Abstract
Our previous data demonstrated that folate receptor β (FR-β) targeted liposomal doxorubicin (FT-L-DOX) showed enhanced cytotoxicity relative to non-targeted liposomal doxorubicin (CON-L-DOX), and the effect was enhanced by selective FR-β upregulation by all-trans retinoic acid (ATRA) in AML blast cells. In this study, the enhanced cytotoxicity was investigated in the proliferating human AML clonogenic cells by combining FT-L-DOX with ATRA. Also, pharmacokinetic properties by pretreatment of ATRA were evaluated using FR-targeted liposomal calcein (FT-L-Calcein). Pharmacokinetic study showed that the area under the concentration curve (AUC) of FT-L-Calcein was decreased and total clearance was increased by pretreatment with ATRA. Meanwhile, the volume of distribution was significantly increased by pretreatment of ATRA. Moreover, calcein level in the liver, spleen and kidney was increased following intravenous administration of FT-L-Calcein by pretreatment of ATRA. In vitro cytotoxicity of FT-L-DOX was higher than that of CON-L-DOX and was increased by pretreatment with ATRA. Colony formation in AML cells was lower due to treatment with FT-L-DOX compared with CON-L-DOX and colony formation further decreased upon pretreatment with ATRA. Moreover, FT-L-DOX was more toxic to AML clonogenic cells than to AML blast cells. The results demonstrate that the efficiency of FR-mediated targeting of FT-L-DOX was preferentially enhanced by ATRA induced FR-β upregulation in AML clonogenic cells.
Keywords: Folate receptor, liposomes, doxorubicin, all-trans retinoic acid, acute myeloid leukemia, clonogenic cell, targeted drug delivery
1. Introduction
Acute myelogenous leukemia (AML) is a clonal disorder involving a hierarchy of leukemic cells that differ in their phenotypic characteristics and proliferation potential. Similar to normal hematopoietic stem cells, leukemic stem cells supposedly reside within the CD34+/CD38- fraction of the leukemic clone (Bonnet and Dick, 1997; Lapidot et al., 1994). They are characterized by indefinite self-renewal and give rise to a population of extensively proliferating progenitor cells which produce the vast pool of aberrantly differentiated and arrested blasts (Passegué et al., 2003; Jordan and Guzman, 2004). Thus, the efficiency of any molecular therapy will ultimately depend on the treatment's ability to eradicate the leukemic stem and progenitor cell compartment.
Standard cytarabine and anthracycline-based chemotherapy results in approximately 70% complete remission rate and 30-40% long-term survival in AML patients (Bishop, 1999). However, treatment with drugs such as anthracycline, is associated with severe side effects such as myelosuppression and dose-limiting cardiotoxicity and also with a high incidence of relapse (Hortoagyi, 1997). Relapsed disease is frequently refractory to chemotherapy due to multidrug resistance (MDR) (Hortoagyi, 1997; List, 1997). Moreover, it has been reported that relapse of AML is associated with survival of leukemic stem cells and progenitor cells (Sperr et al., 2004). Liposomal delivery of anthracycline drugs has been shown to overcome drug efflux in resistant AML cells (Michieli et al., 1999a; Michieli et al., 1999b; Booser et al., 1994). In addition, liposomal drug delivery could selectively target malignant tissues (Pan and Lee, 2004). Therefore, therapeutic strategies for targeting leukemic stem cells or progenitor cells by tissue-targeted liposomal vehicles are promising improvements in the treatment of AML (Taussig et al., 2005; Hong et al., 1999).
Human folate receptor (FR) type α and type β are high-affinity folate binding proteins with a glycosyl phosphatidylinositol (GPI) anchor (Kamen and Smith, 2004; Yan and Ratnam, 1995). Because of their selective expression in solid tumors and in leukemia, these receptors have been investigated as cellular markers for targeted drug delivery (Salazar and Ratnam, 2007; Jackman et al., 2004). Expression of FR-β in normal tissues is restricted to placenta and hematopoietic cells, where it is expressed in the myelomonocytic lineage with an increase in its level of expression during neutrophil maturation or monocyte/macrophage activation (Ross et al., 1999; Nakashima-Matsushita et al., 1999). However, FR-β in neutrophils are unable to bind folate due to aberrant post-translational modifications (Nakamura et al., 2002). FR-β is expressed in approximately 70% of the cases of acute AML blast cells and is frequently co-expressed with CD34 (Pan et al., 2002), a common marker used to enriched populations of human hematopoietic stem cells (HSCs) and progenitors. FR-β is also expressed in bone marrow mononuclear cells of human AML engrafted NOD/SCID mice (Blaser et al., 2007), suggesting that FR-β is expressed in the AML progenitor cells and/or the AML stem cells. In addition, the expression of FR-β can be specifically upregulated by all-trans-retinoic acid (ATRA) in FR-β (+) KG-1 AML cells, MV4-11 AML cells, and primary AML cells (Pan et al., 2002; Blaser et al., 2007; Wang et al., 2000; Qi and Ratnam, 2006). Moreover, enhanced cytotoxicity of FR-targeted liposomal doxorubicin relative to non-targeted control liposomes was further enhanced by selective FR-β upregulation using ATRA (Pan et al., 2002; Lu et al., 2007).
In the present study, we aimed to investigate the targeting of FT-L-DOX on proliferating AML clonogenic cells after pretreatment with ATRA. The targeting efficiency was evaluated by MTT and colony forming unit (CFU) assay. The CFU assay was used to indentify a subpopulation of AML cells that comprise a proliferating pool of leukemic cells that presumably include broadly defined progenitors of leukemic blasts. In addition, it was suggested that prolonged blood circulation time of FT-L-DOX may be important to leukemia cell targeting in vivo (Els et al., 1998; Duncan, 2006). Therefore, the effect of ATRA pretreatment on the pharmacokinetics and tissue distribution was also investigated.
2. Materials and methods
2.1 Reagents
ATRA, folic acid, cholesterol, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT), calcein and doxorubicin hydrochloride (DOX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Iscove's modified Dulbecco's medium (IMDM) and MethoCult™ GF H4434 were obtained from StemCell technology Inc. (Vancouver, Canada). Interleukin 3 (IL-3), human stem cell factor, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were purchased from StemCell Technologies Inc. Hydrogenated phosphatidylcholine from soybean (HSPC) and methoxy-polyehtylene glycol (MW=2000) distearoyl phosphatidylethanolamine (mPEG-DSPE) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Folate-polyethylene glycol (MW=3350)-cholesterol hemisuccinate (F-PEG-CHEMS) was synthesized as described previously (Xiang et al., 2007). All other chemicals used were of analytical grade.
2.2. Cell culture and treatment with ATRA
MV4-11 (human acute myelocytic leukemia cell line), K562 (human erythromyeloblastoid leukemia cell line) and KB (human epidermoid carcinoma cell line derived from HeLa) cells were purchased from the American Type Culture Collection (Rockville, MD, USA). K562 and KB cells were grown in folate-free RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS, Gibco-BRL, Grand Island, NY, USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C in a 5% CO2/95% air humidified atmosphere. MV4-11 cell were grown in folate-free medium containing 20% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. To determine ATRA effect on the upregulation of FR-β expression, cells were treated with 1μmol/L ATRA for 5 days.
2.3. Treatment of AML patient cells with ATRA
AML patient cells were obtained from OSU Leukemia Tissue Bank. Each of the patients signed an informed consent to storing and using his/her leukemia tissue for discovery studies according to institutional guidelines from OSU. Fresh AML samples were fractionated by Ficoll-Hypaque (Nygaard, Oslo, Norway) gradient centrifugation and grown in folate-free RPMI 1640 medium containing with 20% of human serum with the inclusion of 20 ng/ml of interleukin 3 (IL-3), 20 ng/ml of human stem cell factor, and 10 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) at 37°C. For the FR-β upregulation, the cells were treated with ATRA for 24 hr (Qi and Ratnam, 2006).
2.4. Reverse transcription polymerase chain reaction (RT-PCR) analysis for FR-β mRNA
Total RNA from K562, MV4-11 and AML patient cells were treated with vehicle or ATRA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) reagent. Real-time RT-PCR was used to measure endogenous mRNAs for FR-β as well as 36B4 for control in the same sample. The reverse transcription step was carried out using the SuperScript™ III First-Strand Synthesis System from Invitrogen (Carlsbad, CA, USA) following the manufacture's protocol. One microliter of the resulting cDNA was added to 20 μl PCR reaction 10 μL of SYBR containing green PCR Mastermix (Applied Biosystems, USA) and FR-β or 36B4 (acidic ribosomal phosphoprotein) primer. For FR-β, forward primer (GACTGAACTCAGCCAAGGAGCCAGAGTT) and reverse primer (AGAAAGACATGGTCTGGAAATGGATG) were used. PCR amplification was as follows: 2 min at 50 °C, then 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 second, at 60 °C for 1 min second and finally at 72°C for 90 second. Fluorescence data generated were monitored and recorded by the Gene Amp 5700 sequence detection system (Applied Biosystems, USA). All data were set up in triplicate and normalized to 36B4 housekeeping gene.
2.5. Liposome preparation
Liposomes containing DOX were prepared by polycarbonate membrane extrusion methods following by pH-gradient remote loading, as described previously (Mayer et al., 1990; Pan et al., 2003). The lipid composition of CON-L-DOX was HSPC/cholesterol/mPEG-DSPE at molar ratios of 55:40:5. The composition of FT-L-DOX was HSPC/cholesterol/mPEG-DSPE/F-PEG-CHEMS at molar ratios of 55:40:4.5:0.5. For the liposome preparation, lipids were dissolved in chloroform and dried under vacuum. The lipid film was then hydrated with 0.25 M ammonium sulfate (NH4)2SO4 at 65 °C and extruded five times each through 0.2 and 0.1 μm pore size polycarbonate membranes on a nitrogen-driven Lipex lipid extruder (Northern Lipids Inc. Vancouver, B.C., Canada). The (NH4)2SO4 outside of the liposomes was removed by tangential flow diafiltration against phosphate-buffered saline (PBS, pH7.4) using a Millipore Pellicon XL cartridge with a MWCO of 30 kDa. Then DOX solution was added to the empty liposomes and incubated at 65°C for 30 min to enable pH gradient-driven remote loading. Free DOX was removed by size exclusion chromatography on a Sepharose CL-4B column. For calcein encapsulated liposomes, same lipid composition of DOX was used. Calcein containing liposomes were prepared by polycarbonate membrane extrusion method, as described by previously by Lee and Low (Lee and Low, 1995). Size distribution of liposomes was determined by dynamic light scattering on a NICOMP 370 Submicron Particle Sizer (NICOMP, Santa Barbara, CA, USA). To analyze DOX and calcein concentration, liposomes were lysed with ethanol and the concentration in the lysate was determined by measuring absorption at 480 and 495nm, respectively, on a Shimadzu UV-Vis spectrophotometer.
Liposomes containing ATRA were prepared by polycarbonate membrane extrusion methods. The lipid composition of ATRA liposome was EPC/DMPG at molar ratios of 70:30. ATRA encapsulation efficiency was determined by measuring absorption at 395nm on a Shimadzu UV-Vis spectrophotometer.
2.6. Cellular uptake of liposomal calcein
Cellular uptake of liposomal formulations was assessed using MV4-11 AML cells and KB oral carcinoma cells. Approximately 106 cells were incubated with FT-L-Calcein or CON-L-Calcein in folate-free RPMI 1640 media for 2 hr at 37 °C. For receptor blocking studies, 1 mM of free folate was added to media with FT-L-Calcein. Cells were than washed 3 times with cold PBS and analyzed by flow cytometry or fluorescence microscope.
2.7. Pharmacokinetic studies of FT-L-Calcein
The effect of ATRA pretreatment on the pharmacokinetics of FT-L-Calcein was evaluated in Female imprinting control region (ICR) mice (18-22g, purchased from Harlan, USA). Before treatment with liposomal ATRA, mice were placed on a folate-deficient diet (AIN-90G, Dyets, Bethlehem, PA, USA) for at least 1 week. Mice were randomized in 2 experimental groups and treated 5 consecutive days with vehicle (control) or ATRA liposomes at the dose of 10 mg/kg. 3 day after ATRA treatment, mice were intravenously injected with FT-L-Calcein at dose of 1 mg/kg via tail vein. Blood samples were collected in heparin-containing tubes at various time points (5, 60, 240, 360, 480, 960, 1440 min). Plasma was isolated by centrifugation (10 min at 1500×g) and stored at –20 °C. Calcein was extracted with 10 % SDS and quantified by fluorescence spectrometry. The excitation and emission wavelength was 490 and 515 nm, respectively, as described previously. In all analysis, a calibration curve relating fluorescence intensity to the plasma calcein concentration was established. Using WinNonlin software, pharmacokinetic parameters were determined, including area under the curve (AUC), mean residence time (MRT), total body clearance (CL), volume in steady state (Vss) and plasma half-life for the distribution and elimination phases.
2.8 Biodistribution of FT-L-Calcein
Female ICR mice were randomized in 2 experimental groups and treated 5 consecutive days with vehicle (control) or ATRA liposomes at the dose of 10 mg/kg. Before treatment, mice were placed on a folate-deficient diet for at least 1 week. 3 day after last ATRA treatment, mice were intravenously injected with FT-L-Calcein at dose of 1 mg/kg via tail vein. After 24 hr treatment with FT-L-Calcein, the mice were humanely killed and blood was obtained by cardiac puncture. Subsequently, tissues (lung, liver, spleen and kidney) were dissected and added to the tissue digestion buffer. Tissue digestion buffer consisted of 100 mM Tris/HCl (pH 8.5), 1 mM EDTA, 0.5% sodium dodecyl sulfate, and 200 mM sodium chloride. Prior to tissue digestion, 0.1 U/mg of proteinase inhibitor was added. The tumors were homogenized with tissue homogenizer and the concentration of calcein was determined by fluorescence spectrometry.
2.9. In vitro cytotoxicity by MTT assay
In vitro cytotoxicity of liposomal DOX was determined by seeding 5×104 MV4-11, and 1×104 K562 in 96 well plates. Cells were incubated with 1:4 serial diluted free DOX, CON-L-DOX and FT-L-DOX for 2 hrs at 37 °C. After incubation, cells were washed 2 times with cold PBS and cultured for an additional 72 hrs in fresh medium. For cell viability determination, 20 μL of 5 mg/ml MTT was added to each well, and incubated for 4 hrs at of 37 °C. Cells were then sedimented by centrifugation at 1000 g for 8 min, and the formazan crystals were dissolved in 200 μL of DMSO. The absorbance was determined at 570 nm on a Dynatech MR-600 microplates reader. For receptor blocking studies, folic acid (1mM) was added to media during drug exposure.
2.10. Targeting AML clonogenic cells by CFU assay
The medium for MV4-11 cells and AML patient cells were prepared by StemCell Technologies (Vancouver, BC, Canada) according to manufacture's protocol. Cells were incubated with 50 μM of CON-L-DOX and FT-L-DOX for 2 hrs at 37 °C. The effect of different concentrations on the colony forming was also investigated in MV4-11 cells. After treatment, cells were washed 3 times with serum-free RPMI medium and harvested by centrifugation and resuspended in 2% IMDM medium. 5000 cells were plated in MethoCult™ GF H4434 medium in 35 mm Petri dishes (Falcon, Cockeysville, MD, USA) in duplicate. For the AML patient cells, 2.5×106 cells were plated in MethoCult™ GF H4434 medium in 35 mm Petri dishes. Dishes were incubated at 37 °C with 5% CO2 and 95% humidity for 10-14 days. Colonies were scored using an inverted microscope.
2.11 Statistical analysis
Data were represented as mean ± standard deviations and analyzed by 2-tailed Student's t-test using MiniTAB Program (Minitab Inc., State College, PA). p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of ATRA on the expression of FR-β in MV4-11 and K562 cells
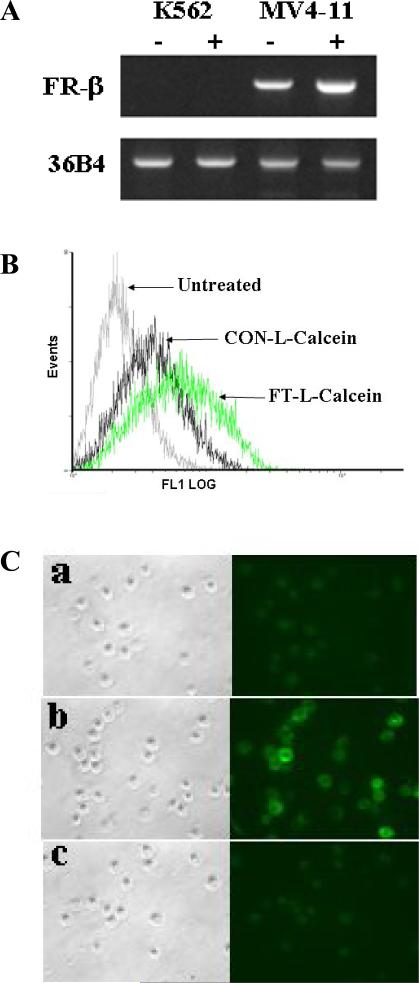
To determine the effect of ATRA on the upregulation of FR-β, FR-β positive AML cells (MV4-11) and FR-β negative AML cells (K562) were used. The FR-β mRNA expression of MV4-11 and K562 cells was determined by RT-PCR after treatment with 1μM ATRA for 5 days, previously known to produce the maximum increase in the level of expression of the endogenous FR-β. MV4-11 cells express FR-β and the expression was elevated up to 4.8-fold by treatment of ATRA. Theses results are consistent with previously reported FR-β upregulation by treatment ATRA in MV4-11 cells (Lu et al., 2007). However, the FR-β expression did not detected in K562 cells, even treated by ATRA for 5 days (Fig. 1A).
Fig. 1.
Effect of ATRA on the FR-β expression (A) and cellular Uptake of FT-L-Calcein in MV4-11 (B) and KB (C) cells. (a) CON-L-Calein, (b) FT-L-Calcein, (c) FT-L-Calcein plus 1mM free folate.
3.2. Liposome characterization
The particle size of liposomes was determined by NICOMP 370 Submicron Particle Sizer. The mean particle size used in this study was about 120 nm for all liposomes. DOX and calcein loading efficiency were determined by UV absorption at 480 and 495 nm, respectively. The results showed that DOX loading efficiency was greater than 95% and calcein loading efficiency was between 7% and 10%. ATRA encapsulation efficiency was also greater than 95%.
3.3. Effect of ATRA on the cellular uptake
The cellular uptake of FT-L-Calcein was determined in FR-β (+) MV4-11 AML cells and KB oral carcinoma cells. CON-L-Calcein was used as a non-targeted control. As shown in Fig. 1B, cellular uptake of FT-L-Calcein was significantly higher than that of CON-L-Calcein in MV4-11 cells, and the uptake of FT-L-Calcein was further increased by treatment of 1 μM ATRA. These results indicate that up-regulation of FR-β could further increase the cellular uptake of FT-L-Calcein. However, cellular uptake of FT-L-Calcein and CON-L-Calcein did not change upon treatment with 1 μM ATRA in FR-β (-) K562 cells (data not shown). In addition, significantly increased cellular uptake of FT-L-Calcein was observed in KB cells by fluorescence microscopy. The cellular uptake of FT-L-Calcein was blocked by 1 mM free folate (Fig. 1C). These data suggest that the novel ligand, F-PEG-CHEMS could be used for targeting FR-β (+) AML cells.
3.3. Pharmacokinetic profile of FT-L-Calcein following treatment with ATRA
To assess pharmacokinetic properties of FT-L-Calcein after pretreatment with ATRA, FT-L-Calcein was intravenously administrated in ICR mice. The plasma concentration profiles of calcein after intravenous injection of FT-L-Calcein are shown in Fig. 2 and pharmacokinetic parameters summarized in Table 1. Calcein in folate targeted liposomes showed a biphasic pattern with a rapid distribution phase (t1/2α=0.46 hr) and a slow terminal elimination phase (t1/2β=7.4 hr). The Area under the concentration-time curve (AUC) of calcein in FT-L-Calcein was significantly decreased by pretreatment with ATRA (P<0.05). The mean residence time (MRT) and t1/2β of calcein was also decreased by pretreatment of ATRA (1.6- and 1.3-fold decrease, respectively). In contrast, the steady-state volume of distribution (Vss) was significantly increased by treatment of ATRA (p<0.05). In general, Folate targeted liposomes exhibited faster clearance compared to non-targeted liposomes, due to FR-β expression in the phagocytic cells of the reticuloendothelial system (RES) (Gabizon et al., 2003; Gabizon et al., 2004). Therefore, decreased AUC following treatment with ATRA might be due to increased tissue distribution via FR upregulation.
Fig. 2.
Temporal plasma profiles of calcein following intravenous administration of FT-L-Calcein at dose of 1mg/kg by pretreatment of ATRA. Each data represents the mean ± SD (n=3).
Table 1.
Pharmacokinetic parameters of calcein by pretreatment of ATRA following intravenous (i.v.) administration of FT-L-Calcein to ICR mice at a dose of 1 mg/kg.
| Group | Control | ATRA |
|---|---|---|
| AUC (ng·hr/ml) | 33.4±4.5 | 12.6±2.9* |
| t½ (hr) | 7.4±2.8 | 5.9±1.9 |
| CL (ml/hr/kg) | 29.9±12.4 | 79.4±9.1 |
| MRT (0-24hr) | 10.3±3.5 | 6.4±1.0 |
| Vss (ml/kg) | 307.1±94.0 | 506.5±101.3* |
Data represent the mean±SD (n=3).
p<0.01 compared with control.
3.4. Biodistribution of FT-L-calcein following treatment with ATRA
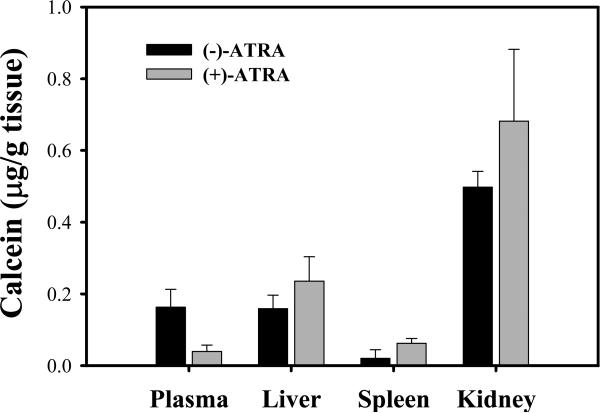
In order to investigate the effect of ATRA on the biodistribution of FT-L-Calcein, the tissue concentration of calcein was determined after 24 hr intravenously administration of FT-L-Calcein in mice. As shown in Fig. 3, calcein level in the liver and spleen was increased by pretreatment of ATRA. However, no calcein was detected in the lung. In addition, increased calcein level was observed in the kidney by pretreatment of ATRA. Therefore, decreased plasma level of calcein by treatment of ATRA might be due to the increased calcein uptake in the liver, spleen and kidney.
Fig. 3.
Tissue distribution of calcein following intravenous administration of FT-L-Calcien at dose of 1 mg/kg by pretreatment of ATRA. Each data represents the mean ± SD (n=3).
3.5 In vitro cytotoxicity by MTT assay
The cytotoxicity FT-L-DOX to AML cells and the effect of ATRA induced FR-β up-regulation on the cytotoxicity was determined by MTT assay. The half maximal inhibitory concentration (IC50) values of free DOX, CON-L-DOX, FT-L-DOX in MV4-11 and K562 cells are summarized in Table 2. In MV4-11 and K562 cells, free DOX showed the highest toxicity. In contrast, liposomal DOX showed reduced cytotoxicity in both MV4-11 and K562 cells. However, cytotoxicity of FT-L-DOX was significantly increased (3.4-fold) compared with CON-L-DOX and ATRA pretreatment further increased (6.8-fold) cytotoxicty of FT-L-DOX in MV4-11 cells. In addition, the cytotoxicity of FT-L-DOX was significantly reduced by addition of 1 mM free folate in MV4-11 cells. In FR-β (-) K562 cells, similar cytotoxicity was observed all liposome-treated groups (Table 1). These results demonstrate that increased FR-β expression will increase the cytotoxicity of FT-L-DOX.
Table 2.
Cytotoxicity of various liposomal DOX formulations to K562 and MV4-11 cells by treatment of ATRA.
| IC50 (μM) | K562 | MV4-11 | ||
|---|---|---|---|---|
| (-)ATRA | (+)ATRA | (-)ATRA | (+)ATRA | |
| Free DOX | 11.4±3.4 | 11.9±2.9 | 5.3±1.5 | 8.0±2.0 |
| CON-L-DOX | 203±18 | 206±12 | 251±15 | 242±48 |
| FT-L-DOX | 189±17 | 159±36 | 74±8a | 36±5a,b |
| FT-L-DOX+1 mM folate | 196±20 | 200±12 | 223±23 | 216±49 |
Cytotoxicity was determined using MTT assay as described in Materials and Methods. IC50 is the half maximal inhibitory concentration. Data represent the mean±SD (n=4).
p<0.01 compared with CON-L-DOX treatment.
p<0.01 compared with no ATRA treatment.
3.6. Targeting AML clonogenic cells in MV4-11 cells
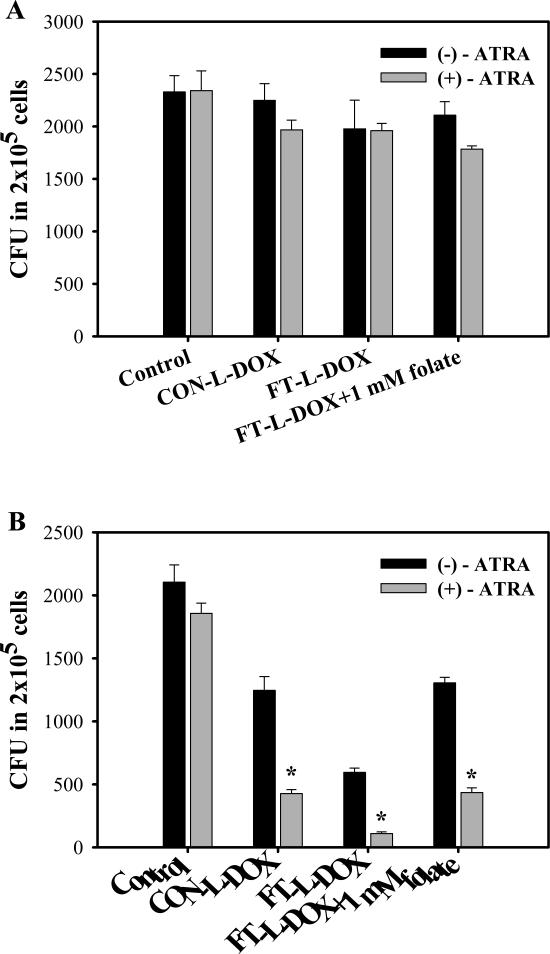
To evaluate cytotoxic effect of FT-L-DOX on the leukemic clonogenic cells, colony growth was determined by CFU assay following pretreatment with ATRA in K562 and MV4-11 cells. Both of K562 and MV4-11 cells formed CFU in methylcellulose culture and the number of CFU did not significantly change upon pretreatment with ATRA (Fig. 4). In FR-β (-) K562 cells, liposomal DOX had no effect colony formation even after pretreatment with ATRA (Fig. 4A). In FR-β (+) MV4-11 cells FT-L-DOX significantly decreased the CFU number compared with CON-L-DOX in a manner that was blocked by the addition of 1 mM free folate (Fig. 4B). A similar pattern was observed in ATRA-treated in MV4-11 cells where the effects of the liposomal treatments were more pronounced. The results suggest that FT-L-DOX targets the AML clonogenic cells through to the FR-β. However, ATRA pretreatment significantly decreased the CFU number in all the treatment groups including CON-L-DOX, FT-L-DOX and FT-L-DOX plus free folate, suggesting that ATRA may also enhance the cytotoxicity of DOX on the clonogenic cells. Nevertheless, the CFU number was more effectively decreased in FT-L-DOX group (5.4-fold decrease) than in the CON-L-DOX group (3.8-fold decrease) due to pretreatment with ATRA, presumably reflecting the effect of FR-β upregulation. Dose response studies in MV4-11 cells showed that the IC50 value of FT-L-DOX to clonogenic cells was 23.2±5.4 and 40.5±3.4 μM with or without pretreatment with ATRA, respectively. The IC50 of FT-L-DOX from the CFU assay was 1.6-fold lower than that from the MTT assay by pretreatment with ATRA. In other words, targeting efficiency of FT-L-DOX to AML clonogenic cells was more potent than AML blast. The results demonstrate that ATRA pretreatment is more effective in the clonogenic cells than in blast cells.
Fig. 4.
Effect of ATRA on colony growth in K562 (A) and MV4-11 (B) cells. Each data represents the mean ± SD (n=3). * indicates significant difference between with or without treatment of ATRA (P<0.01).
3.7. Targeting AML clonogenic cells in AML patient cells
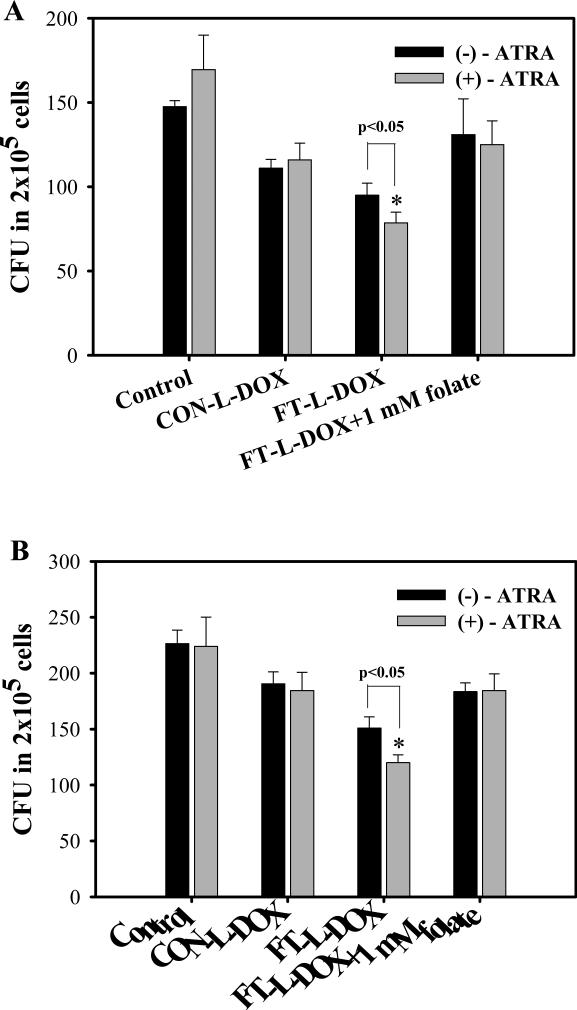
FR-β expression in AML patient cells was determined by real-time RT-PCR. Two AML patient samples, in which the leukemic cells expressed FR-β, were selected to evaluate targeting efficiency of FT-L-DOX to the AML clonogenic cells. FR-β expression in two AML patient cells was elevated 1.7- and 2.5-fold by a brief (24hr) pretreatment with ATRA. In the AML patient samples, only 0.075~0.094 % of AML cells were able to form CFU in the Methocult medium (Fig. 5). In Patient 1 AML cells, FT-L-DOX significantly decreased the number of CFU compared with CON-L-DOX and the addition of 1mM free folate blocked this decrease, indicating FT-L-DOX could be use to target AML clonogenic cells in AML patient cells. Moreover, the number of CFU in the FT-L-DOX treatment group was further decreased by pretreatment with ATRA in both the AML patient samples (Fig. 5A). These results are presumably due to ATRA induced FR-β upregulation. Similar results were observed in Patient 2 AML cells (Fig. 5B). However, in contrast to the MV4-11 cells, no additional ATRA effect was evident from the cytotoxicity of CON-L-DOX, possibly because of the relatively brief period (24h) of the ATRA pretreatment. It may also be noted that the duration of ATRA treatment was sub-optimal for FR-β up-regulation up but the results provide proof-of-principle for the utility of ATRA treatment in enhancing FT-L-DOX cytotoxicity in primary AML clonogenic cells.
Fig. 5.
Effects of ATRA on colony growth in AML patient cells. (A) Patient 1, (B) Patient 2. Each data represents the mean ± SD (n=3). * indicates significant difference between with or without treatment of ATRA (P<0.05).
4. Discussion
We have previously reported that FR-β targeted liposomal doxorubicin showed enhanced cytotoxicity relative to non-targeted liposomes, and the effect was enhanced by selective FR-β upregulation by ATRA both in vitro and in vivo (Pan et al., 2002; Lu et al., 2007). In the present study, FT-L-Calcein and FT-L-DOX were prepared by using folatepolyethylene glycol (MW=3350)-cholesterol hemisuccinate (F-PEG-CHEMS), which have better FR-targeting activity during prolonged storage compared with folate-PEG-cholesterol and folate-PEG-DSPE (Xiang et al., 2008; Zhao et al., 2007). Pharmacokinetic profile and tissue distribution of FT-L-Calcein was investigated in combination with ATRA. Calcein was chosen for the pharmacokinetic and tissue distribution studied because it is membrane impermeable and, therefore, less prone to redistribution in vivo. Furthermore, the effect of ATRA on the targeting of FT-L-DOX on AML clonogenic cells was investigated by using the colony forming unit assay.
Selective folate receptor targeting was observed in FR-positive MV4-11 and KB cells (Fig. 1B, 1C). The pharmacokinetic study have shown that the AUC was significantly decreased and total clearance was significantly increased by pretreatment of ATRA (p<0.05). Meanwhile, the volume of distribution was significantly increased by pretreatment ATRA (Table 1). These results indicated that decreased AUC following treatment with ATRA might be due to increased tissue distribution via FR upregulation. Therefore, tissue concentration was determined after intravenously administration of FT-L-Calcein. Calcein level in the liver and spleen was increased by pretreatment of ATRA (Fig. 3). Previous studies demonstrated that folate targeted liposomes had faster clearance compared to non-targeted liposomes possibly due to direct liposome uptake via the liver FR (Gabizon et al., 2003; Gabizon et al., 2004). Therfore, the increased calcein uptake in the liver by pretreatment of ATRA, might be due to the ATRA induced FR-β upregulation. Although FR-β expression in the spleen is low, ATRA induced FR-β upregulation might be contributed to the increased calcein uptake in the spleen. On the other hand, calcein level in the kidney was also increased by pretreatment of ATRA. Previous studies demonstrated that FR in the kidney should not have contributed to the accelerated clearance of FT-L-calcein, because FT-L-Calcein does not have access to the luminal side of kidney tubular cells where FR is expressed (Gabizon et al., 2003; Gabizon et al., 2004). Moreover, FR-α upregulated by ATRA or not is unclear. Recently, Nese et al. reported acute renal failure case during ATRA therapy (Nese et al., 2008). Taken together, increased calcein uptake in the kidney by pretreatment of ATRA might explained by impaired kidney function. Thus, patients should be carefully monitored for kidney function before treatment with folate targeted liposomes because the potential risk of side effect. Although the detailed mechanism needs to further investigation, the increased calcein uptake in the liver and spleen might be contributed to the decreased plasma concentration of calcein by pretreatment of ATRA.
Since maintenance of AML is dependent on a smaller population of leukemic stem cells and progenitor cells that have the ability to form colonies, it is important to test whether FT-L-DOX is able to targeting the pool of colony forming cells. Therefore, FR-mediated targeting efficiency of FT-L-DOX combined with ATRA in AML blast and clonogenic cells was determined by MTT and CFU assay. The results showed that FT-L-DOX significantly decreased the number of CFUs compared with CON-L-DOX and the CFU number was further decreased by pretreatment with ATRA in MV4-11 cells and AML patient cells (Fig. 3 & Fig.4). These results suggested that FT-L-DOX could be used to target AML clonogenic cells and that ATRA pretreatment increased the FR-targeting efficiency via selective FR-β upregulation. Interestingly, the IC50 from the CFU assay was 2-fold lower than that from MTT assay in MV4-11 cells, indicating FT-L-DOX is more toxic to AML clonogenic cells than AML blast cells.
Prolonged blood circulation and FR-β expression is an important factor for therapeutic efficacy of FT-L-DOX in AML patient. Prolonged blood circulation relative to the DOX free drug was indeed confirmed by pharmacokinetic studies. Although folate receptor targeted liposomes were distributed to normal tissues such as liver and spleen, this was mostly due to the natural RES clearance pathway for liposomes rather than FR-β expression. In contrast, 70% of AML cells expressed FR-β and the expression was upregulated by pretreatment of ATRA. Moreover, ATRA enhanced cytotoxicity of FT-L-DOX was reported in AML KG-1 and MV4-11 cells, suggesting that folate targeted liposomes might be predominantly accumulated FR-β overexpressed leukemia cells. Currently, the most effective treatment of acute promyelocytic leukemia is retinoid differentiation therapy. However, some AML cells are refractory to ATRA-differentiation. Therefore, ATRA induction of FR-β should be particularly beneficial in the therapeutic targeting of FR-β in these AML patients.
In conclusion, the pharmacokinetic profile and tissue distribution was changed by pretreatment of ATRA. Decreased plasma concentration of calcein by treatment ATRA might be due to the increase calcein uptake in the liver and spleen. Moreover, FR-mediated targeting efficiency of FT-L-DOX was enhanced by ATRA induced FR-β upregulation in AML clonogenic cells. Therefore, FR-mediated targeting is a promising strategy for treating human AML in combination with receptor induction using ATRA.
Acknowledgment
This work was supported by NIH R01grants CA095673 and CA080183, and NCI SPORE grant P50 CA140158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bishop JF. Adult acute myeloid leukemia: update on treatment. Med. J. Aust. 1999;170:39–43. doi: 10.5694/j.1326-5377.1999.tb126866.x. [DOI] [PubMed] [Google Scholar]
- Blaser B, Gonit M, Qi H, Shatnawi A, Guimond M, Lee RJ, Ratnam M. Induction of folate receptor type β in a bone marrow engraftment model of acute myelogenous leukemia. Leukemia. 2007;21:2233–2235. doi: 10.1038/sj.leu.2404786. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Booser DJ, Hortobagyi GN. Anthracycline antibiotics in cancer therapy. Focus on drug resistance. Drugs. 1994;47:223–258. doi: 10.2165/00003495-199447020-00002. [DOI] [PubMed] [Google Scholar]
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003;9:6551–6559. [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipidsanchored folic-PEG conjugates. Adv. Drug Delivery Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Hong K, Kirpotin DB, Park JW, Shao Y, Shalaby R, Colbern G, Benz CC, Papahadjopoulos D. Anti-HER2 immunoliposomes for targeted drug delivery. Ann. N. Y. Acad. Sci. 1999;886:293–296. doi: 10.1111/j.1749-6632.1999.tb09440.x. [DOI] [PubMed] [Google Scholar]
- Hortoagyi GN. Anthracyclines in the treatment of cancer: an overview. Drugs. 1997;54:1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- Jackman AL, Theti DS, Gibbs DD. Antifolates targeted specifically to the drug and gene delivery. Adv. Drug Deliv. Rev. 2004;56:1111–1125. doi: 10.1016/j.addr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML. Mechanisms controlling pathogenesis and survival of leukemic stem cells. Oncogene. 2004;23:7178–7187. doi: 10.1038/sj.onc.1207935. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Smith AK. A review of folate α cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv. Drug Deliv. Rev. 2004;54:1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim. Biophys. Acta. 1995;1233:134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- List AF. Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia. 1997;10:937–942. [PubMed] [Google Scholar]
- Lu Y, Wu J, Wu J, Gonit M, Yang X, Lee A, Xiang G, Li H, Liu S, Marcucci G, Ratnam M, Lee RJ. Role of formulation composition in folate receptor-targeted liposomal doxorubicin delivery to acute myelogenous leukemia cells. Mol. Pharm. 2007;4:707–712. doi: 10.1021/mp070058l. [DOI] [PubMed] [Google Scholar]
- Mayer LD, Tai LC, Bally MB, Mitilenes GN, Ginsberg RS, Cullis PR. Characterization of liposomal systems containing doxorubicin entrapped in response to pH gradients. Biochim. Biophys. Acta. 1990;1025:143–151. doi: 10.1016/0005-2736(90)90091-2. [DOI] [PubMed] [Google Scholar]
- Michieli M, Damiani D, Ermacora A, Masolini P, Michelutti A, Michelutti T, Russo D, Pea F. Baccarani, M. Liposome-encapsulated daunorubicin for PGP-related multidrug resistance. Br. J. Haematol. 1999a;106:92–99. doi: 10.1046/j.1365-2141.1999.01505.x. [DOI] [PubMed] [Google Scholar]
- Michieli M, Damiani D, Ermacora A, Masolini P, Michelutti A, Baccarani M. Liposome encapsulated daunorubicin doubles anthracycline toxicity in cell lines showing a non-PGP related multidrug resistance. Haematologica. 1999b;84:1151–1152. [PubMed] [Google Scholar]
- Nakamura M, Nagayoshi R, Ijiri K, Nakashima-Matsushita N, Takeuchi T, Matsuyama T. Nitration and chlorination of folic acid by peroxynitrite and hypochlorous acid, and the selective binding of 10-nitro-folate to folate receptor β. Biochem. Biophys. Res. Commun. 2002;297:1238–1244. doi: 10.1016/s0006-291x(02)02359-8. [DOI] [PubMed] [Google Scholar]
- Nakashima-Matsushita N, Homma T, Yu S. Selective expression of folate receptor β and its possible role in methotrexate transport in synovial macrophages form patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yarali N, Tavil B, Kara A, Ozkasap S, Tunç B. Acute renal failuar during ATRA treatment. Pediatr Hematol Oncol. 2008;25:115–118. doi: 10.1080/08880010801888287. [DOI] [PubMed] [Google Scholar]
- Pan X, Lee RJ. Tumour-selective drug delivery via folate receptor-targeted liposomes. Expert. Opin. Drug Deliv. 2004;1:7–17. doi: 10.1517/17425247.1.1.7. [DOI] [PubMed] [Google Scholar]
- Pan XQ, Wang H, Lee RJ. Antitumor activity of folate receptor-targeted liposomal doxorubicin in a KB oral carcinoma murine xenograft model. Pharm. Res. 2003;20:417–422. doi: 10.1023/a:1022656105022. [DOI] [PubMed] [Google Scholar]
- Pan XQ, Zheng X, Shi G, Wang H, Ratnam M, Lee RJ. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood. 2002;100:594–602. doi: 10.1182/blood.v100.2.594. [DOI] [PubMed] [Google Scholar]
- Passegué E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. USA. 2003;100:11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Ratnam M. Synergistic induction of folate receptor beta by all-trans retinoic acid and histone deacetylase inhibitors in acute myelogenous leukemia cells: mechanism and utility in enhancing selective growth inhibition by antifolates. Cancer Res. 2006;66:5875–5882. doi: 10.1158/0008-5472.CAN-05-4048. [DOI] [PubMed] [Google Scholar]
- Ross JF, Wang H, Behm FG. Folate receptor type β is neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Salazar MD, Ratnam M. The folate receptor: What does it promise in tissue-targeted therapeutics? CancerMetastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- Sperr WR, Hauswirth AW, Florian S, Ohler L, Geissler K, Valent P. Human leukaemic stem cells: a novel target of therapy. Eur. J. Clin. Invest. 2004;34:31–40. doi: 10.1111/j.0960-135X.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, Luongo JL, Danet-Desnoyers GA, Bonnet D. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106:4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten EW, Snijders SV, van Vianen W, Bakker-Woudenberg IA. Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic mice. Antimicrob. Agents. Chemother. 1998;42:2431–2433. doi: 10.1128/aac.42.9.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zheng X, Behm FG, Ratnam M. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood. 2000;96:3529–3536. [PubMed] [Google Scholar]
- Xiang G, Wu J, Lu Y, Liu Z, Lee RJ. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int. J. Pharm. 2008;356:29–36. doi: 10.1016/j.ijpharm.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ratnam M. Preferred sites of glycosylphosphatidylinositol modification in folate receptors and constraints in the hydrophobic portion of the signal. Biochemistry. 1995;34:14594–14600. doi: 10.1021/bi00044a039. [DOI] [PubMed] [Google Scholar]
- Zhao XB, Muthusamy N, Byrd JC, Lee RJ. Cholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomes. J. Pharm. Sci. 2007;96:2424–2435. doi: 10.1002/jps.20885. [DOI] [PubMed] [Google Scholar]