Abstract
Purpose
The mammalian target of rapamycin (mTOR) functions within the PI3K/Akt signaling pathway as a critical modulator of cell survival.
Methods
The mTOR inhibitor temsirolimus (CCI-779) was combined with chemo-radiotherapy in GBM patients in a dose-escalation Phase I trial. The first 12 patients were treated with CCI-779 combined with RT/TMZ and adjuvant TMZ. A second cohort of 13 patients was treated with concurrent CCI-779/RT/TMZ followed by adjuvant TMZ monotherapy.
Results
Concomitant and adjuvant CCI-779 was associated with a high rate (3 of 12 patients) of Grade 4/5 infections. By limiting CCI-779 treatment to the RT/TMZ phase and using antibiotic prophylaxis, the rate of infections was reduced, although 2 of 13 patients developed exacerbation of pre-existing fungal or viral infections. DLTs were observed in 2 of 13 patients with this modified schedule. Weekly CCI-779 (50 mg/week) combined with RT/TMZ is the recommended Phase II dose and schedule. The immune profile of patients in the second cohort was assessed before, during, and after CCI-779 therapy. There was robust suppression of helper and cytotoxic T-cells, B-cells, natural killer (NK) cells and elevation of regulatory T (Tregs) cells during CCI-779/RT/TMZ therapy with recovery to baseline levels during adjuvant TMZ of cytotoxic T-cells, NK cells and Tregs.
Conclusions
The increased infection rate observed with CCI-779 combined with chemo-radiotherapy in GBM was reduced with antibiotic prophylaxis and limiting the duration of CCI-779 therapy. The combined suppressive effects of CCI-779 and TMZ therapy on discrete immune compartments likely contributed to the increased infectious risks observed.
Keywords: glioblastoma, immunosuppression, temsirolimus, radiation temozolomide, infection
INTRODUCTION
Sirolimus is a potent mTOR inhibitor originally developed as an immunosuppressant. Disruption of mTOR signaling by sirolimus has pleiotropic effects on the immune system and leads to a cellular immune defect. With a favorable side-effect profile and good safety record, sirolimus is an important immunosuppressant used in patients following solid organ transplant.(1, 2) Temsirolimus (CCI-779) is an ester of sirolimus developed for oncology indications and is FDA-approved for treatment of renal cell carcinoma. Treatment with CCI-779 monotherapy in recurrent GBM was associated with radiographic improvement in T2 signal abnormality in 36% of patients in the Phase II NCCTG N997B trial (3), and preclinical data suggest additive to synergistic effects of mTOR inhibitors combined with radiation (RT) or temozolomide (TMZ).(4–6) The current study reports the NCCTG Phase I experience of integrating (CCI-779) with RT and TMZ in patients with newly diagnosed GBM.
METHODS and MATERIALS
Eligibility Criteria
Patients with newly diagnosed WHO grade IV glioma, confirmed by central pathology review, were eligible for participation in this trial. Patients were enrolled after 1 week but no later than 6 weeks following biopsy or resection. Enrollment was limited to patients age 18 years or older with an ECOG performance status of 0–2, acceptable baseline hematologic and chemistry function(7), and a total bilirubin ≤ 2.5 the upper limit of normal, serum total cholesterol < 350 mg/dL and total triglycerides < 400 mg/dL. Patients were excluded if they had prior chemotherapy or radiation for a brain tumor, prior therapy with an mTOR inhibitor, were on enzyme-inducing anticonvulsants, or had uncontrolled intercurrent illness or major surgery within 21 days of registration. Written informed patient consent was obtained prior to enrollment.
Protocol therapy
This clinical trial was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute, and was reviewed and approved by the Mayo Institutional Review Board. Patients were enrolled at Mayo Clinic in Rochester, Minnesota and Jacksonville, Florida. CCI-779 was provided by Wyeth Pharmaceuticals (Madison, NJ) by agreement with CTEP. Intravenous CCI-779 was delivered weekly starting 1 week prior to concomitant RT/TMZ and extending through the end of RT for all patients (Figure 1). After a 4–6 week break, the first 12 patients enrolled were treated with weekly CCI-779 concurrent with adjuvant TMZ for up to 6 cycles. After excessive infectious toxicities were observed in this first cohort, the protocol was amended and 13 additional patients were treated with CCI-779 delivered only during concurrent RT/TMZ followed by adjuvant TMZ monotherapy.
Figure 1. Treatment schema.
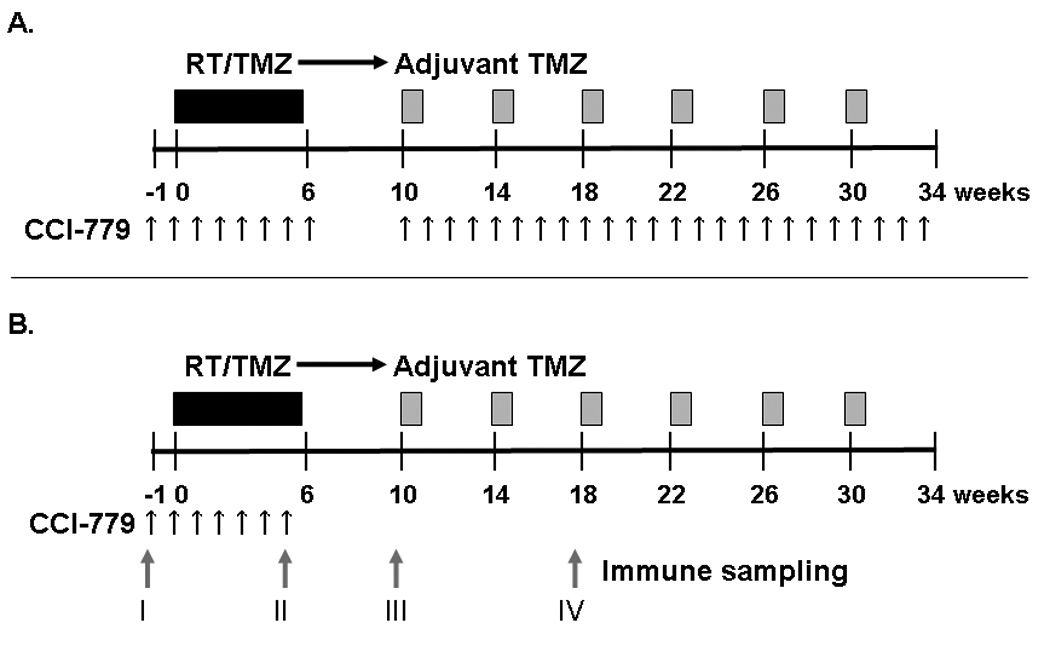
A) The first 12 patients treated on this trial received standard RT/TMZ (black bar) and adjuvant TMZ (gray bars) with weekly CCI-779 (black arrows) given both during RT/TMZ and adjuvant TMZ. B) A second cohort of patients was treated with weekly CCI-779 only during RT/TMZ followed by adjuvant TMZ monotherapy. Blood samples were drawn for immune monitoring at 4 time points labeled I–IV as indicated by gray arrows.
The dose of CCI-779 was escalated in a standard cohorts-of-3 trial design.(8, 9) The starting dose for CCI-779 was 25 mg/week and subsequent dose levels were 50 mg/week and 75 mg/week. Patients were assessed during CCI-779/RT/TMZ therapy for dose-limiting toxicities (DLT). Toxicities observed during the adjuvant phase of TMZ/CCI-779 dosing also were considered when determining the maximally tolerated dose (MTD). Adverse events were reported according to the Common Toxicity Criteria version 3.0. Toxicities were defined as adverse events that were deemed possibly, probably, or definitely related to study treatment. DLTs were defined as failure to deliver greater than 75% of the planned doses of TMZ and CCI-779 due to toxicity or the following specific toxicities: ≥ grade 3 diarrhea, ≥ grade 3 skin rash, ≥ grade 4 neutropenia, leukopenia, or thrombocytopenia, ≥ grade 4 hypertriglyceridemia or hypercholesterolemia, ≥ grade 3 other non-hematological adverse events, or ≥ grade 4 radiation dermatitis. The MTD was defined as the highest dose level at which only 0 or 1 of 6 patients developed DLTs. Once the MTD was defined, an additional 10 patients were enrolled to further establish the tolerability of the regimen.
RT and TMZ were delivered according to the established standard of care. A total dose of 60 Gy in 30 fractions was delivered to partial brain fields using 3-dimensional conformal radiotherapy as described previously.(7) During RT, TMZ was delivered by mouth daily at 75 mg/m2. Adjuvant TMZ was delivered on days 1–5 every 28 days for 6 cycles at 200 mg/m2. The dose of RT was fixed, but dosing of TMZ was adjusted for toxicities that were not clearly related to CCI-779.
Patient Evaluations
Patients underwent a baseline evaluation and were evaluated weekly during RT and prior to each cycle of adjuvant TMZ therapy. Baseline assessments were obtained within 21 days of registration and included history, physical and mini-mental status exam, brain MRI scan, and laboratory testing.(7) Toxicity assessments were performed weekly during RT and with every cycle of adjuvant TMZ and included repeat laboratory and imaging assessments with every other cycle of adjuvant TMZ. For the last 13 patients enrolled, blood samples were obtained for immune profiling at 4 time-points: I) pre-treatment, II) just prior to the last dose of CCI-779 during RT, III) just prior to the first cycle of adjuvant TMZ, and IV) just prior to the third cycle of adjuvant TMZ.
Immune-phenotyping from glioblastoma patients
Leukocytes in whole blood were analyzed by direct antibody staining using MultiTEST TruCOUNT Tube kits or individual antibodies (BD Biosciences, San Jose, CA).(10) Cells were quantitated using a FACSCalibur flow cytometer, and data were analyzed with Cell Quest and Multiset software (BD, Franklin Lakes, NJ).
Statistical considerations
Overall toxicity incidence and toxicity by dose level were summarized using frequency distributions and descriptive measures. The primary endpoint of the trial was MTD, and secondary endpoints included best objective status, time to progression, and changes in immune cell profile. Differences in immune profiles were tested using paired Wilcoxon signed-rank tests comparing each time-point to the pretreatment level.
RESULTS
Patient characteristics
Between July 2006 and August 2009, 27 patients were enrolled on this clinical study. As detailed below, 1 patient received the first dose of CCI-779 and subsequently died from infection prior to initiating RT/TMZ, and CCI-779 was discontinued during cycle 1 for a second patient who was diagnosed with chronic lymphocytic leukemia after initiation of therapy. Both patients were replaced since the toxicity of combined CCI-779, temozolomide and radiation could not be assessed. The patient characteristics of the 25 analyzable patients are shown in Table 1. Excluding those patients who have died, the median follow-up duration is 10.2 months.
Table 1.
Patient characteristics
| (N=25) | |
|---|---|
| Age, median(range) | 58 (46,71) |
| Gender | |
| Female | 10 (40%) |
| Male | 15 (60%) |
| Performance Score | |
| 0 | 8 (32%) |
| 1 | 17 (68%) |
| Corticosteroid Therapy | |
| Yes | 13 (52%) |
| No | 12 (48%) |
| Extent of Resection | |
| Biopsy | 5 (20%) |
| Subtotal Resection | 8 (32%) |
| Gross Total Resection | 12 (48%) |
| Laterality | |
| Right | 11 (44%) |
| Left | 14 (56%) |
Toxicities
The first 12 patients enrolled on this trial received weekly CCI-779 during RT/TMZ and during adjuvant TMZ treatment. An additional 13 patients (all at dose level 1) were treated with weekly CCI-779 only during RT/TMZ. None of the 3 patients treated at Dose level 0 (25 mg/week) nor the 3 patients treated at Dose level 1 (50 mg/week) experienced DLTs during RT. At Dose level 2 (75 mg/week), 2 of 6 patients experienced DLTs with less than 75% of the planned CCI-779 doses delivered due to toxicities in both patients. One patient had grade 2 dysgeusia and grade 3 hypercholesterolemia and hypertriglyceridemia despite medical management, and another patient had grade 2 thrombocytopenia, grade 3 thrombosis, and grade 4 dyspnea related to pulmonary embolus. Thus, treatment was well tolerated at doses of 50 mg CCI-779 per week or lower during the toxicity assessment window of RT/TMZ/CCI-779.
Three fatal infection-related toxicities were observed outside the treatment window of concomitant RT/TMZ/CCI-779, which necessitated significant changes to the treatment regimen. After a patient died from Pneumocystis carinii pneumonia (PCP) on Dose level 0 during the 2nd cycle of adjuvant TMZ/CCI-779, antibiotic prophylaxis with sulfamethoxazole/trimethoprim (Bactrim) or inhaled pentamidine was required throughout treatment. A second patient on Bactrim on dose level 2 developed a retroperitoneal abscess and died from gram negative sepsis after their first dose of CCI-779 before starting RT/TMZ. This infection was attributed to a presumed pre-existing condition, the patient was replaced, and no changes to the protocol were made. A third patient on dose level 2 died during the 4th cycle of adjuvant TMZ and CCI-779 (75 mg/week). This patient discontinued Bactrim prophylaxis a few days prior to presenting with Hemophilus influenza pneumonia and sepsis, and he died following initiation of comfort measures. All 3 patients were on oral dexamethasone (4 mg, 12 mg and 5 mg per day, respectively). After the third death, accrual on the trial was halted for a thorough safety review.
Accrual was resumed following significant alteration to the treatment regimen in order to minimize infectious risks with combined therapy. First, antibiotic prophylaxis with coverage of both PCP and gram-negative organisms was mandated, and experimental treatment with CCI-779 was discontinued if patients could not tolerate prophylaxis. Second, treatment with CCI-779 was limited to 7 weeks, starting 1 week prior to and continuing through the completion of TMZ/RT; CCI-779 was not given in combination with adjuvant TMZ. Third, the dose of CCI-779 was reduced to 50 mg/week. Three additional patients were accrued at this dose level, and 1 of these patients experienced a DLT of Grade 3 fatigue. Thus, with 1 of 6 patients overall experiencing a DLT at Dose level 1, 50 mg/week of CCI-779 was defined as the MTD. As planned, an additional 10 patients then were accrued at dose level 1. Of these 10 patients, 1 patient had Grade 4 hyperlipidemia. A patient with pre-existing onychomycosis developed a Grade 3 rash during CCI-779/RT/TMZ therapy, which was biopsy proven to be dermatophytosis. Another patient developed herpes zoster just after completing RT/TMZ/CCI-779 therapy. Both of these patients were on dexamethasone. Two other patients had grade 3 hyperglycemia and grade 3 lymphopenia, respectively. Thus, this modified treatment regimen was well tolerated without further life-threatening infections, although 2 infectious complications were observed.
A summary of Grade 3, 4, and 5 adverse events potentially related to therapy with TMZ, CCI-779 or radiation therapy is provided in Table 2. For the initial cohort of 12 patients treated with CCI-779 throughout therapy, the most common serious adverse events were hyperlipidemia, thrombocytopenia, leukopenia, while in those patients with CCI-779 treatment limited to RT/TMZ (n=13), the incidence of lipemic and hematologic toxicities appeared lower. In addition to hyperlipidemia and myelosuppression, other common grade 1 or 2 adverse events included fatigue, alopecia, nausea, and anorexia (data not shown). Thus, with the exception of the bacterial infectious complications, treatment with CCI-779, RT and TMZ followed by adjuvant TMZ monotherapy was tolerated well with the expected hematologic and hyperlipemic complications.
Table 2.
Maximum Grade Events Related to Therapy
| Cycles 1–2 | Cycles 3–8 | |||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| Initial regimen (n=12) | 3 | 4 | 5 | 3 | 4 | 5 |
| Elevated ALT | 1 | |||||
| Elevated AST | 1 | 1 | ||||
| Dyspnea | 1 | |||||
| Hyperglycemia | 1 | |||||
| Hypercholesterolemia | 1 | 2 | ||||
| Hypertriglyceridemia | 1 | 1 | 1 | |||
| Leukopenia | 4 | |||||
| Neutropenia | 3 | |||||
| Thrombocytopenia | 1 | 2 | 1 | |||
| Pneumonia | 1 | |||||
| Thrombosis | 1 | 1 | ||||
| Vomiting | 1 | |||||
| Sepsis | 1 | |||||
| Revised regimen (n=13) | ||||||
| Rash/dermatophytosis | 1 | |||||
| Fatigue | 1 | |||||
| Hyperglycemia | 1 | |||||
| Hypercholesterolemia | 1 | |||||
| Hypertriglyceridemia | 1 | |||||
| Leukopenia | 1 | 1 | ||||
| Lymphopenia | 1 | 2 | ||||
| Neutropenia | 1 | |||||
| Thrombocytopenia | 1 | |||||
Cycles 1–2 refers to concomitant RT/TMZ and the subsequent rest-period.
Cycles 3–8 refer to the 6 cycles of adjuvant TMZ.
Survival and progression
The disease status for the 25 patients treated on this trial is shown in Table 3. The best patient response obtained during treatment included 24 stable disease, and 1 progressive disease. Five patients remain on active therapy, and of those no longer on treatment, 11 patients discontinued due to disease progression or death, 1 patient discontinued due to an intercurrent illness (PCP), and 8 patients completed study per protocol. Twelve of 25 patients have died to date, and the median survival for these 12 patients is 13.3 months (95% CI: (7.1, 18.3)). For the 13 patients that remain alive, the median follow-up is 10.2 months (range: 4.4, 39). Given the limited number of patients and short follow-up, no definitive conclusions can be drawn regarding efficacy of the regimen.
Table 3.
Disease status
| (n=25) | |
|---|---|
| Number cycles received, median(range) | 8 (3, 16) |
| Follow-up Status | |
| Alive | 13 (52%) |
| Dead | 12 (48%) |
| Progression Status | |
| No Progression | 8 (32%) |
| Progression | 17 (68%) |
| Best Response | |
| SD | 24 (96%) |
| PD | 1 (4%) |
| Off Active Treatment | |
| Yes | 20 (80%) |
| No | 5 (20%) |
| Reason End Treatment (% out of 20) | |
| Completed Study Per Protocol | 8 (40%) |
| Disease Progression | 10 (50%) |
| Other Medical Problems | 1 (5%) |
| Died on Study | 1 (5%) |
Immune monitoring
Immune profiling was performed for 13 patients with CCI-779 therapy limited to concomitant TMZ/RT. As compared to baseline, levels of circulating CD3+ T-cells were lower at all subsequent time-points (Figure 2A; 46 – 74% of baseline levels, p<0.05 for each time vs. baseline). Similarly, median levels of CD4+ T cells, as compared to baseline (median 836 cells/µl), were markedly suppressed at each time point (387, 390, and 471 cells/µl, respectively: p<0.05 for each time versus baseline) (Figure 2B). CD8+ cytotoxic T-cells were similarly suppressed from baseline (279 cells/µl) at the first two time points (102 and 138 cells/µl, respectively; p<0.05 vs. baseline) but recovered to pre-treatment levels by the last time-point (Figure 2C). The ratio of regulatory T cells (Tregs; CD4+CD25+CD127lo) was significantly elevated only at the end of CCI-779 therapy (15.4% vs. baseline of 4.6%, p=0.03) and also returned towards baseline by the last time-point 3 months following discontinuation of CCI-779 (10.0%; Figure 2D). These data demonstrate a robust suppression of both helper and cytotoxic T-cells and elevation of regulatory T cells during CCI-779/RT/TMZ therapy with recovery of baseline levels in the regulatory and CD8+ cytotoxic T-cells during adjuvant TMZ treatment approximately 3 months after discontinuation of CCI-779.
Figure 2. Therapy induced T cell depletion.
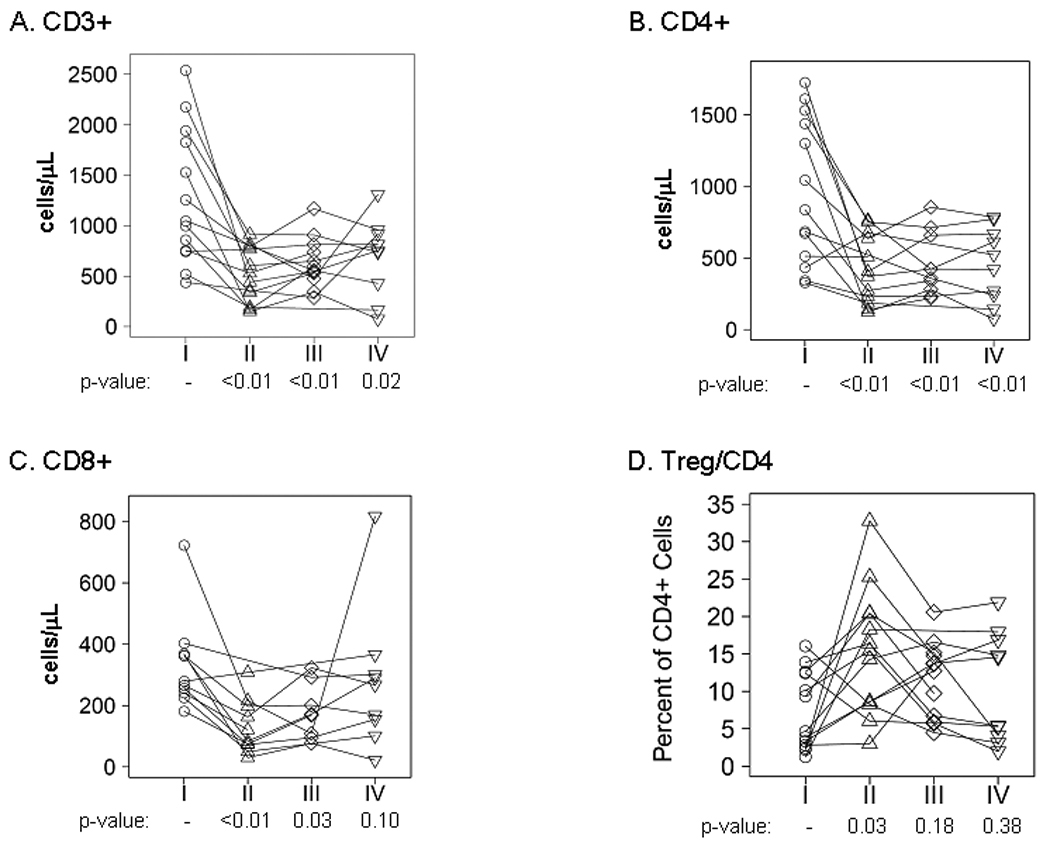
Peripheral blood mononuclear cells (PBMCs) were isolated and analyzed by flow cytometry at the 4 time points indicated in Figure 1B (time points I–IV). Absolute levels for A) CD3+ T cells, B) CD4+ T-cells, C) CD8+ T-cells, and D) the ratio of Tregs to CD4+ T cells are shown at each time point. Each symbol corresponds with an individual patient sample, and the results for each individual patient are connected by a line. The p-values are shown for a comparison to baseline at each time point.
The integrity of the B-cell, natural killer, and monocytic compartments also were assessed. As seen in Figure 3A, CD19+ B cells were significantly suppressed from baseline (212 cells/µl) at all 3 time-points during therapy (103, 62, and 70 cells/µl, respectively; p<0.05 at each time point versus baseline). In contrast, natural killer cells (CD56+CD16+; NK cells) were suppressed from baseline (146 cells/µl) only during CCI-779/RT/TMZ treatment (76 cells/µl; p=0.009) and returned to baseline levels at both subsequent time-points (Figure 3B). Finally, the levels of circulating monocytes, relative to total leukocytes, and the ratios of monocytes positive for HLA-DR and CD86 were suppressed at baseline, as compared to normal healthy donors, but these levels did not change during the course of therapy (data not shown). Thus, combined therapy with CCI-779, TMZ and RT had profound effects on distinct compartments within the immune system, but CD8+ cells and Tregs, but not CD4+ cells recovered to baseline levels during adjuvant TMZ following discontinuation of CCI-779.
Figure 3. Therapy induced NK cell and profound B cell depletion.
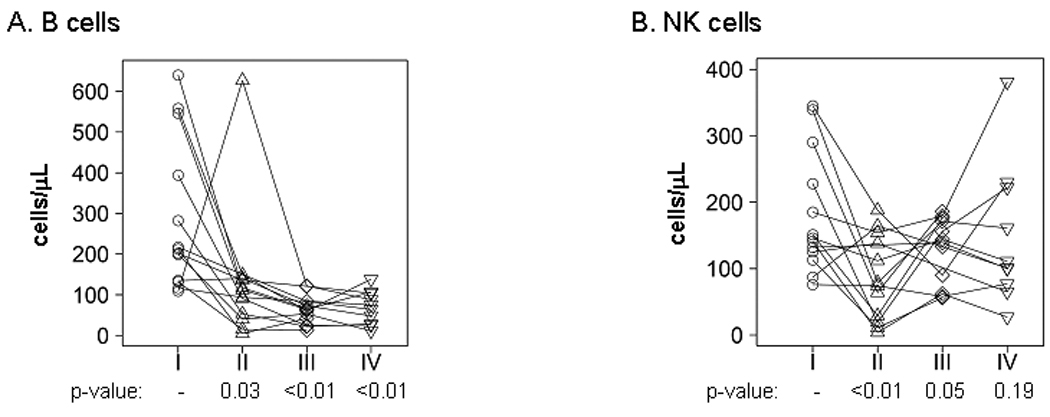
As in Figure 2, PBMCs were analyzed by flow cytometry at 4 time points (I–IV). Absolute levels for A) CD19+ B cells and B) CD56+CD16+ natural killer cells are shown at each time point. Each symbol corresponds with an individual patient sample, and the results for each patient are connected by a line. The p-values are shown for a comparison to baseline at each time point.
DISCUSSION
Preliminary pre-clinical data demonstrate that mTOR signaling can drive GBM tumor cell proliferation, angiogenesis and cell survival, and disruption of these effects with mTOR inhibitor therapy may enhance the efficacy of standard chemo-radiotherapy. Therefore, the safety of CCI-779 combined with concomitant RT/TMZ and adjuvant TMZ was tested in this phase I clinical trial of patients with newly diagnosed GBM. While the combination was relatively well tolerated during concomitant RT/TMZ, there was an excessive risk of severe infections during adjuvant TMZ/CCI-779 therapy. Consequently, antibiotic prophylaxis for both PCP and gram negative organisms was mandated throughout the period of treatment with CCI-779. Moreover, based on previous pre-clinical studies with mTOR inhibitors demonstrate significant radiosensitizing effects in animal models (4, 5), the trial design was modified to limit the duration of CCI-779 therapy 7 weeks during concomitant RT and TMZ. With this modified regimen, no further severe infections were observed. While previous studies in recurrent GBM used temsirolimus monotherapy at 170 to 250 mg/week (3, 11), more recent studies have demonstrated anti-tumor activity and drug levels sufficient for inhibition of mTOR signaling at doses of 25 mg/week (12–14). These data suggest that a dose 50 mg CCI-779 weekly will provide robust inhibition of mTOR activity. Thus, the recommended phase 2 dose and schedule of intravenous temsirolimus in newly diagnosed GBM patients is 50 mg/week combined only during concomitant radiation and TMZ.
The rate of infectious complications on this trial far exceeded the expected rate with standard radio-chemotherapy or CCI-779 monotherapy. Including the patient replaced after developing sepsis with the first dose of CCI-779, 6 patients (of 27 total accrued, 22%) developed an infection. Three of these patients developed lethal infections (PCP, retroperitoneal abscess, and pneumonia, respectively) and a fourth developed grade 3 pneumonia. Of specific note, 2 of these patients developed infections during adjuvant TMZ therapy, when antibiotic prophylaxis has not been routinely required in previous trials.(15) This rate of fatal or potentially life-threatening bacterial infections is much higher than that reported on the randomized EORTC/NCIC trial, in which 21 of 287 (7%) patients treated with RT/TMZ and adjuvant TMZ developed serious infections without any fatal infections.(15) Similarly, in a review of 26 other CTEP-sponsored clinical trials involving 1006 patients treated with CCI-779, there was a 4% rate of Grade 3 (no Grade 4/5) infections. All 4 patients with bacterial infections on the current trial were on at least 4 mg/day of dexamethasone, which likely contributed to their propensity to develop infection. However, no serious infections were observed in 2 clinical trials of CCI-779 monotherapy for recurrent GBM patients, many of whom would have been on dexamethasone.(3, 11) Thus, the data presented suggest an elevated risk for bacterial infections with protracted dosing of CCI-779 in combination with TMZ-based chemo-radiotherapy in GBM patients.
The risk of serious bacterial infections was ameliorated by limiting the duration of combination therapy and mandating prophylactic antibiotics. Although none of the 13 patients treated on the modified protocol developed bacterial infections, 2 patients developed exacerbation of a pre-existing viral or fungal infection, respectively. In a similar trial, reactivation of latent viral infections was observed with a similar mTOR inhibitor everolimus (RAD001) combined with chemoradiotherapy in newly diagnosed GBM patients (NCCTG N057K, unpublished data). This elevated rate of viral and fungal opportunistic infections is similar to observations in patients treated with protracted TMZ dosing regimens for glioma, melanoma, and neuroendocrine tumors.(16–18) Acknowledging the limited numbers of patients treated on this trial, the data suggest that the combination of CCI-779, radiation and TMZ in GBM patients may increase the risk of viral and fungal opportunistic infections in comparison to TMZ and radiation alone.
This study reports the most comprehensive analysis of immune dysfunction in a n y cancer patient population treated with either an mTOR inhibitor or temozolomide-based therapies. Combined therapy with CCI-779/TMZ/RT was associated with profound suppression of cellular, humoral and innate immunity. While B-cell and CD4+ T-cell levels remained suppressed throughout the monitoring period, levels of CD8+ cytotoxic T-cells, regulatory T-cells, and NK cells returned to pre-treatment levels during adjuvant TMZ following discontinuation of RT and CCI-779. Significant CD4+ lymphopenia has been commonly observed in GBM patients treated with RT alone or RT and TMZ, and protracted TMZ dosing was associated with a selective CD4+ and CD19+ lymphopenia without suppression of NK cells or CD8+ lymphocytes in melanoma patients.(18–21) Disruption of PI3K/Akt/mTOR signaling in peripheral CD4+ cells promotes differentiation into Tregs, and mTOR inhibitor therapy is associated with selective expansion of the Treg compartment.(22–25) mTOR inhibitor therapy also is associated with suppression of the proliferative capacity and cytolytic effects of NK cells.(26, 27) Importantly, lymphopenia has been observed in untreated GBM patients, and dexamethasone therapy can exacerbate lymphopenia and increase levels of immune suppressive monocytes, but has no effects on B cells, NK cells, and Tregs (28). In the context of an already immunosuppressive background, these data suggest that the combined immunosuppressive effects of TMZ (suppression of CD4+ helper cells and CD19+ B cells) and CCI-779 (suppression of NK cells and CD8+ cytotoxic T-cells and elevation of immunosuppressive regulatory T-cells) likely contributed to the elevated risk of opportunistic infections observed.
The PI3K/Akt/mTOR pathway is deregulated in the majority of cancers, and there is intense interest in integrating inhibitors of this pathway with cytotoxic chemotherapies in GBM and other tumor types. Although much less is known clinically about the influence of these next-generation small molecule inhibitors on immune function, PI3K activity is critical for T- and B-cell activity.(29) Similar to sirolimus therapy, disruption of PI3K signaling can promote Treg expansion,(30) and PI3K is a critical mediator of NK cell cytotoxicity.(31, 32) These data suggest that second generation PI3K/mTOR inhibitors also may have pleiotropic effects on cellular, humoral and innate immunity and may place patients at increased infectious risks when combined with other immunosuppressive chemotherapies such as TMZ. Thus, future clinical trials testing such potentially synergistic immunosuppressive regimens, in newly diagnosed GBM or other patient populations, should incorporate methods to monitor and ameliorate the potential for increased infectious risks.
Statement of Translational Relevance
The PI3K/mTOR signaling pathway is hyper-activated in a significant proportion of GBM, and there is significant interest in using small molecule inhibitors of this pathway to enhance the efficacy of therapies for GBM. In this study, the mTOR inhibitor temsirolimus, combined with radiation and temozolomide, was associated with a elevated risk of infection and additive immunosuppressive effects. Because next generation PI3K/mTOR inhibitors may have similar immunosuppressive effects, the data presented suggests that specific chemotherapy combinations with novel PI3K/mTOR inhibitors also may place patients at risk for profound immune suppression and severe infectious complications.
Acknowledgments
The authors are grateful to the expert clinical support provided by Sue Steinmetz and Debra Sprau, protocol development support by Janis Wobschall, and biostatistical support by Sara Felton and Keith Anderson, and technical support from Amy L. Mohr and Mary L. Maas from the Human Cellular Therapy Laboratory.
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants: CA-25224, CA-114740, and Brain SPORE CA-108961 from the National Cancer Institute, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
REFERENCES
- 1.Kahan BD. Fifteen years of clinical studies and clinical practice in renal transplantation: reviewing outcomes with de novo use of sirolimus in combination with cyclosporine. Transplant Proc. 2008;40:S17–S20. doi: 10.1016/j.transproceed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Webster AC, Lee VWS, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 3.Galanis E, Buckner JC, Maurer MJ, et al. Phase II Trial of Temsirolimus (CCI-779) in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24:5414–5422. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- 5.Eshleman J, Carlson B, Mladek A, Kastner B, Shide K, Sarkaria J. Inhibition of the Mammalian Target of Rapamycin Sensitizes U87 Xenografts to Fractionated Radiation Therapy. Cancer Res. 2002;62:7291–7297. [PubMed] [Google Scholar]
- 6.Sinnberg T, Lasithiotakis K, Niessner H, et al. Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol. 2009;129:1500–1515. doi: 10.1038/jid.2008.379. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan S, Brown PD, Ballman KV, et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int J Radiat Oncol Biol Phys. 2006;65:1192–1199. doi: 10.1016/j.ijrobp.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- 9.Von Hoff DD, Kuhn J, Clark GM. Design and conduct of phase I trials. In: Buyse ME, Staquet MJ, Sylvester RJ, editors. Cancer clinical trials; method and practice. Oxford: Oxford University Press; 1984. pp. 210–220. [Google Scholar]
- 10.Gustafson MP, Lin Y, New KC. Systemic immunosuppression in glioblastoma: the interplay between CD14+HLA-DRneg monocytes, tumor factors, and dexamethasone. NeuroOncology. 2010 doi: 10.1093/neuonc/noq001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 12.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77:76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized Phase II Study of Multiple Dose Levels of CCI-779, a Novel Mammalian Target of Rapamycin Kinase Inhibitor, in Patients With Advanced Refractory Renal Cell Carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 15.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzberg AB, Stover EH, Sengupta T, et al. Selective lymphopenia and opportunistic infections in neuroendocrine tumor patients receiving temozolomide. Cancer Invest. 2007;25:249–255. doi: 10.1080/07357900701206380. [DOI] [PubMed] [Google Scholar]
- 17.Tosoni A, Cavallo G, Ermani M, et al. Is protracted low-dose temozolomide feasible in glioma patients? Neurology. 2006;66:427–429. doi: 10.1212/01.wnl.0000196465.83423.ec. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Su YB, Sohn S, Krown SE, et al. Selective CD4+ Lymphopenia in Melanoma Patients Treated With Temozolomide: A Toxicity With Therapeutic Implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Grossman SA, Desideri S, Ye X, et al. Iatrogenic immunosuppression in patients with newly diagnosed high-grade gliomas. J Clin Oncol (Meeting Abstracts) 2007;25:2012. [Google Scholar]
- 20.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 21.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro-Oncology. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo M-G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia M, Stabilini A, Roncarolo M-G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 24.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE [Electronic Resource] 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nature Reviews. 2009;9:324–337. doi: 10.1038/nri2546. Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wai L-E, Fujiki M, Takeda S, Martinez OM, Krams SM. Rapamycin, but not cyclosporine or FK506, alters natural killer cell function. Transplantation. 2008;85:145–149. doi: 10.1097/01.tp.0000296817.28053.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo H, Chen H, Daloze P, Wu J. Effects of rapamycin on human HLA-unrestricted cell killing. Clin Immunol Immunopathol. 1992;65:60–64. doi: 10.1016/0090-1229(92)90248-m. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson MP, Lin Y, New KC, et al. Systemic immunosuppression in glioblastoma: the interplay between CD14+HLA-DRneg monocytes, tumor factors, and dexamethasone. NeuroOncology. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 30.Bruno L, Merkenschlager M. Directing T cell differentiation and function with small molecule inhibitors. Cell Cycle. 2008;7:2296–2298. doi: 10.4161/cc.6444. [DOI] [PubMed] [Google Scholar]
- 31.Zebedin E, Simma O, Schuster C, et al. Leukemic challenge unmasks a requirement for PI3Kdelta in NK cell-mediated tumor surveillance. Blood. 2008;112:4655–4664. doi: 10.1182/blood-2008-02-139105. [DOI] [PubMed] [Google Scholar]
- 32.Jiang K, Zhong B, Gilvary DL, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nature Immunology. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]


