Abstract
Bisphenol-A (BPA) is a component of polycarbonate resins, and, lately, concern has been raised about its potential negative effects on human health. BPA is an estrogen analog and, in addition, it can act as a DNA hypomethylator. We examined effects of gestational exposure to BPA on several behaviors in C57BL/6J mice. Because BPA affects maternal care, which, may have long-lasting effects on offspring behavior, we tested mice raised by either biological or fostered dams. Both diet and dam affected behavior in juvenile mice in a social novelty task and the elevated plus maze (EPM). In a social novelty task, the amount of time spent interacting with an adult male was affected by sex and gestational diet, but only in juveniles raised by a foster dam. Control females spent less time sniffing a novel adult than did control males or females exposed to BPA during gestation. In the EPM, juveniles reared by foster dams and exposed to BPA during gestation spent less time in the distal half of the open arm as compared with juveniles gestated on a control diet. Adult offspring raised by their biological dams showed the same response pattern; gestational BPA increased anxiety as compared with control diet. Our results show that prenatal BPA exposure affects social behavior and anxiety in the EPM. Moreover, some facet(s) of the infant-maternal interaction may modify these effects.
Keywords: endocrine disruptive compounds, estrogens, epigenetic, environment, methylation, sex differences, bisphenol A
INTRODUCTION
Bisphenol-A (BPA), an industrial chemical used in the manufacturing of epoxy and polycarbonate resins, has gained attention in recent years because of its potentially detrimental effects on human health (Diamanti-Kandarakis et al., 2009). BPA leaches into foods from plastic wrappings, baby bottles, and coatings inside of food cans (Biles et al., 1999; Brotons et al., 1995; Krishnan et al., 1993). Exposure to BPA is predicted between 1–8 μg/kg body weight (bw)/day in infants (< 11 months of age), and in pregnant women the average exposure is 2.5 μg/kg bw/day (CEHRH, 2008). BPA has been detected in measurable levels in the urine of pregnant women (Fujimaki et al., 2004), in maternal and fetal plasma (Schonfelder et al., 2002), in breast milk, (Sun et al., 2004), and in placental fluid (Ikezuki et al., 2002). For example, in serum of pregnant women, BPA levels range from 0.43–3.1 μg/L with similar values in fetuses (0.64–2.3 μg/L).
There is concern that exposure of embryos and/or infants to BPA may lead to neurological and behavioral disorders (Braun et al., 2009; Kuehn, 2007). In the embryonic mouse brain BPA changes the timing of cell birth in the cortex (Nakamura et al., 2007). In the neonatal rat brain BPA has estrogenic actions and increases dendritic lengths in cerebellar Purkinje cells (Shikimi et al., 2004). In contrast, BPA can block the acts of estrogens on formation of spine synapses in adult primates (Leranth et al., 2008). Gestational exposure to BPA has been shown to influence a variety of behaviors in both rats and mice, often in a sex-specific manner. In male rats, it increases spontaneous locomotor activity (Ishido et al., 2004; Masuo et al., 2004) and rearing-up in the open field (Fujimoto et al., 2006), while in females activity in the open field was unaffected (Xu et al., 2007). BPA exposure also decreases the time spent by male rats in the open arms of the elevated plus maze (Patisaul and Bateman, 2008), and increases play behavior and sociosexual investigation in females (Dessi-Fulgheri et al., 2002). In mice, BPA exposure decreases the time spent by females in the open arms of the elevated plus maze and in the light side of a light/dark chamber (Ryan and Vandenbergh, 2006), in male mice BPA lead to increased time spent in the center of an elevated plus maze (Gioiosa et al., 2007). These data show that basic brain function may be altered by BPA but the direction of change depends on sex, species, and dose.
Here we asked about sex differences and effects of BPA on several behaviors in juvenile male and female C57BL/6J mice, a commonly used inbred strain. We limited BPA exposure to the prenatal period by providing it in the gestating dam’s diet until parturition. We examined self-grooming, activity in the elevated plus maze, and urine marking all of which have been used to indicate anxiety in a novel environment (Lister, 1987; Maruniak et al., 1974). Social preferences were examined because, in adults, this behavior is sexually dimorphic in this strain of mouse (Bodo and Rissman, 2007). Others have used a similar task to assess interest in social interactions in juvenile male mice (Moy et al., 2007). Most of our behavioral tests were done in juveniles, when gonadal hormone levels are uniformly low, thus any deviations in sexually dimorphic behaviors could be caused by BPA actions in utero either via estrogenic (or anti-estrogenic) actions and/or changes in methylation patterns of genes relevant to the observed behaviors. In addition, it has been shown that BPA affects maternal behavior (Palanza et al., 2002). Since maternal care can have long-lasting epigenetic effects on behaviors (Champagne et al., 2001; Francis et al., 1999), we tested animals reared by either their biological or fostered dams. To our knowledge, ours is the first study to examine the influence of the dam as a factor that potentially interacts with gestational exposure to BPA to affect behavior of offspring.
MATERIALS AND METHODS
Breeding and BPA Treatment
One group of female mice (C57BL/6J) was placed on a phytoestrogen free control diet (n=21; Harlan Teklad, TD.95092). Another group received the same diet formulated with added BPA (n=16; 50 mg/kg, Harlan Teklad, TD.06156). BPA was obtained from Sigma Aldrich (purity +99%) and sent to Harlan Teklad for incorporation into the diet. We selected this dose of BPA because it has been shown to hypomethylate the Agouti gene in viable yellow agouti (Avy) mice (Cropley et al., 2006; Dolinoy et al., 2007). One week after starting on the new chow, all females were paired with a male. Seven days later males were removed and females continued to consume their assigned diets (food and water ad libitum). To quantify the amount of BPA consumed during gestation, food from a portion of the dams was weighed daily at the same time each day, from E9 until the end of gestation. We counted the day of parturition as E20 for calculation of gestational days. All females were individually housed on a 12:12 light-dark cycle (lights off at 1300 h). All procedures were in compliance with the University of Virginia Animal Use and Care Committee.
When the pups were born, all BPA-consuming dams were switched to control diet (Harlan Teklad, TD.95092) and pups either remained (BPA: n= 36, control: n=49) with their dam (biological) or were removed and fostered (BPA: n=36, control: n=24) to another dam (foster) that was on the control diet and had also given birth in the past 24 hours. All pups were briefly (no more than 2 minutes) removed from the nest to control for stress related to handling, and biological litters were culled to 3–6 pups (random sexes) to normalize for litter effects. To minimize stress and reduce infanticide, foster dams had mixed litters containing 1–2 of their own pups (not in the study) and test pups (1–4). All the fostered pups were littermates. For identification purposes, either the biological or foster pups had their tails clipped (approximately 0.5 cm) at the time of fostering. All pups (n=145) remained with their dams until PN21, at which time they were group housed by litter and sex and continued on the control diet.
Behavioral Testing
All habituation and behavioral tests were conducted in the dark (between 1300–1800 hours) under red light. Behaviors were recorded and later scored by an observer blind to sex and treatment group. PN20 animals (the day before weaning) were singly housed in a novel cage for 10 minutes. During this time, they were filmed and time spent self-grooming was scored. After the test, mice were returned to their home cages with their siblings and dam. Two separate cohorts were tested in the same battery of juvenile behavior tests, with an additional EPM test for biologically reared adults (n=41 from 6 BPA and 9 Control litters) in cohort two. Due to a computer malfunction resulting in the loss of some of our videos, some data were not scored.
Two days later, the same mice were moved, in their home cages, to a testing room one hour prior to testing to habituate. Each mouse was placed into the middle of the elevated plus maze and tested as previously described (Imwalle et al., 2005). Behavior was filmed for 10 minutes. The total time spent in the closed and open arms and the numbers of crosses through the middle were scored. Time spent in the middle of the maze was calculated based on the total duration of the test less the time in the two arms. The open arm was subdivided into proximal and distal halves and time in each was recorded.
Starting on PN26, mice were habituated one hour prior to testing. Each mouse was placed individually into a round chamber (35.56 cm diam. × 35.56 cm) with a piece of filter paper on the bottom. Mice were allowed to roam freely for 10 minutes, and the number of urine spots on the paper was counted using a black light.
To measure social novelty behavior, (between PN27-30), mice were habituated to a testing room for one hour. Next, they were placed into the center section of a three-chambered Plexiglas box (76.2 cm × 26.67 cm × 17.78 cm, divided by black Plexiglas walls and backed by black Plexiglas so that the center section was darkened on 3 sides) with 2 doors leading to the outer chambers each containing a small metal cylinder with a round top (10.16 cm diam. × 13.97 cm) and vertical bars (spaced 1 cm apart), hereafter referred to as a “jail cell”. Mice were habituated to the test box in the center section with both doors closed for 10 minutes. After 10 minutes the doors were opened and the mouse was allowed to freely explore all three chambers and the empty jail cells for 10 minutes. Mice were once again closed into the center section and a novel adult male mouse was randomly placed in one of the jail cells. The doors were opened to allow mice to explore all three chambers and data were collected for 10 minutes. The time spent in each chamber, the time spent sniffing each jail cell, and the numbers of entries into each side were all scored (adapted from (Moy et al., 2004; Moy et al., 2009).
Gonadal Weights and Plasma Hormone Levels
Between PN70 and PN77 mice from the second cohort were deeply anesthetized with an intraperitoneal injection of euthasol (19.5mg pentobarbital/0.05ml) and blood was collected by cardiac puncture. Animals were then intracardially perfused with 0.5% paraformaldehyde for 25 minutes. After fixation, gonads were removed and weighed. Plasma was collected, frozen and later assayed, in duplicate, for testosterone and corticosterone concentrations using radioimmunoassays (Siemen’s Medical Solutions Diagnostics) performed by the University of Virginia Ligand Assay Core (grant # U54-HD28934). These assays have lower level sensitivities of 0.1 ng/ml and 20 ng/ml, and had intra-assay coefficients of variations (mean +/− SEM) of 4.3 +/− 1.61% and 2.3 +/− 2.1%, respectively.
Statistical Analyses
All data were analyzed using NCSS Software (2000). For food intake, a two-way repeated measures analysis of variance (ANOVA) was used with diet and gestational day as the factors. For behavioral data, gonad weights, and hormone levels, we used three-way ANOVAs to assess the contribution of sex, diet, and rearing dam, two-way and one-way analyses were also performed. To compare juvenile and adult EPM behavior we conducted a three-way ANOVA with age, sex and diet as the factors. Paired comparisons were conducted using Fisher’s LSD multiple comparison tests.
RESULTS
Food Intake of Dams
There was no effect of BPA on overall food intake of pregnant dams (p=0.45, Figure 1A). Food intake averaged about 4 g of chow, or 0.2 mg of BPA, per day. Thus, dams (n=5) on the BPA diet had an average daily BPA intake of 8 mg/kg BW/day. The dams consumed an amount of BPA less than or equal to the established no observable adverse effect level (NOAEL) for mice. Estimated BPA intake increased over gestational days, peaking on E18, which was significantly higher than E9, E10, E11, E12, E13, E14, and E19 (Figure 1B, p<0.004).
Figure 1.
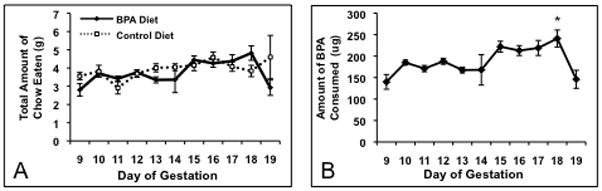
A) Mean food intake (± SEM) on each day of gestation starting at day 9 of pregnancy (E9). No significant differences in overall food intake between dams on the BPA (n=5) or control diets (n=8; p=0.45) were detected. B) Mean BPA intake (± SEM) for dams on the BPA diet. We noted an increase in BPA intake after E11 with a peak on E18. * BPA intake on E18 was significantly higher than E9 through E14 and E19 (p<0.004).
Grooming and Urine Marking
There were no effects of sex, diet, or dam on the amount of grooming shown by any of the juveniles in an empty cage (Table 1). Juveniles from BPA dams showed no difference from controls in the number of urine markings made in an open arena (Table 1) regardless of sex or dam.
Table 1.
Mean +/− SEM time spent grooming (seconds) and number of urine marks in juveniles exposed to BPA or control diet in utero. Juveniles were reared by either their biological or foster dams. No significant effects of sex, diet, or dam were detected for these behaviors.
| Time Grooming (Sec) | Number of Urine Marks | ||
|---|---|---|---|
| Biological | BPA F (14) | 210 +/− 36.8 | 3 +/− 0.56 |
| Con F (23) | 192 +/− 23.9 | 2 +/− 0.28 | |
| BPA M (15) | 215 +/− 28.5 | 3 +/− 0.38 | |
| Con M (17) | 187 +/− 16.4 | 3 +/− 0.56 | |
| Fostered | BPA F (13) | 202 +/− 36.9 | 3 +/− 1.01 |
| Con F (12) | 165 +/− 36.9 | 3 +/− 0.73 | |
| BPA M (14) | 155 +/− 23.6 | 5 +/− 0.87 | |
| Con M (12) | 242 +/− 25.7 | 3 +/− 0.54 | |
Elevated Plus Maze
Three-way ANOVAs (dam x diet x sex) did not reveal any three-way interactions, but significant two-way interactions as well as main effects of dam and diet were noted in the elevated plus maze. Time spent in the closed arm of the EPM was affected by prenatal diet (F(1,62)=14.81; p<0.0003) and dam (F(1,62)=10.59; p<0.002), and we found an interaction between these two factors (F(1,62)= 21.14; p<0.0001). The analysis of the interaction revealed that control juveniles reared by foster dams spent less time in the closed arm than mice in any other group (Figure 2A; p<0.05). An effect of diet (F(1,63)=13.59; p<0.0005), dam (F(1,63)=10.12; p<0.0025), and an interaction (F(1,63)=23.27; p<0.0001) were found on time in the middle of the EPM (Figure 2B). Control juveniles raised by foster dams spent more time in the middle of the EPM than juveniles in any other group (p<0.05). There was also an interaction of sex and diet on the time spent in the distal portion of the open arm (Figure 2C; F(1,63)=3.86; p<0.05), with BPA exposed males spending less time in the open than control males. There was no effect of diet, dam, or sex on number of times the mice crossed through the middle of the EPM (Table 2).
Figure 2.
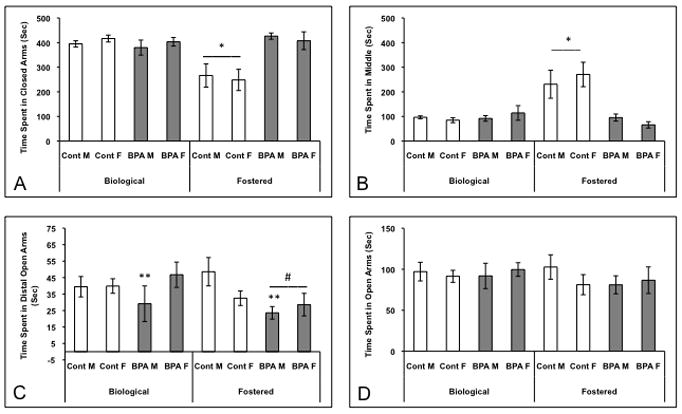
Mean +/− SEM time spent (seconds) by juveniles in different sections of the elevated plus maze. Juveniles were exposed to BPA or control diet in utero and reared by biological or foster dams (Control Biological: 7 males and 9 females, BPA Biological: 7 males and 9 Females, Control Fostered: 7 males and 6 females, BPA Fostered: 11 males and 7 females). A) * Control juveniles reared by foster dams spent less time in the closed arm than mice in any other group (p<0.05). B) * Control juveniles raised by foster dams spent more time in the middle of the EPM than mice in any other group (p<0.002). C) ** Regardless of rearing dam, BPA exposed males spent less time in the distal open arms than control males (p<0.05). # BPA exposed mice, reared by foster dams, spent less time in the distal portion of the open arms than mice given control diet and raised by foster dams (p<0.04). D) Time spent in the entire length of the open arms did not differ among the groups.
Table 2.
Mean +/− SEM number of crosses between chambers in the testing apparatus for juveniles exposed to BPA or control diet in utero. Juveniles were reared by either their biological or foster dams. No significant effects of sex, diet, or dam were detected for overall activity levels in these tasks.
| # Crosses in the EPM | # Crosses in the Social Novelty Task | ||
|---|---|---|---|
| Biological | BPA F (9/14) | 24 +/− 1.9 | 22 +/− 1.5 |
| Con F (9/23) | 23 +/− 1.6 | 21 +/− 1.4 | |
| BPA M (7/15) | 22 +/− 2.9 | 17 +/− 1.7 | |
| Con M (11/17) | 22 +/− 1.5 | 16 +/− 4.5 | |
| Fostered | BPA F (7/13) | 20 +/− 2.7 | 19 +/− 2.1 |
| Con F (6/12) | 21 +/− 3.8 | 23 +/− 2.7 | |
| BPA M (11/14) | 21 +/− 1.7 | 18 +/− 1.9 | |
| Con M (7/12) | 21 +/− 1.7 | 18 +/− 2.9 | |
To examine these effects further, we conducted separate two-way ANOVAs, one for each rearing condition. When we limited the analysis to mice raised by biological dams only, no significant effects of diet or sex were noted. In fostered animals, regardless of sex, prenatal BPA exposure decreased the amount of time spent in the distal portion of the open arm (Figure 2C; F(1,28)=4.59; p<0.04). No differences were noted when we scored the amount of time in the entire open arm (Figure 2D). Diet affected time in the middle of the EPM in a similar manner to the distal open arm (F(1,27)=18.57; p<0.002). BPA exposed juveniles spent less time in the middle of the maze than control juveniles (Figure 2B). The reverse pattern was noted in the closed arm, where diet was also a factor (F(1,28)=19.18; p<0.0002): BPA exposed juveniles spent more time in the closed arms than controls (Figure 2A).
Social Novelty
Behavior of the juveniles exposed to BPA prenatally differed depending on whether they were reared by their biological or by foster dams. Again, there were no three-way interactions. However, several two-way interactions were significant, and a significant effect of sex was observed. We noted a significant diet by dam interaction (Figure 3A; F(1,76)= 4.93; p< 0.03) for time spent in the empty test chambers. BPA treated juveniles reared by their biological dams spent less time in the empty section of the test box as compared with control juveniles reared by their dams and BPA exposed juveniles reared by foster dams (p<0.05). There was also a significant interaction between dam and diet (F(1,75)=3.76; p<0.025) for time spent in the center section. In this case, BPA treated juveniles reared by biological dams spent more time in the center than BPA juveniles reared by foster dams (p<0.05; data not shown). A sex difference was noted for time spent sniffing the male stimulus animal (Figure 3D; F(1,77)=5.63; p<0.02). Regardless of treatment or rearing dam, juvenile males spent more time sniffing the adult male than juvenile females (p<0.05). No other significant effects were noted and, importantly, no differences in the number of crosses in and out of the three sections were observed (Table 2).
Figure 3.
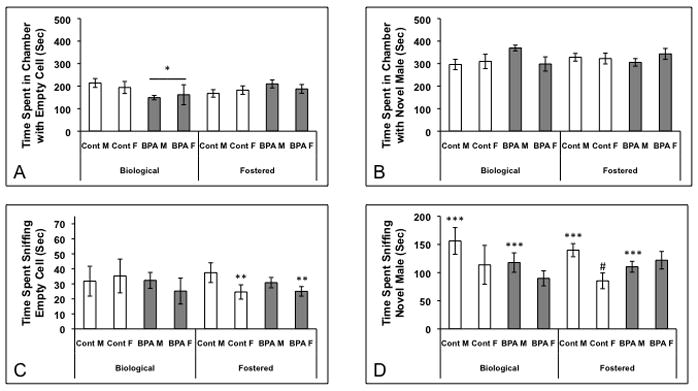
Mean +/− SEM time spent (seconds) in each section of the three-chambered box and sniffing either an empty jail cell or one containing an adult male during a social novelty test. Juveniles were exposed to BPA or control diet in utero and reared by biological or foster dams (Control Biological: 5 males and 4 females, BPA Biological: 13 males and 5 females, Control Fostered: 12 males and 11 females, BPA Fostered: 12 males and 11 females). A) * BPA treated juveniles reared by their biological dams spent less time in the chamber with the empty cell than control diet mice similarly reared by biological dams (p<0.05). B) There were no differences among the groups in time spent with the novel male. C) ** Females reared by foster dams spent less time sniffing the empty jail cell than fostered males (p<0.05). D) *** Juvenile males in all treatment groups spent more time sniffing the adult male than juvenile females (p<0.05). # Females exposed to control diet and raised by a foster dam displayed less sniffing directed toward the novel male than either females exposed to BPA or males given the control diet (p<0.03).
For animals reared by biological dams, in utero BPA exposure affected time spent on the side of the chamber with the empty jail cell (F(1,25)=4.20; p<0.05). BPA exposed mice spent less time than controls on the empty side (Figure 3A; p<0.05). There were no other effects of gestational diet or sex in the biological reared juveniles.
Juveniles reared by foster dams, had a diet by sex interaction (F(1,50)=4.97; p<0.03) for time spent sniffing the novel male. Females gestated on control diet spent less time investigating an adult male than females exposed to BPA or control males (Figure 3D). In addition, a sex difference was found for time sniffing the empty jail cell (Figure 3C; F(1,46)=4.21; p<0.05); females investigated less than males. We also found a main effect of diet on time in the center section (F(1,48)=4.26; p<0.045). Juveniles exposed to the control diet spent more time in the center than BPA exposed mice (p<0.05; data not shown).
Elevated Plus Maze tested in Adults
Because previous studies in adult mice reported that neonatal BPA treatments increased anxiety in the EPM, we examined behavior in a cohort of adults. We restricted this study to mice reared by their biological dams to match the conditions used previously (Gioiosa et al., 2007). We found an effect of diet on the time spent in the most distal portion of the open arm (Figure 4A; F(1,39)=4.21; p<0.05). Similar to previous reports, mice gestated on a BPA diet spent less time in this section of the open arm than control adults. A sex difference in crossing was noted (Figure 4B; F(1,39)=7.58; p<0.01); males were more active in the EPM than females. No other effects of diet were found.
Figure 4.
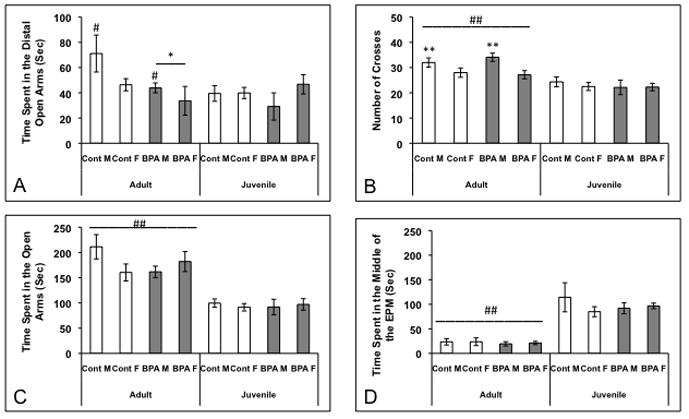
Mean +/− SEM time spent (seconds) in different parts of the elevated plus maze and number of crosses. Mice were exposed to BPA or control diet and reared by biological dams, then tested both as juveniles and in adulthood (Control Adults: 7 males and 16 females, BPA adults: 12 males and 6 females, Control Juveniles: 11 males and 9 females, BPA juveniles: 7 males and 9 females). A) * Adult mice exposed to BPA in utero spent significantly less time than adult controls in the distal portion of the open arms (p<0.05). # Adult males spent more time in the distal open arms than juvenile males (p<0.04). B) ## Adults, particularly males (** p<0.02), crossed through the middle of the EPM more frequently than juveniles (p<0.0001). C) ## Adults spent more time in the open arms than juveniles (p<0.0001). D) ## Adults spent less time in the middle of the EPM than juveniles (p<0.0001).
Since the juvenile and adult data were from independent groups, we used a three-way ANOVA to examine effects of diet, age, and sex on behavior in the EPM. We did not find any three-way interactions. However, significant main effects of age on number of crosses (Figure 4B; F(1,66)= 29.13; p<0.00001), time spent in the open arms (Figure 4C; F(1,66)=52.49; p<0.00001), and time spent in the middle (Figure 4D; F(1,66)=36; p<0.00001). Adults were more active than juveniles regardless of sex or diet. Adults also spent more time in the open arms, and less time in the middle than juveniles. We also found significant interactions of sex and age for time spent in the distal portion of the open arm (Figure 4A; F(1,66)=4.56; p<0.04) and number of crosses (Figure 4B; F(1,66)=5.76; p<0.02). Adult males spent more time in the distal open arm than juvenile males, while there were no differences between adult and juvenile females. Adult males were also more active, in terms of numbers of crosses into the middle, than all other groups.
Adult Gonad Weights and Plasma Hormone Levels
There were no effects of BPA or dam on gonad weights or corticosterone levels among either males or females. There was a trend for BPA exposed males to have higher testosterone levels than controls (Table 3; F(1,28)=3.75; p=0.06).
Table 3.
Mean +/− SEM gonadal weights and hormone levels in adult mice exposed to BPA or control diet in utero and reared by either their biological or foster dams.
| Uterine Weight (mg) | Ovarian Weight (mg) | Seminal Vesicle Weight (mg) | Testes Weight (mg) | Plasma Testosterone (ng/mL) | Plasma Corticosterone (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Biological | BPA F (5) | 78 +/− 20 | 12 +/− 1 | -- | -- | 0.10 +/− 0.02 | 227.4 +/− 37.70 |
| Con F (18) | 77 +/− 9 | 13 +/− 1 | -- | -- | 0.01 +/− 0.02 | 219.177 +/− 15.24 | |
| BPA M (12) | -- | -- | 150 +/− 6 | 166 +/− 4 | 1.87 +/− 0.80* | 222.229 +/− 19.66 | |
| Con M (9) | -- | -- | 160 +/− 9 | 157 +/− 14 | 0.15 +/− 0.03 | 181.596 +/− 29.09 | |
| Fostered | BPA F (10) | 85 +/− 12 | 14 +/− 1 | -- | -- | 0.11 +/− 0.03 | 229.35 +/− 36.82 |
| Con F (6) | 85 +/− 17 | 12 +/− 1 | -- | -- | 0.13 +/− 0.03 | 229.954 +/− 39.30 | |
| BPA M (6) | -- | -- | 161 +/− 4 | 167 +/− 6 | 0.16 +/− 0.05* | 196.618 +/− 37.43 | |
| Con M (5) | -- | -- | 150 +/− 9 | 174 +/− 24 | 0.09 +/− 0.01 | 256.091 +/− 49.72 | |
BPA exposed males showed a trend for higher testosterone levels than controls (p=0.06).
DISCUSSION
We characterized several behaviors in juveniles in response to gestational exposure to BPA and asked whether maternal rearing environment influenced the effects of BPA. Our results suggest that not only does BPA exposure in utero affect behavior, but also that these effects depend upon rearing conditions. Two of the four juvenile behaviors we observed were affected by gestational diet: responses to conspecifics and anxiety (in the EPM). Primarily juvenile mice raised by foster dams were susceptible to actions of BPA in utero. Mice reared by their biological dams were largely resilient.
BPA Exposure Counteracts Sex Differences in Juvenile Social Behavior
In fostered animals, diet and sex interacted to affect juvenile social behaviors. Because foster dams were never exposed to BPA-containing chow during gestation, these data may be the best reflection of actions of prenatal diet on the juveniles themselves, but as discussed later, it is possible that fostering introduces another, previously unaddressed, variable. Control females spent less time sniffing a novel adult than control males and BPA exposed females, while males gestated with BPA were intermediate. BPA exposed males were not statistically different from BPA exposed females; therefore sex differences were only present in control mice. This result agrees with other findings in the literature, in both rats and mice, which have shown that neonatal BPA exposure tends to reduce sex differences for a variety of behaviors including novelty, adult EPM, and open field (Farabollini et al., 1999; Gioiosa et al., 2007; Rubin et al., 2006).
BPA Exposure Increases Juvenile Anxiety
Regardless of type of rearing dam, BPA exposure decreased the amount of time males spent in the distal portion of the open arm of the elevated plus maze, while females were less affected. The difference in time spent in the most distal portion of the open arm, which is the most exposed part of the maze, suggests a sex-specific increase in anxiety induced by BPA exposure. Interestingly here, in contrast to the social behavior test, males are the affected sex. Because anxiety is reportedly elevated in adults exposed to BPA shortly after birth, we also tested adults in the EPM. We found that BPA exposed mice raised by their biological dams, and tested as adults, spent less time in the distal open arm of the EPM than controls, regardless of sex. These results are in agreement with studies in rats that found that BPA exposure increased anxiety in adulthood (Farabollini et al., 1999; Fujimoto et al., 2006). The mechanisms by which BPA acts on anxiety are not clear. One candidate is the dopamine system (Laviola et al., 2005) as BPA has been shown to decrease numbers of tyrosine hydroxylase neurons (Tanida et al., 2009) and alter D1 receptor expression in the midbrain of male mice (Suzuki et al., 2003). Another candidate is estrogen receptor (ER) beta which is involved in anxiety in mice; ER-beta knockout mice are less anxious than wildtype controls (Imwalle et al., 2005). In a dose-response study, the three highest of four doses of BPA given to ICR mouse dams resulted in decreased ER-beta protein in PN21 male offspring hippocampi (Xu et al, 2010). In addition, BPA treatment during gestation and lactation eliminates a sex difference in corticosterone releasing hormone containing neurons in the bed nucleus of the stria terminalis of rats (Funabashi et al., 2004).
In contrast to juveniles, there was no sex difference in adults tested in the EPM. These results differ from those of Gioiosa and colleagues (2007). Several procedural differences may account for this including; a different mouse strain, tests on a single day of the estrus cycle, a different dose of BPA (10 μg/kg/day given orally in corn oil), which was administered to the dams in a bolus, and was given during a different neonatal interval. It is also not clear why a sex difference in EPM behavior exists in juveniles, but not in adults, in response to BPA. Perhaps the sex differences in adult hormone levels mask the sex differences in the EPM. Alternatively, juveniles and adults act differently in the EPM; juveniles (reared by their biological dams) spent significantly less time in the open arms and middle of the EPM and were less active in the maze than adults. Differences in activity level in the EPM between juveniles and adults have been found before (Hefner and Holmes, 2007), in studies using CD-1 (Macri et al., 2002) and C57BL/6 mice (Hefner and Holmes, 2007).
The two other measures of anxiety we observed, grooming and urine marking, were unaffected by either BPA treatment or rearing dam. This is not novel since others have reported similar disparities in behavioral results using different types of “anxiety” tests (Henderson et al., 2004). For example BPA treated male rats were no different than controls in the open-field and the EPM, but were more anxious in a passive avoidance task (Negishi et al., 2004). Along with our results, this suggests that in order to model anxiety in mice it may be necessary to have them perform more than one behavioral task.
Rearing Dam Modifies Effects of BPA Exposure on Anxiety
Perhaps our most interesting finding was that type of dam interacted with BPA exposure to have significant effects on juvenile social responses and anxiety in the EPM. There are several possible scenarios by which this interaction might occur. The most straightforward is that, although we switched biological dams to control phytoestrogen free diet at parturition, it is possible that they had significant levels of BPA remaining in their blood. BPA is rapidly cleared in rats (Yoo et al., 2000), but in humans exposed to BPA in food the half-life was as long as 43 hours (Stahlhut et al., 2009). Therefore, pups reared by biological dams could have been exposed to BPA for 1–2 days longer than control pups. This hypothesis would predict that the critical period for these effects of BPA is just after birth.
Another possible explanation is that biological dams display differences in their maternal behaviors produced by BPA exposure. Females exposed to BPA during the final few days of gestation and tested as adults with their own litters spent more time alone, and less time doing nest related activities, as compared with control females (Palanza et al., 2002). Interestingly, in cynomologus monkeys, which have only one infant at a time, the behavior of the moms and the infant males are both affected by BPA consumption (Nakagami et al., 2009). Control male infants spent more time clinging to their mothers and less time looking outward than BPA males or females in either group. Mothers behaved in a similar fashion. In the future we plan to directly assess behavior of foster dams to ask if interactions with pups are affected by BPA.
In addition, foster dams may treat BPA exposed pups differently than their own or control fostered pups. Our data in fact suggest that this is the case since control diet fostered juveniles spend less time in the closed arm and more time in the middle of the EPM than BPA-treated foster raised juveniles. While some studies indicate that intra-strain cross-fostering does not impact mouse maternal behavior (van der Veen et al., 2008), recent findings show that C57BL/6 dams lick and groom fostered pups more than with their own pups (Curley et al., 2009). These results suggest that, regardless of BPA exposure, the fostered pups could have been exposed to different levels of maternal behavior. In fact, control foster-reared and biological dam-reared mice did display behavioral differences on two measures; time spent in the closed arms and time spent in middle of the EPM. In rats, unstressed foster dams that were given prenatally stressed pups spent more time in the nest and more time licking the pups than dams given unstressed pups (Del Cerro et al., 2010). Further studies are needed to explore whether the BPA exposure and/or fostering alone can change maternal behavior.
Conclusion
In conclusion, our data demonstrate that gestational BPA exposure can influence both juvenile and adult behaviors. Behavioral responses to BPA are also dependent upon maternal/pup interactions. It is therefore important when designing future studies on BPA exposure to consider both sex differences in response to BPA and effects of maternal environment.
Acknowledgments
The authors thank Aileen Wills and Lucia Tejada for assistance with fostering and testing animals and Michelle Edwards for scoring adult behaviors. This work was supported by NIH grant RO1 MH086711 and Autism Speaks grant # 4802; KHC was supported by NIH T32 HD007323. Hormone assays were performed by the University of Virginia Ligand Assay Core (U54-HD28934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WORKS CITED
- Biles JE, White KD, McNeal TP, Begley TH. Determination of the diglycidyl ether of bisphenol A and its derivatives in canned foods. J Agric Food Chem. 1999;47:1965–9. doi: 10.1021/jf9810867. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–90. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–52. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–12. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEHRH. Draft NTP Brief on Bisphenol-A. 2008. [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley JE, Suter CM, Beckman KB, Martin DI. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A. 2006;103:17308–12. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental Shifts in the Behavioral Phenotypes of Inbred Mice: The Role of Postnatal and Juvenile Social Experiences. Behav Genet. 2009 doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cerro MC, Perez-Laso C, Ortega E, Martin JL, Gomez F, Perez-Izquierdo MA, Segovia S. Maternal care counteracts behavioral effects of prenatal environmental stress in female rats. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–7. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Dessi-Fulgherit F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol Biochem Behav. 1999;64:687–94. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Fujimaki K, Arakawa C, Yoshinaga J, Watanabe C, Serizawa S, Imai H, Shiraishi H, Mizumoto Y. [Estimation of intake level of bisphenol A in Japanese pregnant women based on measurement of urinary excretion level of the metabolite] Nippon Eiseigaku Zasshi. 2004;59:403–8. doi: 10.1265/jjh.59.403. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29:475–85. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–16. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–5. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–93. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76:423–33. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–86. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kuehn B. Expert panels weigh bisphenol-A risks. JAMA. 2007;298:1499–503. doi: 10.1001/jama.298.13.1499. [DOI] [PubMed] [Google Scholar]
- Laviola G, Gioiosa L, Adriani W, Palanza P. D-amphetamine-related reinforcing effects are reduced in mice exposed prenatally to estrogenic endocrine disruptors. Brain Res Bull. 2005;65:235–40. doi: 10.1016/j.brainresbull.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:14187–91. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Animal Behaviour. 2002;64:541–6. [Google Scholar]
- Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav. 1974;12:1035–9. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ishido M, Morita M, Oka S. Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast. 2004;11:59–76. doi: 10.1155/NP.2004.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–42. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami A, Negishi T, Kawasaki K, Imai N, Nishida Y, Ihara T, Kuroda Y, Yoshikawa Y, Koyama T. Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology. 2009;34:1189–97. doi: 10.1016/j.psyneuen.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420:100–5. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112:1159–64. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(Suppl 3):415–22. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–8. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikimi H, Sakamoto H, Mezaki Y, Ukena K, Tsutsui K. Dendritic growth in response to environmental estrogens in the developing Purkinje cell in rats. Neurosci Lett. 2004;364:114–8. doi: 10.1016/j.neulet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117:784–9. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Irie M, Kishikawa N, Wada M, Kuroda N, Nakashima K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed Chromatogr. 2004;18:501–7. doi: 10.1002/bmc.345. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mizuo K, Nakazawa H, Funae Y, Fushiki S, Fukushima S, Shirai T, Narita M. Prenatal and neonatal exposure to bisphenol-A enhances the central dopamine D1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience. 2003;117:639–44. doi: 10.1016/s0306-4522(02)00935-1. [DOI] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi WM, Inamoto T, Yokoyama T, Kitagawa H, Hoshi N. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett. 2009;189:40–7. doi: 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Abrous DN, de Kloet ER, Piazza PV, Koehl M. Impact of intra- and interstrain cross-fostering on mouse maternal care. Genes Brain Behav. 2008;7:184–92. doi: 10.1111/j.1601-183X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu Y, Sadamatsu M, Tsutsumi S, Akaike M, Ushijima H, Kato N. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci Res. 2007;58:149–55. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010;58:326–33. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Shin BS, Kwack SJ, Lee BM, Park KL, Han SY, Kim HS. Pharmacokinetic disposition and tissue distribution of bisphenol A in rats after intravenous administration. J Toxicol Environ Health A. 2000;61:131–9. doi: 10.1080/00984100050120415. [DOI] [PubMed] [Google Scholar]


