drug resistance and natural product screening for T. brucei sp. [7], P. falciparum [8], and Leishmania sp. [9], with the main focus on plant metabolites. Natural products from medicinal plants with activity against T. cruzi were also highlighted [10], and two publications summarizing the discovery and development of marine antimalarials [4••,5••] recently appeared.
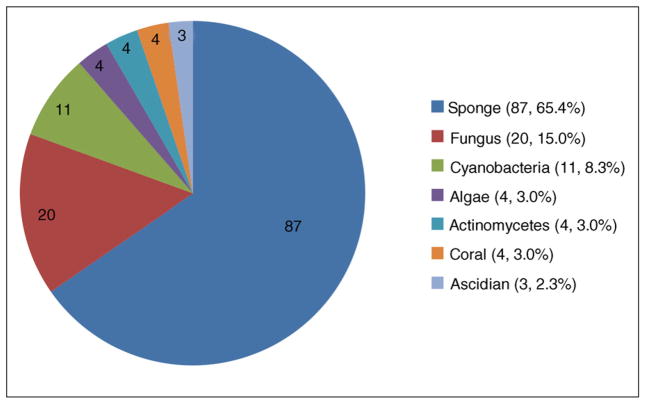
The synopsis in Figure 2 reflects these previous reviews and our supplemental literature searches. The 133 compounds included in this collection, discovered from 2008 to 2010, all displayed an IC50 below 30 μM against one or more parasites during in vitro screening. It is evident that marine sponges provide the majority of antiparasitic MDSMs with 87 compounds included in 30 publications between 2008 and 2010 [11–14,15•,16,17,18•,19,20•,21–27,28•,29–31,32•,33,34•,35,36•,37•,38•,39•,40]. Marine-derived fungi have produced the next largest set of 20 compounds, reported in only four papers [41–44] and cyanobacteria follow with 11 compounds included in eight reports [45–52]. Marine algae [53], actinomycetes [54–56], and hard corals [57–60] supplied four antiparasitic MDSMs each, and the final three metabolites were isolated from an ascidian [61•]. The remainder of this account will be focused on compounds from marine invertebrates (sponges, corals, and ascidians: 94 compounds, 70.7%) versus microorganism-derived molecules (fungi, cyanobacteria, actinomycetes, algae: 39 compounds, 29.3%) based on the special interest of this review.
Figure 2.
Overview of marine organism sources of 133 antiparasitic small molecules parasite targets include all entries from Table 1 except River Blindness.
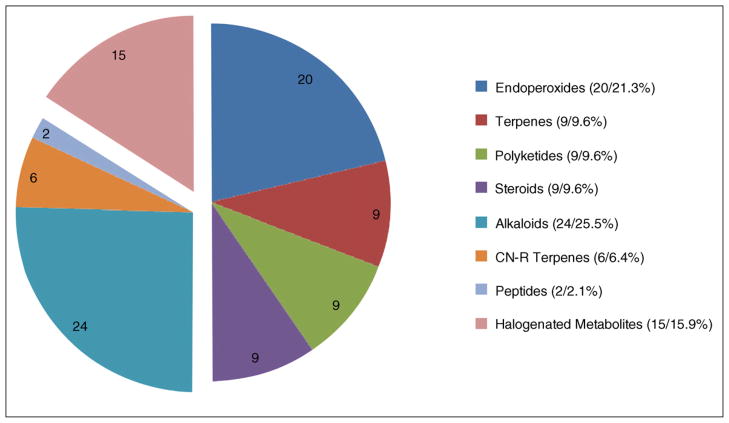
The 94 invertebrate-derived structures are divided into eight classes on the basis of their molecular formulas and structural motifs as shown in Figure 3. Compounds containing only carbon, hydrogen and oxygen (47 compounds, 50%) are the most abundant and have been divided into four classes: endoperoxides, oxoterpenes, polyketides, and steroids. Nitrogen-containing compounds (32 compounds, 34%) are also divided into subclasses of alkaloids, peptides, and isonitrile (CN-R) terpenes and the unique structures possessing halogen atoms comprise their own class (15 compounds, 16%).
Figure 3.
Structure types and the tally among eight classes of MDMS’s from invertebrates reported 2008–2010.
A snapshot of selected natural products
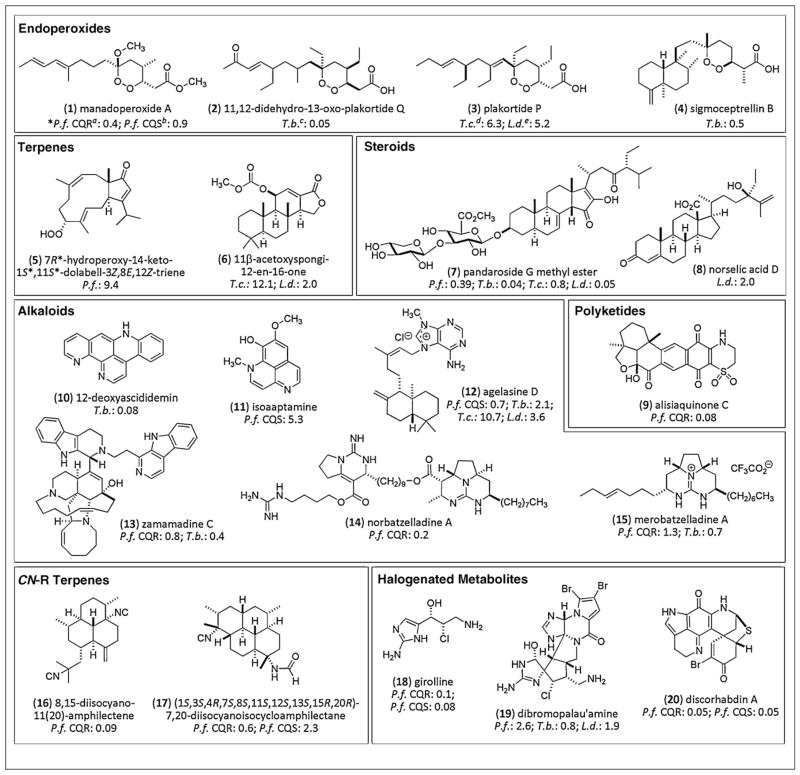
Twenty compounds were chosen to represent the outstanding structural diversity of antiparasitic MDSMs from invertebrates and they are displayed in Figure 4 along with the IC50 (μM) results obtained from in vitro screening. Our discussion will be limited to the most potent compounds of each molecular type, and the reader is encouraged to examine the cited literature for a more comprehensive overview. Assay standards listed in Table 2 provide the benchmark for comparison of in vitro bioassay data for the MDSMs discussed below.
Figure 4.
Twenty selected lead structures from 94 MDSM’s and their antiparasitic IC50’s (μM) *Parasite: IC50 (μM) result from in vitro screening; aP.f. CQR = P. falciparum chloroquine-resistant strain; bP.f. CQS = P. falciparum chloroquine-sensitive strain; cT.b. = T. brucei; dT.c. = T. cruzi; eL.d. = L. donovani.
Table 2.
Examples of in vitro bioactivity data for assay standards
| Disease target | Assay standard | IC50 (μM) | Organism target | Reference |
|---|---|---|---|---|
| Malaria | Chloroquine | 0.05 | P. falciparum (D6 CQSa) | [27] |
| 5.3 | P. falciparum (W2 CQRb) | [27] | ||
| Artemesinin | 0.01 | P. falciparum (K1 CQR) | [37•] | |
| Atovaquone | 0.0005 | P. falciparum (D6 CQS) | [38•] | |
| 0.002 | P. falciparum (W2 CQR) | [38•] | ||
| Schistosomiasis | Niclosamide | 4.6c | Biomphalaria glabrata | [50] |
| Chagas | Benznidazole | 1.2 | T. cruzi | [33] |
| Nifurtimiox | 10.0 | T. cruzi | [52] | |
| River Blindness | None | |||
| Leishmaniasis | Amphotericin B | 0.07 | L. donovani | [27] |
| Miltefosine | 0.5 | L. donovani | [33] | |
| Sodium stibogluconate | 44.7 | L. donovani | [51] | |
| Sleeping Sickness | Pentamidine | 0.03 | T. brucei brucei | [20•] |
| Melarsoprol | 0.01 | T. brucei rhodesiense | [33] |
CQS = chloroquine sensitive strain.
CQR = chloroquine-resistant strain.
LC100 value.
Compounds containing carbon, hydrogen and oxygen
Endoperoxides [18•,20•,22,27,32•,35]. These polyketide and terpenoid compounds display activities against P. falciparum and T. brucei in the nanomolar range, and also inhibit growth of T. cruzi at concentrations that are comparable with current assay standards. These multiple activities mirror the use of pentamidine and nifurtimox for the treatment of more than one parasitic disease. Interestingly, the antimalarial endoperoxy polyketide manadoperoxide A (1) showed a greater growth inhibition of a chloroquine resistant (CQR) strain of P. falciparum versus a chloroquine sensitive (CQS) strain [18•]. Compounds isolated from Plakortis sp. and Diacarnus bismarckensis displayed activity against T. brucei, although the polyketide 2 [20•] surpassed other terpenes in potency, including compound 4 [32•]. Limited screening of this compound class has been completed versus T. cruzi; however, endoperoxide 3 demonstrated growth inhibition equal with that of nifurtimox (Table 2) [22] and also showed modest activity against L. donovani.
Terpenes [21,30,59,60], Steroids, [26,31,37•], and Polyketides [17,25,35]. Other oxygen-containing terpenes display broad spectrum antiparasitic activities similar to the endoperoxy group, albeit with much less effectiveness. Two examples are the coral-derived compound 5 [60] and compound 6 from a Spongia sp. [30]. Compound 7, from a group of steroidal saponins, showed high potency against L. donovani. This represents a 20-fold improvement over the assay standard benznidazole; however, the selectivity for 7 between mammalian cells (IC50 = 0.2 μM, rat myoblast) and the parasite was relatively low (SI = 4.0) [31]. Another antileishmanial steroid 8 was isolated from a Crella sp. sponge collected in Antarctica, but was less potent than 7. Recent reports on antiparasitic polyketides focus on screening against P. falciparum. One highlight is the sulfonated compound 9 that showed excellent growth inhibition of CQR strains and little cytotoxicity versus mammalian cells (IC50 = 45.0 μM, MCF-7, SI = 512).
Compounds containing nitrogen
Alkaloids [11,13,15•,23,34•,36•,39•,57,61•] and CN-R terpenes [37•,38•]. Like the endoperoxide group of structures, alkaloids from invertebrates exhibited powerful bioactivity in multiple antiparasitic screens, with greatest potency against T. brucei and P. falciparum. Notable compounds include the pyridoacridone compounds 10 [61•] and 11 [13], a terpene alkaloid (12) [36•], the β-carboline 13, and guanidine alkaloids 14 [23] and 15 [34•]. Other nanomolar growth inhibitors of P. falciparum include the isonitrile-containing terpenes 16 [37•] and 17 [38•]. An outstanding selectivity index of (>154) was observed between the P. falciparum CQR strain and human fibroblast cells (IC50 = 13.9 μM) for compound 16.
Compounds containing halogen atoms
Halogenated metabolites [13,14,16,19,24,28•,33,40]. Secondary metabolites containing halogen atoms are commonly isolated from marine organisms and these compounds often elicit biological responses. Girolline (18), a relatively simple structure, is no exception, showing strong growth inhibition effects in several strains of P. falciparum in the nanomolar range. A similar pattern was reported for discorhabdin A (20), which showed equal activity in CQS and CQR strains of P. falciparum. The complex polycyclic alkaloid dibromopalau’amine (19) exhibited low micromolar IC50’s for P. falciparum, T. brucei rhodiense and L. donovani and showed modest selectivity (SI = 9.8) for T. brucei rhodesiense versus mammalian cells (rat myoblast, IC50 = 7.8 μM) [33]. A greater selectivity index of 27.2 was reported for compound 18 between Vero cells (IC50 = 2.1 μM) and a CQR strain P. falciparum [12].
Concluding remarks
The prospects for structure–activity relationship (SAR)-driven mining of MDSM-derived pharmacophores as antiparasitics against the targets shown in Table 1 are significant. Considerable structural diversity is represented in the 94 relevant structures we examined (as illustrated by the subset in Figure 4) and is the basis for the recommendation of six structural classes that are ripe for further development. This final list is based on biological properties of potency and/or selectivity, on new insights gained during the last 2.5 years on previously studied pharmacophores, plus new insights obtained for analogs based on structures examined in the past. The scaffolds among this list shown in Table 3 include: endoperoxides, guanidine alkaloids, β-carboline alkaloids, pyridoacridone alkaloids, isonitrile (CN-R) terpenes, and terpene alkaloids.
Table 3.
MDSM lead compound classes for antimalarial and trypanocidal development
| Target parasite | Lead compound classes and compound numbersa | Legacy compound(s) and year of antiparasitic discovery | References |
|---|---|---|---|
| P. falciparum; T. brucei sp. | Endoperoxides (1–4, 21) | plakortin (2002) | [18•,20•,22,27,32•,62] |
| P. falciparum | Guanidine alkaloids (14, 15, 24) | crambesidin 800 (2006) | [23,34•,64] |
| P. falciparum | β-Carboline alkaloids (13, 23) | manzamine A (2000) | [39•,63] |
| P. falciparum | Pyridoacridone alkaloids (10, 11, 22) | ascididemin (2003) | [13,28•,61•,65] |
| P. falciparum | CN-R terpenes (16, 17, 25) | kalihinol (1998) | [37•,38•,66] |
| P. falciparum; T. brucei sp. | Terpene alkaloids (12) | agelasine D (2008) | [11,15•,36•] |
See Figure 3 and structures below.
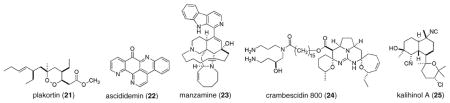
Comparing the information in the two center columns of Table 3 illustrates that, while no significant new structure types have been identified, significant interest and effort is being devoted to enhancing the lead potential of legacy pharmacophores. Here are some specific examples to underscore this point. The antimalarial activity of plakortin (21) was discovered in 2003 (IC50 = 0.87 μM (P. falciparum D10-CQS), IC50 = 0.41 μM (P. falciparum W2-CQR)) [62] and its potency against P. falciparum has not been surpassed by any other endoperoxide (such as 1–4). Significantly, endoperoxide 2 represents a new lead compound for Sleeping Sickness; it possesses nanomolar potency against T. brucei and 100-fold selectivity versus mammalian cells (HEK293) [20•]. Similarly, in 2000 manzamine A (23) (original structure published in 1986) was found to have antimalarial properties [63]. Its recent re-isolation along with the zamamadine (13) series of compounds [39•] not only showed that manzamine A is still the most potent antimalarial β-carboline alkaloid, but also uncovered its trypanocidal properties (IC50 = 0.07 μM, T. brucei rhodesiense). However, concerns about the cytotoxicity of 23 remain unresolved. Among the guanidine alkaloids, crambescidin 800 (24) (IC50 = 0.16 μM (P. falciparum 3D7-CQS), IC50 = 0.24 μM (P. falciparum FCR3-CQR)) [64] is more potent than batzelladines (compounds 14, 15). New prospects have been discovered for the pyridoacridone class, whose analogs have been poorly studied. In particular, ascididemin (22) exhibits nM activity against Plasmodium and Trypanosoma [61•,65]. The isonitrile-containing terpenoids represent a class known since 1973 and while kalihinol A (25) is exquisitely potent (P. falciparum IC50 = 0.001 μM) [66] this activity level is matched by that of amphilectenes (16, 17). The isonitrile functionality is the warhead for activity, putatively by disruption of heme [67]. The known agelasine class represents a new opportunity for further study; however, compound 12 is less potent than other legacy compounds.
In summary, our analysis of the discovery of active invertebrate-derived MDSMs suggests the following trends: First, most of the information gained from in vitro screening studies in the last three years has provided additional structure–activity information for established antimalarial compounds [4••]. Second, several known antimalarial MDSMs and their congeners also have potent trypanocidal activity versus T. brucei. Third, there are no significant lead compounds under advanced evaluation against the other four parasitic diseases of Table 1 (Entries 2–5).
Gazing into our crystal ball
Scientists and philanthropists are becoming united in their belief that curing the six parasite diseases discussed here can occur by a fusion of the structural diversity inherent in natural products and the new insights being accumulated from breakthroughs in molecular biology. Thus, we expect that the investigation of new and known MDSMs in the context of antiparasitic research will thrive in coming years. Furthermore, such research activities will expand beyond the core tasks of natural product-derived library screening. As illustrated above, MDSM leads are in hand for Malaria and Sleeping Sickness, therefore a shift in effort is needed on two different fronts. First, accelerated screening must occur against Leishmania sp. and T. cruzi using well-established assays. Second, programs need to be initiated employing the schistosomiasis parasite utilizing assays for medium-throughput [68] and high-throughput [69••] screening that have recently been described. Likewise, current awareness searches should be directed to assays employing the River Blindness causative organisms — Wolbachia pipientis and Onchocercus volvulus.
The climate to promote rapid discovery has arrived. The impact of genomics and the availability of publicly accessible, open and shared databases such as Collaborative Drug Discovery (CDD) (URL: http://www.collaborative-drug.com/) represent new important milestones to facilitate future research based on deeper understanding of parasite biology and host interactions, and the potential for small molecules to modulate these processes. The CDD database and similar tools are rapidly expanding with voluminous screening and compound data being added by researchers worldwide, such as the GlaxoS-mithKline open-access collection of 13 500 antimalarial compounds. The complete sequences for the genomes of each parasite in Table 1 have been published [70,71••,72–75]. Transcriptome and proteomic analyses provide additional insights into parasite life cycles, environmental responses, parasite–host interactions, and identification of new druggable targets [71••]. Indeed, the synergy of efforts by the research community and the age of genomics have already greatly enhanced our understanding of these diseases and potential curative agents. Thus, our summary of the recent literature here serves not only as an overview of what has been accomplished, but also as a wake-up call to the global natural products community in the tasks that remain before us!
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Crews P, Hunter LM. The search for antiparasitic agents from marine animals. In: Attaway DH, Zaborsky OR, editors. Marine Biotechnology. Plenum Press; 1993. pp. 343–389. [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70 :461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 3.Naik G. New malaria drug candidate holds promise. Wall Street J. 2010 [Google Scholar]
- 4••.Peach K, Linington RG. New innovations for an old infection: antimalarial lead discovery from marine natural products during the period 2003–2008. Future Med Chem. 2009;1:593–617. doi: 10.4155/fmc.09.56. The authors review the development of lead antimalarial MDSMs, including the discovery of new lead scaffolds and synthetic efforts toward simplified analogs. [DOI] [PubMed] [Google Scholar]
- 5••.Fattorusso E, Taglialatela-Scafati O. Marine antimalarials. Mar Drugs. 2009;7:130–152. doi: 10.3390/md7020130. This review is focused on the structures and activities of three antimalarial MDSM families including isonitrile-containing derivatives, alkaloids, and endoperoxides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioset J-R. Natural products for neglected diseases: a review. Curr Org Chem. 2008;12:643–666. [Google Scholar]
- 7.Gehrig S, Efferth T. Development of drug resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of human African trypanosomiasis with natural products (review) Int J Mol Med. 2008;22:411–419. [PubMed] [Google Scholar]
- 8.Turschner S, Efferth T. Drug resistance in Plasmodium: natural products in the fight against malaria. Mini-Rev Med Chem. 2009;9 :206–214. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]
- 9.Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products (review) Int J Mol Med. 2008;22:277–286. [PubMed] [Google Scholar]
- 10.Uchiyama N. Antichagasic activities of natural products against Trypanosoma cruzi. J Health Sci. 2009;55:31–39. [Google Scholar]
- 11.Appenzeller J, Mihci G, Martin M-T, Gallard J-F, Menou J-L, Boury-Esnault N, Hooper J, Petek S, Chevalley S, Valentin A, et al. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J Nat Prod. 2008;71:1451–1454. doi: 10.1021/np800212g. [DOI] [PubMed] [Google Scholar]
- 12.Benoit-Vical F, Salery M, Soh PN, Ahond A, Poupat C. Girolline: a potential lead structure for antiplasmodial drug research. Planta Med. 2008;74:438–444. doi: 10.1055/s-2008-1034348. [DOI] [PubMed] [Google Scholar]
- 13.Bowling JJ, Pennaka HK, Ivey K, Wahyuono S, Kelly M, Schinazi RF, Valeriote FA, Graves DE, Hamann MT. Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine. Chem Biol Drug Des. 2008;71 :205–215. doi: 10.1111/j.1747-0285.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcul L, Inman WD, Morris AA, Tenney K, Ratnam J, McKerrow JH, Valeriote FA, Crews P. Additional insights on the bastadins: isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configurations. J Nat Prod. 2010;73:365–372. doi: 10.1021/np9005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Calcul L, Tenney K, Ratnam J, McKerrow JH, Crews P. Structural variations to the 9-N-methyladeninium diterpenoid hybrid commonly isolated from Agelas sponges. Aust J Chem. 2010;63:915–921. Two terpene alkaloids from a Papua New Guinea Agelas sp. sponge, agelasine F and agelasine M, display low micromolar activity against T. brucei. The unique core structure of agelasine M, containing a bridgehead aldehyde, imparts 10 times greater potency for the trypanosome versus Jurkat cells. [Google Scholar]
- 16.Davis RA, Duffy S, Avery VM, Camp D, Hooper JNA, Quinn RJ. (+)-7-Bromotrypargine: an antimalarial beta-carboline from the Australian marine sponge Ancorina sp. Tetrahedron Lett. 2010;51:583–585. [Google Scholar]
- 17.Desoubzdanne D, Marcourt L, Raux R, Chevalley S, Dorin D, Doerig C, Valentin A, Ausseil F, Debitus C. Alisiaquinones and alisiaquinol, dual inhibitors of plasmodium falciparum enzyme targets from a new caledonian deep water sponge. J Nat Prod. 2008;71:1189–1192. doi: 10.1021/np8000909. [DOI] [PubMed] [Google Scholar]
- 18•.Fattorusso C, Persico M, Calcinai B, Cerrano C, Parapini S, Taramelli D, Novellino E, Romano A, Scala F, Fattorusso E, et al. Manadoperoxides A–D from the Indonesian sponge Plakortis cfr. simplex. Further insights on the structure–activity relationships of simple 1,2-dioxane antimalarials. J Nat Prod. 2010;73:1138–1145. doi: 10.1021/np100196b. The authors describe the structure elucidation of four new endoperoxy polyketides. While none are as potent as plakortin against P. falciparum, the authors provide insight into the mode of action of the endoperoxide antiparasitic activity. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Davis RA, Sykes ML, Avery VM, Camp D, Quinn RJ. Pseudoceratinazole A: a novel bromotyrosine alkaloid from the Australian sponge Pseudoceratina sp. Tetrahedron Lett. 2010;51:4847–4850. [Google Scholar]
- 20•.Feng Y-J, Davis RA, Sykes M, Avery VM, Camp D, Quinn RJ. Antitrypanosomal cyclic polyketide peroxides from the Australian marine sponge Plakortis sp. J Nat Prod. 2010;73:716–719. doi: 10.1021/np900535z. Four polyketide endoperoxides in the plakortide family represent new leads for Sleeping sickness. 11,12-Didehydro-13-oxo-plakortide Q shows 100-fold selectivity versus mammalian cells. [DOI] [PubMed] [Google Scholar]
- 21.Johnson TA, Amagata T, Sashidhara KV, Oliver AG, Tenney K, Matainaho T, Ang KK-H, McKerrow JH, Crews P. The Aignopsanes, a new class of sesquiterpenes from selected chemotypes of the sponge Cacospongia mycofijiensis. Org Lett. 2009;11:1975–1978. doi: 10.1021/ol900446d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kossuga MH, Nascimento AM, Reimao JQ, Tempone AG, Taniwaki NN, Veloso K, Ferreira AG, Cavalcanti BC, Pessoa C, Moraes MO, et al. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J Nat Prod. 2008;71:334–339. doi: 10.1021/np0705256. [DOI] [PubMed] [Google Scholar]
- 23.Laville R, Thomas OP, Berrue F, Marquez D, Vacelet J, Amade P. Bioactive guanidine alkaloids from two Caribbean marine sponges. J Nat Prod. 2009;72:1589–1594. doi: 10.1021/np900244g. [DOI] [PubMed] [Google Scholar]
- 24.Lebouvier N, Jullian V, Desvignes I, Maurel S, Parenty A, Dorin-Semblat D, Doerig C, Sauvain M, Laurent D. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a Vanuatu marine sponge Pseudoceratina sp. Mar Drugs. 2009;7:640–653. doi: 10.3390/md7040640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longeon A, Copp BR, Roue M, Dubois J, Valentin A, Petek S, Debitus C, Bourguet-Kondracki M-L. New bioactive halenaquinone derivatives from South Pacific marine sponges of the genus Xestospongia. Bioorg Med Chem. 2010;18:6006–6011. doi: 10.1016/j.bmc.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 26.Ma WS, Mutka T, Vesley B, Amsler MO, McClintock JB, Amsler CD, Perman JA, Singh MP, Maiese WM, Zaworotko MJ, et al. Norselic acids A–E, highly oxidized anti-infective steroids that deter mesograzer predation, from the Antarctic sponge Crella sp. J Nat Prod. 2009;72:1842–1846. doi: 10.1021/np900382x. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed R, Peng J, Kelly M, Yousaf M, Winn E, Odde S, Bie Z, Xie A, Doerksen RJ, Hamann MT. Polyketide-peroxides from a Species of Jamaican Plakortis (Porifera: Demospongiae) Aust J Chem. 2010;63:877–885. [Google Scholar]
- 28•.Na MK, Ding Y, Wang B, Tekwani BL, Schinazi RF, Franzblau S, Kelly M, Stone R, Li X-C, Ferreira D, et al. Anti-infective discorhabdins from a deep-water Alaskan sponge of the Genus Latrunculia. J Nat Prod. 2010;73:383–387. doi: 10.1021/np900281r. This article describes the structures of two new discorhabdin analogs and the screening of eight compounds in this structural family. Discorhabdin A, discorhabdin C and dihydrodiscorhabdin C display selective antimalarial activity; however, in vivo studies in mice failed due to compound toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakao Y, Kawatsu S, Okamoto C, Okamoto M, Matsumoto Y, Matsunaga S, van SRWM, Fusetani N. Ciliatamides A–C, bioactive lipopeptides from the deep-sea sponge Aaptos ciliata. J Nat Prod. 2008;71:469–472. doi: 10.1021/np8000317. [DOI] [PubMed] [Google Scholar]
- 30.Orhan I, Sener B, Kaiser M, Brun R, Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regalado EL, Tasdemir D, Kaiser M, Cachet N, Amade P, Thomas OP. Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J Nat Prod. 2010;73:1404–1410. doi: 10.1021/np100348x. [DOI] [PubMed] [Google Scholar]
- 32•.Rubio BK, Tenney K, Ang K-H, Abdulla M, Arkin M, McKerrow JH, Crews P. The marine sponge Diacarnus bismarckensis as a source of peroxiterpene inhibitors of Trypanosoma brucei, the causative agent of sleeping sickness. J Nat Prod. 2009;72:218–222. doi: 10.1021/np800711a. Using careful examination of spectral data and modified Mosher’s method, the absolute structures of two new endoperoxy terpenes are reported. Screening of these compounds and five known structures resulted in sigmoceptrellin B as the most potent analog. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scala F, Fattorusso E, Menna M, Taglialatela-Scafati O, Tierney M, Kaiser M, Tasdemir D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar Drugs. 2010;8 :2162–2174. doi: 10.3390/md8072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Takishima S, Ishiyama A, Iwatsuki M, Otoguro K, Yamada H, Omura S, Kobayashi H, van SRWM, Matsunaga S. Merobatzelladines A and B, anti-infective tricyclic guanidines from a marine sponge Monanchora sp. Org Lett. 2009;11:2655–2658. doi: 10.1021/ol9006794. Two new guanidine alkaloids related to crambescidin 800 are discussed. The activity of the compounds is tested against T. brucei, and this is the first assessment of trypanocidal activity of this alkaloid class. [DOI] [PubMed] [Google Scholar]
- 35.Ueoka R, Nakao Y, Kawatsu S, Yaegashi J, Matsumoto Y, Matsunaga S, Furihata K, van SRWM, Fusetani N. Gracilioethers A–C, antimalarial metabolites from the marine sponge Agelas gracilis. J Org Chem. 2009;74:4203–4207. doi: 10.1021/jo900380f. [DOI] [PubMed] [Google Scholar]
- 36•.Vik A, Proszenyak A, Vermeersch M, Cos P, Maes L, Gundersen L-L. Screening of agelasine D and analogs for inhibitory activity against pathogenic protozoa; identification of hits for visceral leishmaniasis and chagas disease. Molecules. 2009;14:279–288. doi: 10.3390/molecules14010279. Agelasine D and a series of 32 synthetic analogs were screened against four protozoan parasites. The highest potency of this compound class is revealed against T. brucei, and one simplified analog shows greater selectivity than agelasine D while maintaining trypanocidal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Wattanapiromsakul C, Chanthathamrongsiri N, Bussarawit S, Yuenyongsawad S, Plubrukarn A, Suwanborirux K. 8-Isocyanoamphilecta-11(20),15-diene, a new antimalarial isonitrile diterpene from the sponge Ciocalapata sp. Can J Chem. 2009;87:612–618. The authors describe four CN-R terpenes in the amphilectene family that were isolated from a Thai sponge. 8,15-Diisocyano-11(20)-amphilectene showed antimalarial and cytotoxic properties in the same range as artemesinin. [Google Scholar]
- 38•.Wright AD, Lang-Unnasch N. Diterpene formamides from the tropical marine sponge Cymbastela hooperi and their antimalarial activity in vitro. J Nat Prod. 2009;72:492–495. doi: 10.1021/np800654w. In this study of diterpenes containing both isonitrile and formamide functionalities, the authors uncovered an amphilectene analog with potent antimalarial activity. The structure–activity relationship that emerges from this study identifies the isonitrile as the warhead for activity. [DOI] [PubMed] [Google Scholar]
- 39•.Yamada M, Takahashi Y, Kubota T, Fromont J, Ishiyama A, Otoguro K, Yamada H, Omura S, Kobayashi J. Zamamidine C, 3,4-dihydro-6-hydroxy-10,11-epoxymanzamine A, and 3,4-dihydromanzamine J N-oxide, new manzamine alkaloids from sponge Amphimedon sp. Tetrahedron. 2009;65:2313–2317. The isolation and structure elucidation of four new compounds in the β-carboline family are described. This is the first report of trypanocidal activity of the manzamine class of MDSMs. [Google Scholar]
- 40.Yang X, Davis RA, Buchanan MS, Duffy S, Avery VM, Camp D, Quinn RJ. Antimalarial bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J Nat Prod. 2010;73 :985–987. doi: 10.1021/np900834g. [DOI] [PubMed] [Google Scholar]
- 41.Kasettrathat C, Ngamrojanavanich N, Wiyakrutta S, Mahidol C, Ruchirawat S, Kittakoop P. Cytotoxic and antiplasmodial substances from marine-derived fungi, Nodulisporium sp. and CRI247–01. Phytochemistry (Elsevier) 2008;69:2621–2626. doi: 10.1016/j.phytochem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Pontius A, Krick A, Kehraus S, Brun R, Koenig GM. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J Nat Prod. 2008;71 :1579–1584. doi: 10.1021/np800294q. [DOI] [PubMed] [Google Scholar]
- 43.Watts KR, Ratnam J, Ang K-H, Tenney K, Compton JE, McKerrow J, Crews P. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorg Med Chem. 2010;18 :2566–2574. doi: 10.1016/j.bmc.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu L, He Z, Xue J, Chen X, Wei X. Beta-resorcylic acid lactones from a Paecilomyces fungus. J Nat Prod. 2010;73:885–889. doi: 10.1021/np900853n. [DOI] [PubMed] [Google Scholar]
- 45.Balunas MJ, Linington RG, Tidgewell K, Fenner AM, Urena L-D, Della TG, Kyle DE, Gerwick WH. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J Nat Prod. 2010;73:60–66. doi: 10.1021/np900622m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbaras D, Kaiser M, Brun R, Gademann K. Potent and selective antiplasmodial activity of the cyanobacterial alkaloid nostocarboline and its dimers. Bioorg Med Chem Lett. 2008;18 :4413–4415. doi: 10.1016/j.bmcl.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez M, Tidgewell K, Capson TL, Engene N, Almanza A, Schemies J, Jung M, Gerwick WH. Malyngolide dimer, a bioactive symmetric cyclodepside from the Panamanian marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2010;73 :709–711. doi: 10.1021/np9005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linington RG, Edwards DJ, Shuman CF, McPhail KL, Matainaho T, Gerwick WH. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J Nat Prod. 2008;71:22–27. doi: 10.1021/np070280x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linington RG, Clark BR, Trimble EE, Almanza A, Urena L-D, Kyle DE, Gerwick WH. Antimalarial peptides from marine cyanobacteria: isolation and structural elucidation of gallinamide A. J Nat Prod. 2009;72:14–17. doi: 10.1021/np8003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira AR, McCue CF, Gerwick WH. Cyanolide A, a glycosidic macrolide with potent molluscicidal activity from the Papua New Guinea cyanobacterium Lyngbya bouillonii. J Nat Prod. 2010;73:217–220. doi: 10.1021/np9008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez LM, Lopez D, Vesely BA, Della TG, Gerwick WH, Kyle DE, Linington RG. Almiramides A–C: discovery and development of a new class of Leishmaniasis lead compounds. J Med Chem. 2010;53:4187–4197. doi: 10.1021/jm100265s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons TL, Engene N, Urena LD, Romero LI, Ortega-Barria E, Gerwick L, Gerwick WH. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigroviridis. J Nat Prod. 2008;71 :1544–1550. doi: 10.1021/np800110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin A-S, Stout EP, Prudhomme J, Le RK, Fairchild CR, Franzblau SG, Aalbersberg W, Hay ME, Kubanek J. Bioactive bromophycolides R-U from the Fijian red alga Callophycus serratus. J Nat Prod. 2010;73:275–278. doi: 10.1021/np900686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Na M, Meujo DAF, Kevin D, Hamann MT, Anderson M, Hill RT. A new antimalarial polyether from a marine Streptomyces sp. H668. Tetrahedron Lett. 2008;49:6282–6285. doi: 10.1016/j.tetlet.2008.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pimentel-Elardo SM, Kozytska S, Bugni TS, Ireland CM, Moll H, Hentschel U. Anti-parasitic compounds from Streptomyces sp. strains isolated from Mediterranean sponges. Mar Drugs. 2010;8 :373–380. doi: 10.3390/md8020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prudhomme J, McDaniel E, Ponts N, Bertani S, Fenical W, Jensen P, Le RK. Marine actinomycetes: a new source of compounds against the human malaria parasite. PLoS One. 2008;3:e2335. doi: 10.1371/journal.pone.0002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer M, Delberghe F, Liron F, Guillaume M, Valentin A, Guyot M. An antiplasmodial new (bis)indole alkaloid from the hard coral Tubastraea sp. Nat Prod Res A. 2009;23:178–182. doi: 10.1080/14786410801925134. [DOI] [PubMed] [Google Scholar]
- 58.Ospina CA, Rodriguez AD. Corallolides A and B: bioactive diterpenes featuring a novel carbon skeleton. Org Lett. 2009;11 :3786–3789. doi: 10.1021/ol901577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez II, Rodriguez AD, Zhao H. Aberrarone: a Gorgonian-derived diterpene from Pseudopterogorgia elisabethae. J Org Chem. 2009;74:7581–7584. doi: 10.1021/jo901578r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei X, Rodriguez AD, Baran P, Raptis RG. Dolabellane-type diterpenoids with antiprotozoan activity from a Southwestern Caribbean Gorgonian octocoral of the genus Eunicea. J Nat Prod. 2010;73:925–934. doi: 10.1021/np100074r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Feng Y, Davis RA, Sykes ML, Avery VM, Carroll AR, Camp D, Quinn RJ. Antitrypanosomal pyridoacridine alkaloids from the Australian ascidian Polysyncraton echinatum. Tetrahedron Lett. 2010;51:2477–2479. The structure elucidation of a new highly unsaturated alkaloid, 12-deox-yascididemin, is described. Although pyridoacridones have been previously known to interact nonspecifically as DNA intercalators, the new compound displays 100-fold selectivity for T. brucei versus mammalian cells. [Google Scholar]
- 62.Fattorusso E, Parapini S, Campagnuolo C, Basilico N, Taglialatela-Scafati O, Taramelli D. Activity against Plasmodium falciparum of cycloperoxide compounds obtained from the sponge Plakortis simplex. J Antimicrob Chemother. 2002;50:883–888. doi: 10.1093/jac/dkg008. [DOI] [PubMed] [Google Scholar]
- 63.Ang KKH, Holmes MJ, Higa T, Hamann MT, Kara UAK. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob Agents Chemother. 2000;44:1645–1649. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazaro JEH, Nitcheu J, Mahmoudi N, Ibana JA, Mangalindan GC, Black GP, Howard-Jones AG, Moore CG, Thomas DA, Mazier D, et al. Antimalarial activity of crambescidin 800 and synthetic analogues against liver and blood stage of Plasmodium sp. J Antibiot. 2006;59:583–590. doi: 10.1038/ja.2006.78. [DOI] [PubMed] [Google Scholar]
- 65.Copp BR, Kayser O, Brun R, Kiderlen AF. Antiparasitic activity of marine pyridoacridone alkaloids related to the ascididemins. Planta Med. 2003;69:527–531. doi: 10.1055/s-2003-40640. [DOI] [PubMed] [Google Scholar]
- 66.Miyaoka H, Shimomura M, Kimura H, Yamada Y, Kim H-S, Wataya Y. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge, Acanthellasp. Tetrahedron. 1998;54:13467–13474. [Google Scholar]
- 67.Wright AD, Wang H, Gurrath M, Koenig GM, Kocak G, Neumann G, Loria P, Foley M, Tilley L. Inhibition of heme detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J Med Chem. 2001;44 :873–885. doi: 10.1021/jm0010724. [DOI] [PubMed] [Google Scholar]
- 68.Abdulla M-H, Ruelas DS, Wolff B, Snedecor J, Lim K-C, Xu F, Renslo AR, Williams J, McKerrow JH, Caffrey CR. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis. 2009;3:e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Peak E, Chalmers IW, Hoffmann KF. Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability. PLoS Negl Trop Dis. 2010;4:e759. doi: 10.1371/journal.pntd.0000759. The quantitative screen discussed here can be formatted for industrial (384-well plate) or academic (96-well plate) throughputs, and can be adapted for use with other parasitic worm species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Han Z-G, Brindley PJ, Wang S-Y, Chen Z. Schistosoma genomics: new perspectives on schistosome biology and host–parasite interaction. Ann Rev Genomics Hum Genet. 2009;10:211–240. doi: 10.1146/annurev-genom-082908-150036. The genomic analysis of Schisotosoma sp. using transcriptomic and proteomic high-throughput techniques is reviewed. Of great interest is the discussion of identifying drug targets for Schistosomiasis control. [DOI] [PubMed] [Google Scholar]
- 72.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran A-N, Ghedin E, Worthey EA, Delcher AL, Blandin G, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 73.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004;2:e69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, Peters N, Adlem E, Tivey A, Aslett M, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]