Abstract
Effects of massed repetition on the modulation of the late positive potential elicited during affective picture viewing were investigated in two experiments. Despite a difference in the number of repetitions across studies (from 5 to 30), results were quite similar: the late positive potential continued to be enhanced when viewing emotional, compared to neutral, pictures. On the other hand, massed repetition did prompt a reduction in the late positive potential that was most pronounced for emotional pictures. Startle probe P3 amplitude generally increased with repetition, suggesting diminished attention allocation to repeated pictures. The blink reflex, however, continued to be modulated by hedonic valence, despite massive massed repetition. Taken together, the data suggest that the amplitude of the late positive potential during picture viewing reflects both motivational significance and attention allocation.
Affect reliably modulates the magnitude of a late positive potential (LPP) measured over centro-parietal sensors, with the largest LPPs elicited when viewing either pleasant or unpleasant, compared to neutral, pictures (e.g., Cacioppo, Crites, & Gardner, 1996; Codispoti, Mazzetti, & Bradley, 2009; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Johnston, Miller, & Burleson, 1986; Palomba, Angrilli, & Mini, 1997). Several recent studies have found that, despite a decrease in overall amplitude of the LPP with repeated presentation of the same picture, emotional pictures continue to elicit a larger late positive potential than neutral pictures (Codispoti, Ferrari & Bradley, 2006, 2007). One interpretation is that the LPP reflects, in part, motivational significance, defined as activation of cortico-limbic appetitive and defensive systems that mediate the sensory and motor processes that support perception and action (Bradley, 2009).
In our previous studies, however, repeated pictures were always presented intermixed among other (repeated) pictures. Thus, although the pictures were highly familiar, initial encoding and attention allocation processes were still necessary for picture identification on each presentation and it may be these processes that underlie continued modulation of the LPP. We tested this hypothesis in two experiments by repeatedly presenting the same picture over and over with no intervening stimuli between repetitions. If it is motivational significance that mediates the persistent modulation found for emotionally engaging picture despite repetition, we expected to continue to find differences in LPP amplitude between emotional and neutral pictures; to the extent that the LPP primarily indexes heightened initial attention allocation, we expected the differences to disappear.
We employed two other reliable measures of attention and emotion in picture viewing to aid in interpreting changes in the late positive potential with massed repetition. Presentation of an acoustic startle probe during affective picture viewing modulates both the reflexive eyeblink and the amplitude of the P3 component of the event-related potential to the probe. Probe P3 amplitude is smaller for startle stimuli presented in the context of emotional, compared to neutral, picture viewing. One interpretation is that heightened resource allocation during affective picture processing results in fewer resources available for processing the secondary acoustic probe (Bradley, Codispoti & Lang, 2006; Bradley, Cuthbert & Lang, 1999; Cuthbert, Schupp, Bradley, McManis & Lang, 1998). This interpretation is consistent with data finding that reaction times to secondary probes are also slower when viewing emotional, compared to neutral pictures (Bradley et al., 1999; De Cesarei & Codispoti, 2008). If repetitive contiguous presentation decreases attention allocation to the picture, we expected the difference in probe P3 amplitude between emotional and neutral pictures to decrease with massed repetition. Moreover, the specific comparison between modulation of the LPP and the probe P3 as a function of repetition is also quite informative: If the LPP during picture viewing and the probe P3 index the same attentional process, we expected that the effects of picture repetition on affective modulation of these electrocortical potentials would be similar.
Unlike the probe P3, the reflexive blink response to a startle probe presented during picture perception is modulated by hedonic valence, with larger reflexes elicited when viewing unpleasant, compared to pleasant, pictures (e.g., Bradley et al., 1999; Vrana, Spence & Lang, 1988). When affective pictures are repeatedly presented intermixed with other (repeated) pictures, the modulatory effects of emotion remain intact (Bradley, Lang & Cuthbert, 1993). On the other hand, the reflexive blink response is significantly smaller shortly after picture onset, compared to later in the viewing interval, suggesting blink magnitude is inhibited when pictures are novel and attention-getting (Bradley et al., 2006). If repetition decreases this inhibitory effect on blink magnitude, startle reflexes are expected to be generally smaller for novel, compared to repeated, pictures. Nonetheless, because the blink reflex is differentially modulated by pleasant and unpleasant content (i.e., unlike the LPP or the P3), we expected affective modulation to remain with massed repetition.
Experiment 1
Participants
Participants were 25 students (13 women) from the University of Florida introductory psychology courses who participated for course credit. Of these, two subjects (1 man and 1 woman) were excluded from analysis of startle data because of virtually no startle responses.
Materials and design
Stimuli were 168 pictures selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008), consisting of 56 pleasant, 56 neutral, and 56 unpleasant pictures. Of these, 144 pictures were presented only once throughout the study (“novel” presentation), whereas each of the remaining 24 pictures (8 for each hedonic content) was repeatedly presented 5–8 times in a row (“massed” repetition); block length for massed pictures was variable to reduce predictability. In total, there were 6 blocks each of 5, 6, 7, or 8 massed repetitions (156 trials). Novel pictures were arranged such that there were two pictures of each hedonic content in each block of 6. Blocks of novel pictures were alternated with blocks of massed repetitions, for a total of 300 trials (144 novel pictures + 156 repeated pictures).
The acoustic startle probe consisted of a 98-dB, 50-ms burst of white noise with instantaneous rise time. The stimulus was generated by a Coulbourn S81-02 white-noise generator and presented binaurally over earphones (Eartone A3 Audiometric Insert Earphones, Aearo Company, Indianapolis, USA). A total of 48 startle probes were presented (1200 ms following picture onset) in the study, 24 during viewing of novel pictures (8 of each hedonic content), and 24 during viewing of massed repetitions (8 of each hedonic content). During massed presentation, a startle probe could be presented at either the 3rd, the 4th or the 5th repetition of the picture. Using the same 168 pictures, three presentation orders were constructed that varied, across participants, the specific pictures presented in the novel and massed conditions as well as the pictures that were probed.
Each trial consisted of a fixation-cross presented at the center of the screen for 500 ms prior to picture onset. Each picture was displayed for 2 s, followed by 2 s of inter-trial interval. Pictures were presented on a 19-in. CRT monitor, situated approximately 100 cm from the participant.
Physiological recording and data reduction
Electroencephalographs were collected from the scalp using a 129-channel system (Electrical Geodesics, Inc., Eugene, OR) running NetStation software on a Macintosh computer. Scalp impedance for each sensor was kept below 50 kΩ. The EEG was recorded continuously with a sampling rate of 250 Hz, the vertex sensor as reference electrode, and on-line bandpass filtered from 0.01 to 100 Hz. EEG data were analyzed offline using a MATLAB based program (Junghofer, Elbert, Tucker, & Rockstroh, 2000) in which continuous EEG data were low-pass filtered at 40 Hz using digital filtering and artifact detection was performed by means of a dedicated algorithm that uses statistical parameters to determine trials with artifacts (Junghofer et al., 2000).
For both picture onset and startle probe ERP epochs, a 100 ms pre-stimulus data segment was subtracted as baseline. Processed data were averaged by hedonic content and novel/massed presentation1 for each participant. The average number of trials per condition employed was approximately 40 for picture onset ERPs and approximately 8 for the startle probe ERPs. Based on previous studies (Bradley, Hamby, Loew, & Lang, 2007; Codispoti et al, 2006) and ERP visual inspection, statistical analysis were performed on mean amplitude values computed in a 400–700 ms window for the late positive potential and a 250–350 ms window for the probe P3. For both analysis, amplitudes were measured over a group of centro-parietal sensors (sensor number: 7,32,38,54,55,61,62,68,79,80,81,88,107,129).
The eyeblink component of the startle response was measured by recording EMG activity from the orbicularis oculi muscle beneath the left eye. The raw EMG signal was amplified (× 30,000) and frequencies below 90 Hz and above 250 Hz were filtered with a Coulbourn S75-01 bioamplifier. The raw signal was rectified and integrated using a Coulbourn S76-01 contour-following integrator using a normal time constant of 123 ms. The blink response was sampled at 1000 Hz for 50 ms prior to the onset of the startle probe and for 250 ms after probe onset. The startle data were reduced off-line using a VPM program (Cook, 1997) that implements a peak-scoring algorithm (Balaban, Losito, Simons, & Graham, 1996) that scores the peak response for onset latency and amplitude. Trials with artifacts were rejected, while trials with no responses were scored as zero magnitude blinks.
Procedure
After arrival at the laboratory, participants signed an informed consent form. Participants were then seated in a recliner in a small, sound-attenuated, dimly lit room, and recording electrodes for the blink and the EEG sensor net were attached. The subject was instructed that a series of pictures would be presented in which each picture should be viewed the entire time it was on the screen and that brief noises heard over the headphones could be ignored.
An initial 30-trial practice block, consisting of the same neutral picture repeatedly displayed, was presented together with 12 startle probes, in order to facilitate the basic circuit habituation of the startle reflex (Bradley et al. 1993). Following this initial habituation phase, 300 pictures were presented consisting of novel pictures and massed repetitions. After the picture series was finished, a post-experimental questionnaire was completed and the subject was debriefed.
Data analysis
Each measure (LPP, probe P3, blink magnitude) was analyzed in a repeated measure analysis of variance (ANOVA) using stimulus repetition (2: Massed, Novel) and hedonic content (3: Pleasant, Neutral, Unpleasant) as factors. Greenhouse-Geisser corrections were applied where relevant. The eta squared statistic (η2), indicating the proportion between the variance explained by one experimental factor and the total variance, has been calculated and is reported.
Results
Late Positive Potential
Figure 1 illustrates the ERP waveforms averaged over centro-parietal sensors for pleasant, neutral and unpleasant pictures in the massed and novel repetition conditions2. Significant main effects of hedonic content [F(2,48)=43.7, p<.0001, η2=.64] and repetition [F(1,24)=24.7, p<.0001, η2=.51] were accompanied by a significant interaction between hedonic content and repetition [F(2,48)=14.9, p<.0001, η2=.38]. When viewing novel pictures, hedonic content modulated the magnitude of the centro-parietal late positive potential, as expected, [F(2,48)=42.1, p<.0001, η2=.64] with larger positivity when viewing pleasant or unpleasant pictures, compared to neutral contents [Fs(1,24)>26, ps<.0001, η2>.52], replicating many previous studies. Viewing massed repetitions of emotional pictures also prompted significantly larger LPPs than when viewing neutral pictures [F(2,48)=17.7, p<.0001, η2=.42; pleasant and unpleasant vs neutral, Fs(1,24)>19.3, p<.0001; η2>.45]. The interaction primarily indicates that, whereas the LPP when viewing novel and repeated neutral pictures did not differ, the LPP for emotional pictures was smaller when the pictures were repeated [F(1,24)=10.5, p<.001, η2=.31 for pleasant; F(1,24)=59.1, p<.0001, η2=.71 for unpleasant].
Figure 1.
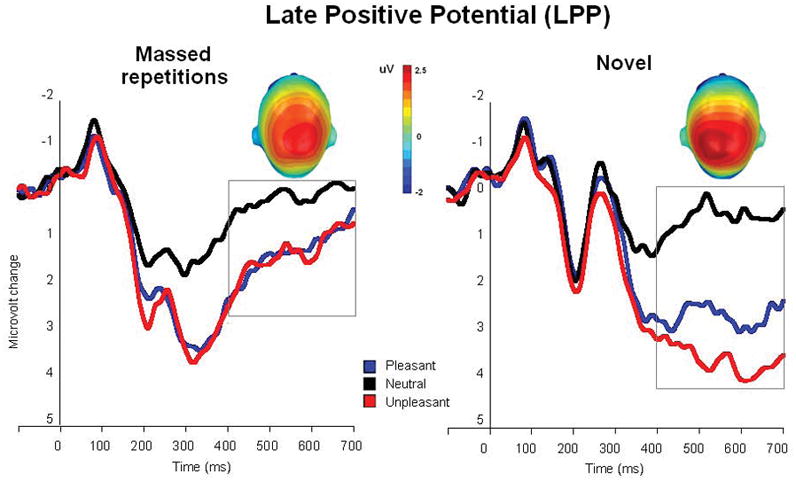
Experiment 1. Grand average ERP waveforms (centro-parietal sensor group) when viewing pleasant, neutral and unpleasant pictures in the massed and novel repetition conditions. Insets are the top view of the scalp distribution of the difference in the 400–700 ms window between emotional (pleasant and unpleasant) and neutral picture processing.
Probe P3
Figure 2 illustrates the ERP waveforms to the startle probe (centro-parietal sensors) presented during viewing of pleasant, neutral and unpleasant pictures in the massed and novel repetition conditions. Repetition of the same picture over and over significantly increased probe P3 amplitude, with larger P3s when viewing repeated, compared to novel, pictures [novel vs massed, F(1,24)=18.6, p<.0001, η2=.44]. A main effect of hedonic content [F(2,48)=3.8, p<.05, η2=.14] indicated smaller P3 amplitude for emotional, compared to neutral pictures [quadratic effect, F(1,24)=4.1, p=.055, η2=.15], as illustrated in Figure 2. Although the interaction of hedonic content and repetition did not reach significance (p=.31), a priori tests exploring modulation for novel and massed pictures indicated that whereas probe P3 was significantly modulated by picture content during viewing of novel pictures [F(2,48)=6.34, p<.01, η2=.2], it was not reliably modulated by emotional content when viewing repeated pictures (p=.3).
Figure 2.
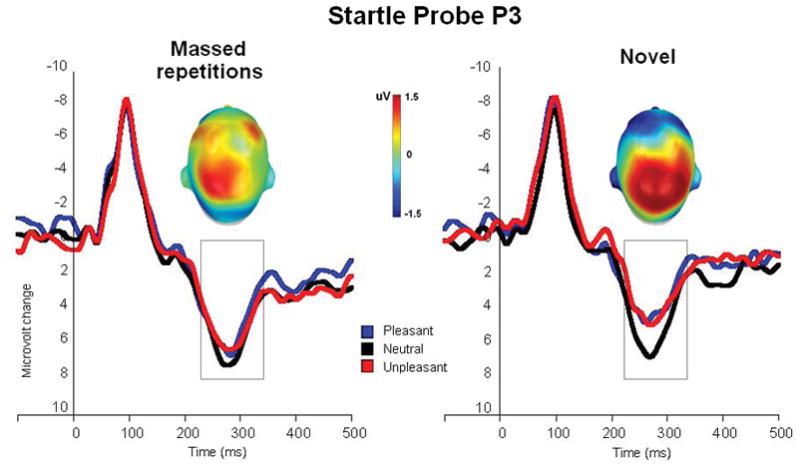
Experiment 1. Grand average ERP waveforms (centro-parietal sensor group) to the startle probe during viewing of pleasant, neutral and unpleasant pictures in the massed and novel repetition conditions. Insets are the top view of the scalp distribution of the difference in the 250–350 ms window between emotional (pleasant and unpleasant) and neutral picture processing.
Startle Reflex
Startle blink magnitude was significantly smaller when viewing novel, compared to repeated, pictures [F(1,22)=5.2, p<.05, η2=.19] (see Table 1). Nonetheless, blink magnitude varied with hedonic content [F(2, 44)=13.88, p<.0001, η2=.39], regardless of picture repetition (hedonic content x stimulus repetition, p>.05). Blinks were larger when viewing unpleasant, compared to pleasant or neutral, pictures [F(1, 22)=22.12, p<.0001, η2=.5; F(1, 22)=26.9, p<.0001, η2=.55, respectively].
Table 1.
Means (standard error) of late positive potential (LPP), the amplitude of the P3 component to the startle probe (Probe P3) and startle blink magnitude, for each hedonic content in Experiment 1. All values are expressed in microvolts (μV).
| LPP (w400–700ms) | Probe P3 (w250–350ms) | Startle blink magnitude | ||||
|---|---|---|---|---|---|---|
| Massed | Novel | Massed | Novel | Massed | Novel | |
| Unpleasant | 1.5 (.4) | 3.4 (.4) | 6.6 (.9) | 5.2 (.7) | 8.1 (1) | 7.7 (.1) |
| Neutral | 0.2 (.4) | 0.4 (.3) | 7.0 (.9) | 5.9 (.9) | 7.2 (1) | 6.3 (.9) |
| Pleasant | 1.5 (.3) | 2.6 (.4) | 6.1 (.8) | 4.2 (.7) | 7.1 (1) | 6.3 (.9) |
Experiment 2
In experiment 1, massed repetition affected all measures -- decreasing the LPP following picture onset and increasing both the amplitude of the P3 to the startle probe during picture viewing as well as startle blink magnitude. Nonetheless, LPP amplitude continued to be significantly larger when viewing emotional, compared to neutral pictures, even following multiple massed repetitions of the same picture. Similarly, affective modulation of the reflexive blink remained significant despite massed repetition of the pictures. On the other hand, although mean probe P3 amplitude was attenuated when viewing emotional, compared to neutral, novel pictures, the difference clearly decreased and was not statistically reliable for pictures that were repeatedly presented, consistent with a hypothesis that probe P3 indexes attention allocation.
In Experiment 2, we extended the number of massed repetitions to 30 contiguous repetitions in order to more completely attenuate attentional resource allocation to emotional pictures. As in Experiment 1, we measured the magnitude of the LPP following picture onset as well as the amplitude of the probe P3 and the startle blink reflex. Moreover, startle probes were also presented in the inter-picture interval in Experiment 2. If probe P3 amplitude primarily indexes attention allocation, as hypothesized, we expected not only that its modulation by emotion would be eliminated by massive, massed repetition, but that probe P3 amplitude following repetition would not differ from that elicited when there was not even a picture in the foreground. To the extent that the startle blink reflects motivational significance, we expected modulation to remain intact regardless of the number of repetitions.
Of particular interest are the effects of massive repetition on the late positive potential: If the heightened positivity primarily reflects differences in attention allocation, we expected it to be eliminated with massive, massed repetition. To the extent it reflects motivational significance, we expected to continue to find a larger LPP for emotional, compared to neutral, pictures. Rather than comparing massed repetition to novel picture presentation, in Experiment 2 we compared massed repetition to a distributed repetition condition in which pictures were repeated across the experiment, but intermixed with other (repeated) pictures. In this comparison, effects specifically due to massed repetition should be highlighted.
Method
Participants
Participants were 25 students (13 women) from the University of Florida introductory psychology courses who participated for course credit. Of these, three subjects (2 men and 1 woman) were excluded from analysis of startle data because of virtually no startle responses.
Materials and design
Thirty-six color pictures depicting 12 pleasant (erotic couple and romance), 12 unpleasant (threat and mutilation) and 12 neutral (objects and people) scenes were selected from the International Affective Picture System (IAPS; Lang et al., 2008). Of these, 6 pictures were presented 30 times in a row (massed) and 30 were presented intermixed with other pictures and repeated 6 times across the study (distributed). Each massed repetition was followed by the series of 30 distributed pictures, resulting in a total of 360 trials.
Six presentation orders were constructed that varied, across participants, the specific pictures presented in the massed and distributed blocks such that each of the 36 pictures was presented in the massed and distributed condition across participants. The order of pictures in the distributed condition was counterbalanced such that there were two pictures of each hedonic content in each subblock of six.
As in Experiment 1, each trial consisted of a fixation-cross presented at the center of the screen for 500 ms prior to picture onset. Each picture was displayed for 2 s, followed by a 2 s inter-trial interval. Pictures were presented on a 19-in. CRT monitor, situated approximately 100 cm from the participant.
Across each of the 30 massed repetitions, 3 startle probes (one every ten trials) were delivered during picture viewing (1200 ms after picture onset) as well as in the post-picture interval (1200 ms after picture offset). Startle probes were similarly presented in the distributed condition.
Procedure
The procedure was equal to that described in Experiment 1.
Physiological recording and data reduction
The startle probe, and measurement of the blink response and electroencephalographic activity, as well as data reduction, were as described in Experiment 1. Blink responses and probe ERPs were averaged across the thirty massed repetition block, separately for probes delivered during picture viewing and those prompted in the post-picture interval (6 trials per condition). For the LPP (400–700 ms window), the thirty massed repetitions were averaged into two subblocks of 15 trials (Massed 1–15, Massed 16–30).
Each measure was analyzed in a repeated measure analysis of variance (ANOVA) involving picture hedonic content (3: Pleasant, Neutral, Unpleasant) and stimulus repetition (2: Massed, Distributed).
Results
Late Positive Potential
Figure 3 illustrates the ERP waveforms for pleasant, neutral and unpleasant pictures in the massed and distributed repetition conditions over centro-parietal sensors. A main effect of hedonic content (F(2,48)=104, p<.0001, η2=.81) again indicated greater positivity for both pleasant and unpleasant pictures, compared to neutral content [F(1,24)=120, p<.0001, η2=.83; F(1,24)=175, p<.0001, η2=.88, respectively]. As illustrated in Figure 3, emotional (pleasant and unpleasant) pictures prompted a larger LPP than neutral pictures both in the massed repetition condition [Fs(1,24)>19.6, ps<.0001, η2>.45] and in the distributed repetition condition [Fs(1,24)>82.9, ps<.0001, η2>.78]. For massed repetition, in fact, the magnitude of affective modulation was similar when assessed either during the first or last 15 repetitions in the massed condition, F(1,24)<1 (see Figure 3, inset). Again, effects of repetition [F(1,24)=138.3, p<.0001, η2=.85] and a significant interaction between repetition and hedonic content [F(2,48)=19.8, p<.0001, η2=.45] indicated a larger decrease in the LPP following massed repetition for emotional, compared to neutral, pictures, resulting in significantly smaller affective modulation for massed, compared to distributed, repetitions, F(1,24)=32, p<.0001, η2=.57.
Figure 3.
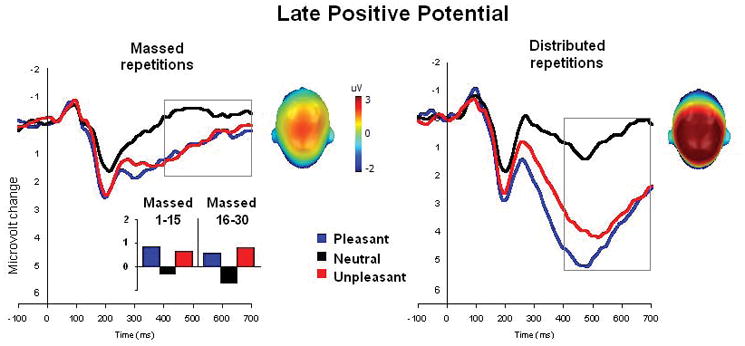
Experiment 2. Grand average ERP waveforms (centro-parietal sensor group) to pleasant, neutral and unpleasant pictures in the massed (averaged over 30 repetitions) and distributed repetition conditions. Insets are the top view of the scalp distribution of the difference in the 400–700 ms window between emotional (pleasant and unpleasant) and neutral picture processing. Massed condition also shows the mean amplitude of the late positive potential (400–700 ms) for massed repetitions averaged over two subblocks of fifteen repetitions each.
Probe P300
As in Experiment 1, probe P3 amplitude was significantly larger for massed repetitions [F(1,24)=9.9, p<.005, η2=.3]. Unlike Experiment 1, however, massive massed repetition now resulted in a significant interaction between repetition and hedonic content [F(2,48)=6.25, p<.005, η2=.21]. As illustrated in Figure 4 (top), probe P3 amplitude was significantly attenuated when viewing emotional, compared to neutral, pictures, when repetitions were distributed, similar to the pattern found for novel pictures in Experiment 1 [F(2,48)=10, p<.0001, η2=.29]. Massed repetition, however, again eliminated the difference in probe P3 amplitude when viewing emotional and neutral pictures [F(2,48)<1]. Whereas the type of repetition did not affect the amplitude of the probe P3 when viewing neutral pictures, massed repetition significantly increased probe P3 amplitude when viewing emotional pictures, consistent with an interpretation of less attention allocation as repetition increased, [Fs(1,24)>5, p<.05. η2>.2].
Figure 4.
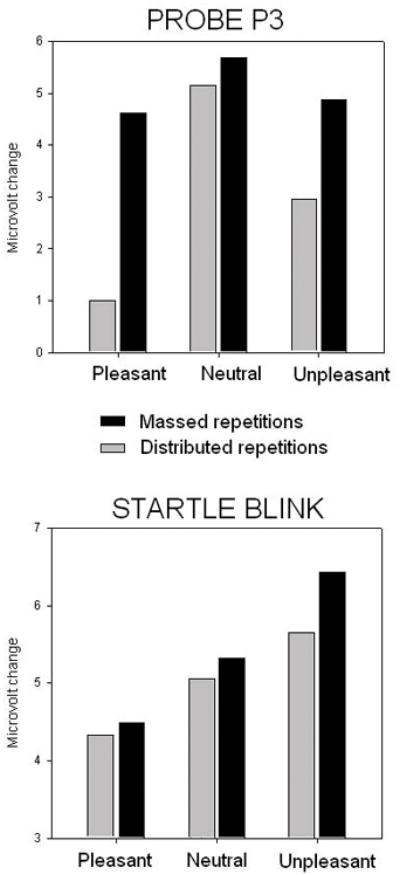
Experiment 2. (Top) Probe P3 amplitude in the 250–350 ms window over centro-parietal sensors for pleasant, neutral and unpleasant pictures in the massed and distributed repetition conditions. (Bottom) Blink magnitude for startle probes presented during viewing of pleasant, neutral and unpleasant pictures in the massed and distributed repetition conditions.
The amplitude of the probe P3 in the inter-picture interval differed for massed and distributed repetitions, consistent with an interpretation that probe P3 indexes attention allocation: Probe P3 amplitude in the picture viewing (5.05 μV) and post-picture interval (4.48 μV) did not differ for massed repetitions. On the other hand, probe P3 amplitude was smaller (3.04 μV) during picture viewing, compared to the inter-picture interval (4.49 μV) when the repetition was not massed [F(1,24)=24.8, p<.0001, η2=.5].
Startle Reflex
Figure 4 (bottom) illustrates blink magnitude for startle probes presented during viewing of pleasant, neutral and unpleasant pictures. A significant main effect of hedonic content [F(2,42)=13.8, p<.0001, η2=.39], indicated significant startle potentiation when viewing unpleasant pictures [F(1,21)=9.9, p<.01, η2=.32], and significant attenuation when viewing pleasant pictures [F(1,21)=7.9, p<.05, η2=.27], compared to neutral pictures. Similar to Experiment 1, affective modulation of blink magnitude was intact even in the massed condition in which the same picture was repeated thirty times in a row, [F(2,42)=8.87, p<.01, η2=.29]. Moreover, the type of repetition (massed vs distributed) did not affect overall blink magnitude3.
Discussion
Effects of massed repetition on the modulation and the amplitude of the late positive potential (LPP) during affective picture viewing were investigated. Despite a difference in the absolute number of contiguous repetitions in two studies (from 5 to 30), results were strikingly similar: Even following many contiguous repetitions of the same picture, larger late positive potentials were associated with viewing emotional, compared to neutral, pictures. These data disconfirm a hypothesis that intermixing pictures in previous studies (Codispoti et al. 2006; 2007) was responsible for the sustained modulation following repetition. Rather, modulatory differences remained intact even following the massive massed repetition in Experiment 2, in which the same picture was presented 30 times in a row. In both studies, a reduction in the amplitude of the late positive potential with massed repetition was most pronounced for emotional, compared to neutral, pictures. Thus, although viewing emotional pictures consistently prompted a larger late positive potential than when viewing neutral pictures, the absolute amplitude of the late positive potential was greatly reduced for emotional pictures following massed repetition.
Two additional indices of attention allocation and motivational significance shed light on this pattern of LPP findings. The amplitude of the P3 component to a secondary startle probe presented during picture viewing, a measure of attention allocation, significantly increased with repetition in both studies, consistent with the hypothesis that more attention is available for processing the secondary startle probe when the picture has been previously processed. Indeed, with the massive massed repetition in Experiment 2, probe P3 amplitude during picture viewing was no longer different from that elicited in the inter-picture interval, implying equivalent processing of the startle probe regardless of whether a (repeated) picture was even present in the visual foreground.
Moreover, the increase in probe P3 amplitude with repetition was specifically related to emotional picture processing -- repetition did not change probe P3 amplitude when viewing neutral pictures, suggesting these stimuli attract few attentional resources even when novel. Thus, whereas probe P3 amplitude is typically attenuated when viewing novel emotional, compared to neutral, pictures (Cuthbert et al., 1998), massed repetition effectively eliminated affective modulation of this electrocortical component in both studies. Taken together then, the probe P3 data suggest that “motivated attention” (Lang et al., 1997) -- the heightened attention allocation directed towards motivationally significant (pleasant or unpleasant) stimuli -- decreases with massed repetition.
Affective modulation of the reflexive eyeblink to the secondary startle probe, on the other hand, was preserved despite picture repetition. Even after 30 repetitions, blink reflexes were significantly potentiated for startle probes presented during unpleasant picture viewing (relative to neutral) and reduced when viewing pleasant pictures. Again, the persistence of affective modulation of the startle reflex, also found previously for distributed repetitions (Bradley et al., 1993), is particularly striking in Experiment 2, given repetition of the same picture 30 times in a row. Consistent with a multi-process model of affective blink modulation (Bradley et al., 2006), startle magnitude when viewing novel pictures (Experiment 1) was significantly smaller overall than those elicited when viewing repeated pictures, supporting the thesis that attention allocation can generally inhibit reflex magnitude. Unlike the probe P3 data, however, the attenuating effects of repetition on blink magnitude were not confined to emotional stimuli, and, in Experiment 2, in which all pictures were repeated (massed or distributed), the type of repetition did not differentially affect blink magnitude. These data suggest that perceptual novelty may be the critical mediator of repetition effects on the overall magnitude of the blink reflex, rather than motivated attention. In any case, it is clear that the blink reflex continues to reflect motivational significance and that this modulation is not affected by repetition.
Taken together, these data support a two-process account of LPP modulation during affective picture viewing, in which the amplitude of the late positive potential reflects both motivational significance and differences in initial motivated attention. Thus, like the blink reflex, affective modulation of the LPP persists despite massed repetition, suggesting it indexes motivational significance - the activation of fundamental appetitive and defensive motivational systems that are the foundation of emotion (Lang et al., 1997). On the other hand, like probe P3 amplitude, massed repetition affected the amplitude of the LPP specifically for emotional stimuli, reflecting a decrease in attention allocation.
A dual process interpretation is consistent with our previous study in which the amplitude of the LPP was independently affected by task relevance and motivational significance (Ferrari, Codispoti, Cardinale & Bradley, 2008). In that study, when emotional pictures were targets in a categorization task, the LPP was heightened, compared to emotional non-targets. On the other hand, LPP amplitude continued to be enhanced for emotional, compared to neutral, pictures, regardless of target status. In the current study, attention was manipulated by stimulus repetition, rather than by task-relevance, and the data are consistent in showing independent effects of motivational significance and attention allocation. Taken together, the data indicate that the ‘natural selective attention’ that is engaged by the presentation of emotionally evocative cues in the absence of overt tasks or specific instructions (Bradley, 2009; Lang et al., 1997) is greatest for novel pictures and decreases with massed repetition.
We have suggested that cues acquire motivational significance from their associations to appetitive and defensive neural circuits that mediate attention, arousal, and action in the service of protecting and sustaining life (e.g., Lang et al., 1997; Lang & Bradley, 2008). For pictures, the similarity of perceptual (visual) features to real-world objects and events serve as the cues that activate the relevant motivational circuit (Bradley & Lang, 2007). It is not surprising that sheer repetition alone is not sufficient to destroy these long-standing associations. Indeed, it has long been recognized that extinction -- repetitive presentation of cues with existing appetitive or defensive associations -- does not eliminate these associations, as evidenced by spontaneous recovery, reinstatement, and their reappearance in a different context (see Myers & Davis, 2007, for an overview).
In the present studies, the data suggest that despite repeated massed exposure to an aversive or appetitive picture, affective cues continue to activate the neural circuits mediating appetitive or defensive motivation. That these motivational associations are indexed, in part, by the late positive potential is supported by its continued modulation despite repetition. We have suggested that the activation of fundamental motivational systems mediating appetitive and defensive behavior is the foundation of emotion, giving events and objects “motivational significance” and prompting heightened attention in the service of action (Lang et al., 1997; Lang & Bradley, 2008; Ferrari et al., 2010). The probe P3 data indicate that the initial orienting and heightened attention allocation to emotional cues can be attenuated by repetitive exposure. The persistence of startle modulation, however, is strong evidence that motivational activation continues despite massive repetition. Taken together, then, the data suggest that motivational significance remains following repetitive massed exposure, whereas motivated attention is attenuated or eliminated, and that the amplitude of the late positive potential is sensitive to both emotion and attention.
Acknowledgments
This research was supported in part by a grant from the National Institute of Mental Health (P50 MH 72850) to the Center for the Study of Emotion and Attention (CSEA) at the University of Florida. Vera Ferrari is now at the University of Bologna, Italy.
Footnotes
Based on a previous analysis (Ferrari, Bradley, Codispoti & Lang, 2010), trials which signaled a change in the experimental structure (i.e., the first novel picture following a block of massed repetitions and the first repetition of a picture in the block of massed repetitions) were excluded from the averaging because these trials prompt an enhanced P3 amplitude that reflects stimulus meaning, regardless of repetition.
Analysis of the large N2 component in the ERP to pictures that is dramatically present for all of the novel pictures and greatly attenuated for all of the massed pictures (i.e., independent of hedonic content) is presented in a separate report (Ferrari et al., 2010).
A follow-up test indicated that startle potentiation during viewing of unpleasant pictures was even larger during massed repetition, compared to when viewing of distributed unpleasant pictures, F(1,21)= 4.8, p<.05, η2=.19.
References
- Balaban MT, Losito BDG, Simons RF, Graham FK. Off-line latency and amplitude scoring of the human reflex eye blink with Fortran IV. Psychophysiology. 23:612. [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–97. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell A, Boehmelt A, editors. Startle Modification: Implications for Neuroscience, Cognitive Science and Clinical Science. Stanford, CA: Cambridge; 1999. pp. 157–183. [Google Scholar]
- Bradley MM, Hamby S, Loew A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty and the startle reflex: habituation in humans. Behavioral Neuroscience. 1993;107:970–80. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Gardner WL. Attitudes to the right: Evaluative processing is associated with lateralized late positive event-related brain potentials. Personality and Social Psychology Bulletin. 1996;22:1205–1219. [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and ERPs: Distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Mazzetti M, Bradley MM. Unmasking emotion:exposure duration and emotional engagement. Psychophysiology. 2009;46:731–8. doi: 10.1111/j.1469-8986.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- Cook EW., III . VPM reference manual. Birmingham, Alabama: Author; 1997. [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: activation and emotion. Psychophysiology. 1996;33:103–11. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, McManis M, Lang PJ. Probing affective pictures: attended startle and tone probes. Psychophysiology. 1998;35:344–7. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Fuzzy picture processing: effects of size reduction and blurring on emotional processing. Emotion. 2008;8:352–63. doi: 10.1037/1528-3542.8.3.352. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Detecting novelty and significance. Journal of Cognitive Neuroscience. 2010;22:404–11. doi: 10.1162/jocn.2009.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Cardinale R, Bradley MM. Directed and motivated attention during processing of natural scenes. Journal of Cognitive Neuroscience. 2008;20:1753–61. doi: 10.1162/jocn.2008.20121. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Johnston VS, Miller DR, Burleson MH. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23:684–694. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–32. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Appetitive and defensive motivation as the substrate of emotion. In: Elliott A, editor. Handbook of approach and avoidance motivation. Erlbaum; 2008. pp. 51–66. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert MM. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1997. [Google Scholar]
- Myers K, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;2:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–61. [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]


