Abstract
The photodissociation of CO from HbCO at ambient temperature is studied by means of a femtosecond IR technique. The bleaching of the FeCO absorption and the appearance of a new IR absorption near that of free CO are both observed at 300 fs after optical excitation. The bleach does not recover on the time scale of a few picoseconds but does recover by approximately 4% within 1 ns, which suggests that a barrier to recombination is formed within a few picoseconds. The CO spectrum does not change significantly between 300 fs and 1 ns, suggesting that the CO quickly finds some locations in the heme pocket that are not more than a few angstroms from the iron. The de-ligated CO appears in its ground vibrational level. There is evidence that 85 +/- 10% of this CO remains in the heme pocket at 1 ns; it probably resides there for 50 ns. The flow of excess vibrational energy from the heme to the solvent was directly observed in the IR experiments. The heme cools within 1-2 ps while thermal disruption of the surrounding solvent structure requires approximately 30 ps.
Full text
PDF
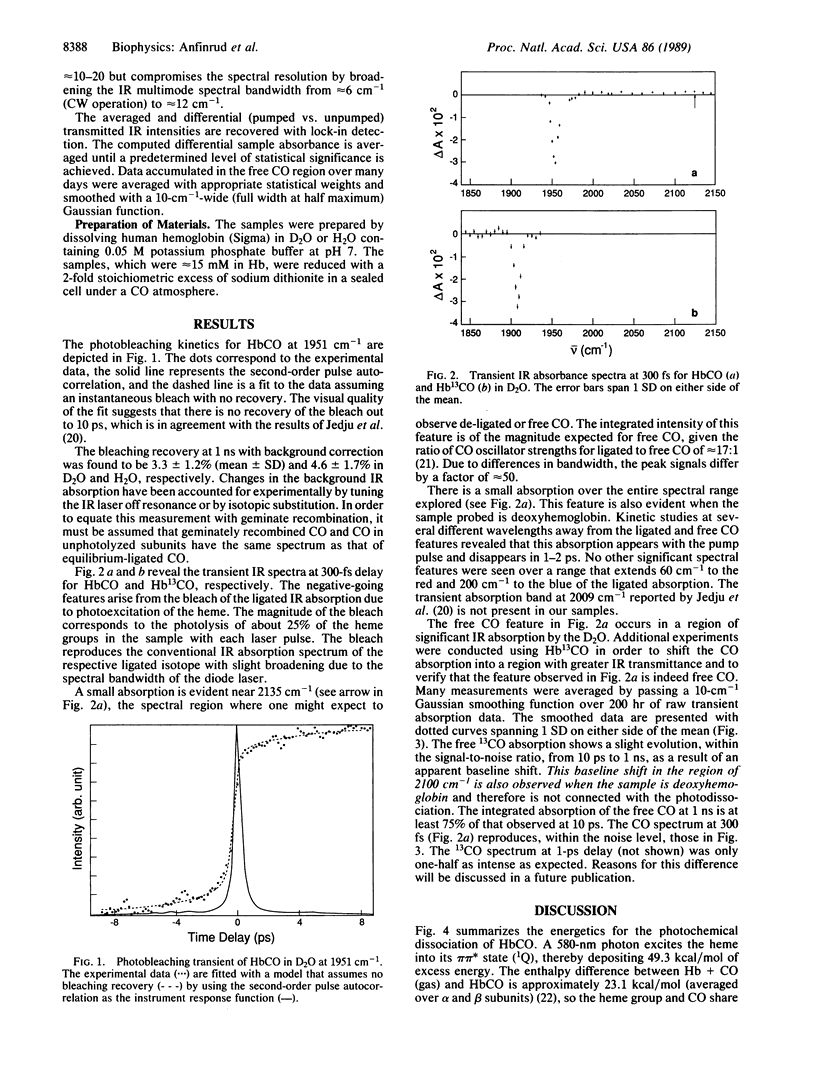
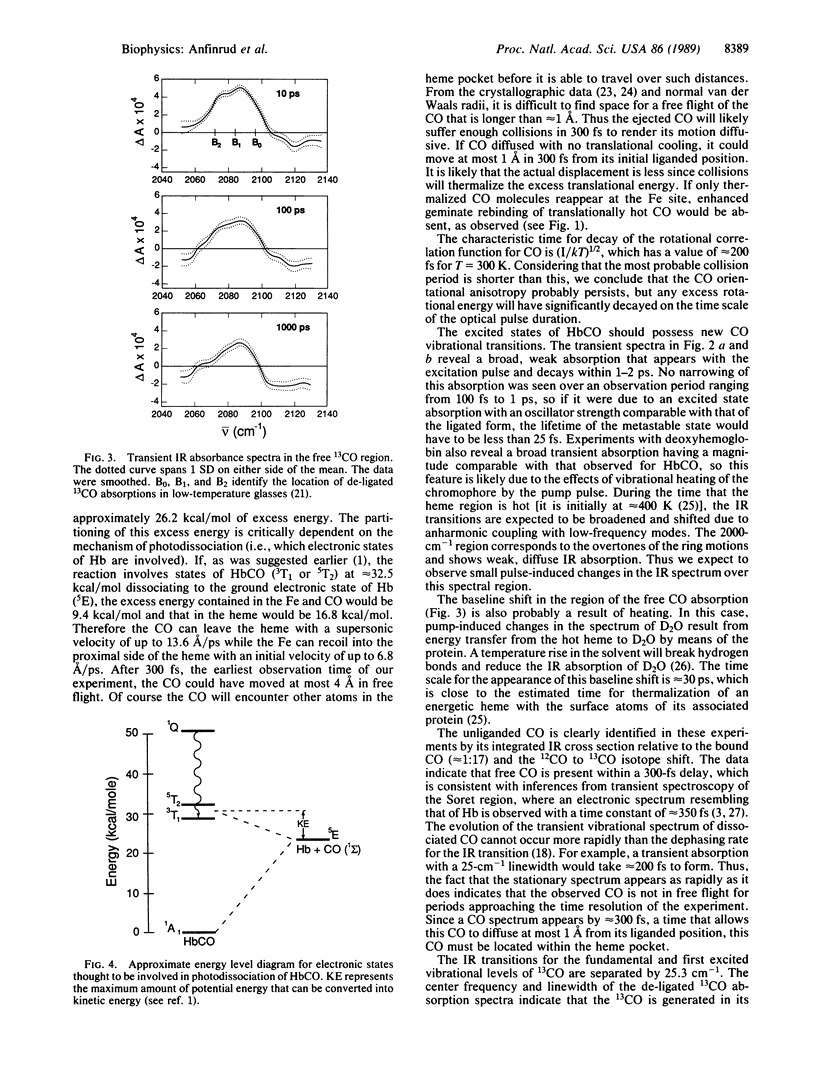


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., DiIorio E. E., Dlott D. D., Frauenfelder H., Iben I. E., Langer P., Roder H., Sauke T. B., Shyamsunder E. Ligand binding to heme proteins: relevance of low-temperature data. Biochemistry. 1986 Jun 3;25(11):3139–3146. doi: 10.1021/bi00359a011. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979 Aug 15;132(3):343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- Chance B., Ravilly A., Rumen N. Reaction kinetics of a crystalline hemoprotein: an effect of crystal structure on reactivity of ferrimyoglobin. J Mol Biol. 1966 Jun;17(2):525–534. doi: 10.1016/s0022-2836(66)80162-6. [DOI] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius P. A., Hochstrasser R. M., Steele A. W. Ultrafast relaxation in picosecond photolysis of nitrosylhemoglobin. J Mol Biol. 1983 Jan 5;163(1):119–128. doi: 10.1016/0022-2836(83)90032-3. [DOI] [PubMed] [Google Scholar]
- Fiamingo F. G., Alben J. O. Structures of photolyzed carboxymyoglobin. Biochemistry. 1985 Dec 31;24(27):7964–7970. doi: 10.1021/bi00348a019. [DOI] [PubMed] [Google Scholar]
- Findsen E. W., Friedman J. M., Ondrias M. R., Simon S. R. Picosecond time-resolved resonance Raman studies of hemoglobin: implications for reactivity. Science. 1985 Aug 16;229(4714):661–665. doi: 10.1126/science.4023704. [DOI] [PubMed] [Google Scholar]
- Greene B. I., Hochstrasser R. M., Weisman R. B., Eaton W. A. Spectroscopic studies of oxy- and carbonmonoxyhemoglobin after pulsed optical excitation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Eaton W. A., Hochstrasser R. M. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8982–8986. doi: 10.1073/pnas.83.23.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Levitt M., Eaton W. A. Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2034–2038. doi: 10.1073/pnas.82.7.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F. C., Ackers G. K., Gaud H. T., Gill S. J. Thermodynamic studies on ligand binding and subunit association of human hemoglobins. Enthalpies of binding O2 and CO to subunit chains of hemoglobin A. J Biol Chem. 1979 Apr 25;254(8):2875–2880. [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Eaton W. A. Time-resolved optical spectroscopy and structural dynamics following photodissociation of carbonmonoxyhemoglobin. Biophys Chem. 1988 Feb;29(1-2):63–76. doi: 10.1016/0301-4622(88)87025-x. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Mathews F. S. An x-ray study of azide methaemoglobin. J Mol Biol. 1966 Oct 28;21(1):199–202. doi: 10.1016/0022-2836(66)90088-x. [DOI] [PubMed] [Google Scholar]
- Petrich J. W., Martin J. L., Houde D., Poyart C., Orszag A. Time-resolved Raman spectroscopy with subpicosecond resolution: vibrational cooling and delocalization of strain energy in photodissociated (carbonmonoxy)hemoglobin. Biochemistry. 1987 Dec 1;26(24):7914–7923. doi: 10.1021/bi00398a056. [DOI] [PubMed] [Google Scholar]
- Petrich J. W., Poyart C., Martin J. L. Photophysics and reactivity of heme proteins: a femtosecond absorption study of hemoglobin, myoglobin, and protoheme. Biochemistry. 1988 May 31;27(11):4049–4060. doi: 10.1021/bi00411a022. [DOI] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]


