Abstract
Hypertension contributes significantly to worldwide cardiovascular morbidity and mortality. Hypertension appears to have a complex association with endothelial dysfunction, a phenotypical alteration of the vascular endothelium that precedes the development of adverse cardiovascular events and portends future cardiovascular risk. This review concentrates on recent findings with respect to the mechanisms of hypertension-associated endothelial dysfunction, the interrelationship between these two entities, and the relationship of the efficacy of antihypertensive therapies to improvements in vascular homeostasis beyond blood pressure reduction. Current evidence suggests that hypertension and endothelial dysfunction are integrally related with respect to pathophysiologic mechanisms. Future studies will need to identify the key connections between hypertension and endothelial dysfunction to allow novel interventions to be designed and promulgated.
Keywords: Hypertension, Endothelium, Cardiovascular risk, Sodium, Nitric oxide, Mitochondria, NADPH oxidase, Mineralocorticoid receptor, Oxidative stress, Inflammation
Introduction
With a prevalence of nearly 30% in the US adult population [1], hypertension remains a central pathophysiologic contributor to cardiovascular morbidity and mortality [2]. Although the precise cascade of events from the development of hypertension to adverse cardiovascular events remains to be elucidated, cardiovascular risk factors, including hypertension, are clearly associated with the development of vascular endothelial dysfunction [3]. Endothelial dysfunction is a phenotypical alteration of the endovascular lining of blood vessels that is characterized by a pro-thrombotic, pro-inflammatory, and pro-constrictive phenotype [4]. Endothelial function is readily measurable through multiple modalities and is an established barometer of cardiovascular risk [5]. Further, interventions aimed at reducing cardiovascular risk, including antihypertensive therapy, are more effective if they concomitantly improve endothelial function [6, 7••]. These data support the provocative hypothesis that reductions in cardiovascular risk secondary to antihypertensive therapy may relate independently to a particular intervention’s beneficial effects on endothelial function as well as to its absolute effect on blood pressure. This review focuses on recent findings related to the pathophysiology of hypertension-related endothelial dysfunction, including insights into the mechanisms of hypertension-associated endothelial dysfunction, the relationship between hypertension and endothelial dysfunction, and the effects of antihypertensive interventions on endothelial function.
Measuring Endothelial Function
Endothelial function is readily measured using both invasive and noninvasive modalities that have been subject of recent thorough reviews [8, 9]. These methods are designed to assess vasodilation to pharmacologic stimuli (eg, acetylcholine or bradykinin), mechanical stimuli (shear), or both. Further, the vasodilator responses to these stimuli are primarily related to nitric oxide (NO) production capacity. Key research over the past several decades has identified NO as a central regulator of vascular endothelial function, with a loss of NO bioavailability identified as a central phenotypic characteristic of endothelial dysfunction [10, 11].
Early studies measuring endothelial function were invasive, being performed in the coronary arteries using acetylcholine or pharmacologic flow manipulation [12, 13]. The cost and invasiveness of these methods now limits their use, and endothelial function is currently more widely measured using validated methods employing venous plethysmography, high-resolution ultrasound in the peripheral circulation, or, more recently, digital pulse arterial tonometry [9]. Brachial artery reactivity testing using high-resolution ultrasound to measure vasodilation of the brachial artery to hyperemic shear has emerged as one of the most common methods of assessing endothelial function, based on both its noninvasive nature and its proven validity [14]. Endothelium-dependent vasodilation of the brachial artery is NO-dependent, correlates with endothelial function in the coronary artery, and independently predicts future cardiovascular risk in patients with and without established atherosclerotic disease [3]. The severity of hypertension correlates with increasing impairment of endothelial function as measured by brachial artery reactivity testing, and antihypertensive therapy that concomitantly reverses brachial artery endothelial dysfunction reduces cardiovascular risk [6, 15]. Thus, endothelial function is readily measurable in a reproducible, valid, and noninvasive manner in hypertensive patients.
Mechanisms of Hypertension-Associated Endothelial Dysfunction
Emerging data implicate increases in systemic oxidative stress and vascular inflammation in the pathogenesis of hypertension [16, 17]. Excessive vascular oxidative stress and vascular inflammation are central characteristics of phenotypical endothelial dysfunction, and reductions in both have been shown to reverse endothelial dysfunction [3].
Excessive Reactive Oxygen Species Production in Hypertension
Recent studies have sought to further characterize the mechanisms behind hypertension-induced oxidative stress and inflammation. Multiple sources of oxidative stress have been implicated in the pathogenesis of hypertension-related endothelial dysfunction [17]. Investigations over the past year have gone further to investigate the potential mechanisms regulating two important sources of hypertension-associated oxidative stress: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondria [18, 19]. Isolated carotid arteries from mice were exposed to increasing intraluminal pressure and showed concomitant reductions in endothelium-dependent vasodilation to acetylcholine, increases in vascular superoxide production, and increased NADPH oxidase activity [19]. Additional studies using small interfering RNA (siRNA) and suppression of Rac-1 activity implicate overexpression of integrin-kinase 1 as a key first step in the mechanotransduction of hypertension-induced vascular superoxide production through NADPH oxidase. Transgenic mice overexpressing thioredoxin 2, a key peroxidase in the mitochondrial matrix important in converting hydrogen peroxide to water, are resistant to angiotensin II–induced hypertension, oxidative stress, and endothelial dysfunction [18]. Taken together with recent reports demonstrating coordinated expression of reactive oxygen species (ROS) from NADPH oxidase and mitochondria in hypertensive states [20], a paradigm of hypertension-induced ROS originating from coordinated mitochondrial sources and NADPH oxidase appears to be emerging. Elucidation of the mechanisms of this coordinated expression of ROS and the coordination of ROS expression with other cellular sources will require further work.
Inflammatory Regulation of Hypertension-Associated Endothelial Dysfunction
Over the past decade, researchers and clinicians have increasingly recognized the important role of adipose tissue in regulating metabolism and inflammation through the production of both inflammatory and anti-inflammatory adipokines [21]. Inflammation in adipose tissue, apparent in visceral fat depots, is associated with impaired endothelial function in obese patients [22]. Though most investigations relating adipose inflammation to vascular endothelial function concentrate on insulin resistance and obesity, recent studies have evaluated the effect of perivascular adipose tissue on vascular homeostasis in hypertension. Adipose tissue from hypertensive rats applied to thoracic aorta segments failed to suppress phenylephrine-induced vasoconstriction, in contrast to adipose tissue from normotensive animals [23]. Similarly, obese, hypertensive rats with perivascular inflammation show impaired endothelial function relative to control animals [24]. Overall, these data suggest that perivascular adipose tissue and inflammation within adipose tissue play important roles in regulating local and systemic vascular homeostasis. Future work in this area will need to identify mechanisms by which perivascular fat regulates endothelial function in hypertension, as well as the relative impact on endothelial function in humans of perivascular adipose tissue inflammation.
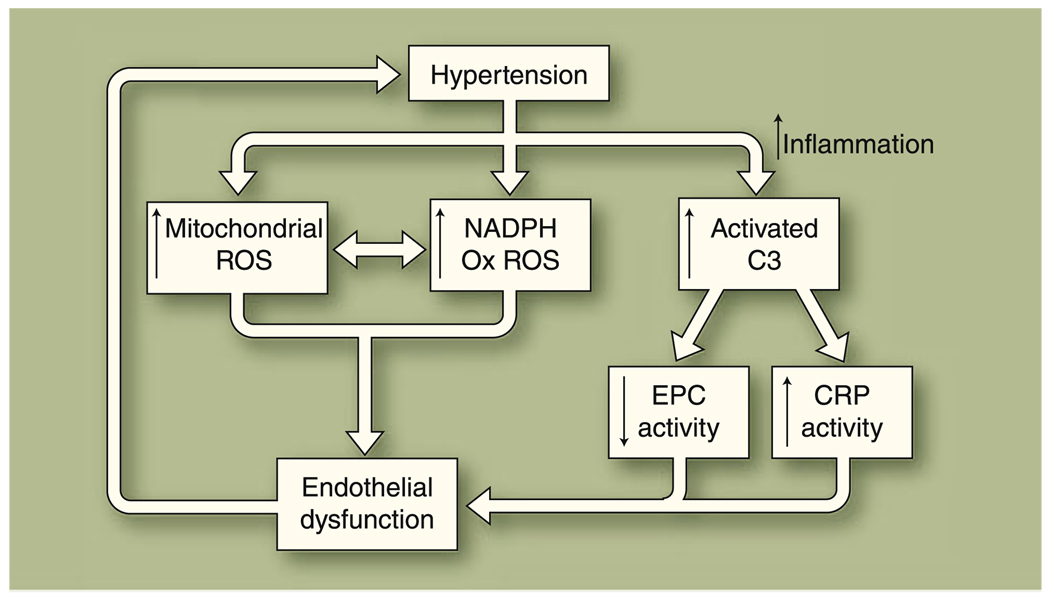
Recent data also delineate novel roles for elements of both innate and adaptive immune responses in regulating endothelial function under hypertensive conditions. Activation of innate immunity’s complement pathway may negatively impact vascular endothelial function in hypertension [25], whereas increased anti-inflammatory interleukin-10 expression from the adaptive immune response blunts the adverse effects on endothelial function of angiotensin II–associated hypertension [26]. Circulating endothelial progenitor cells (EPCs), derived from myeloid pluripotent stem cells that also give rise to mature mononuclear cells, also play significant roles in maintaining endothelial homeostasis through their regenerative and repair mechanisms. Reduced levels of EPCs correlate with impaired vascular endothelial function [27], whereas infusions of EPCs help to reverse endothelial dysfunction in an atherosclerosis-prone mouse model [28]. The regenerative and repair capacity of EPCs in newly diagnosed prehypertensive and hypertensive humans is impaired relative to healthy controls [29]. Further, the level of circulating EPCs is negatively influenced by activated complement fragment C3a in hypertensive humans [25]. Impairment of vascular endothelial function and vascular injury related to C-reactive protein depends on the presence of C3 [30]. Overall, these newer data suggest that hypertension-associated vascular endothelial dysfunction relates to local vascular inflammation as well as to systemic inflammation. Figure 1 summarizes the recent findings with respect to the pathophysiology of hypertension-associated endothelial dysfunction.
Fig. 1.
Potential mechanism of hypertension-associated endothelial dysfunction. Recent publications suggest that hypertension is associated with increased production of reactive oxygen species (ROS) from mitochondria and NADPH oxidase in the vascular endothelium. Local and systemic inflammation also occurs, leading to an increase in activated complement factor 3 (C3) and subsequent increased vascular damage through reduced endothelial progenitor cell (EPC) activity and inflammation-related damage. The resultant excessive oxidative stress and inflammation result in endothelium-dependent vasomotor dysfunction. Endothelial dysfunction may subsequently worsen hypertension. CRP—C-reactive protein
Linkage of Clinical Hypertension with Endothelial Dysfunction
An association between endothelial dysfunction and hypertension is well established [31–35]. Data from the Framingham offspring cohort suggest that the severity of hypertension is positively associated with the degree of impairment of endothelial function [15]. Whether endothelial dysfunction is a cause or an effect of hypertension remains controversial; current data support a complex and potentially bidirectional relationship. Evidence that endothelial dysfunction precedes hypertension arises from mechanistic data showing that exogenous infusion of inhibitors of endothelium-derived nitric oxide synthase (eNOS) results in hypertension in humans [36]. In addition, Rossi et al. [37] showed in a cohort study of postmenopausal women that normotensive women with impaired endothelial function have a nearly sixfold increased risk of developing hypertension. Data in support of hypertension preceding endothelial dysfunction include a report demonstrating that acute, large increases in blood pressure acutely impair endothelial function as measured by brachial artery flow-mediated dilation (FMD) in young adults who were not athletically trained but were otherwise healthy [38]. In another study, higher systolic blood pressure in adolescence was associated with an increased incidence of endothelial dysfunction in adulthood [39].
To further investigate this important question, Shimbo and colleagues [40••] recently reported data on the incidence of future hypertension related to a baseline FMD measurement in a cohort of 3500 ethnically diverse persons in the Multiethnic Study of Atherosclerosis (MESA) study. The investigators found that impaired FMD was not a significant independent predictor of the future development of hypertension, following adjustment for covariables. As the largest and most diverse study to address the question of directionality of the relationship of hypertension and endothelial dysfunction, these data support the concept that hypertension is a source, rather than a consequence, of endothelial dysfunction. As discussed in an accompanying editorial [41], these data, though clearly suggesting that endothelial dysfunction is an effect rather than a cause of hypertension, do not completely settle this controversy, given the differences in risk and ethnic profiles of the populations studied by Shimbo et al. [40••] and Rossi et al. [37].
Therapeutic Interventions: New Insights on Their Impact on Endothelial Function
Reduced Sodium Intake
Substantial reductions in morbidity, mortality, and health care costs can be achieved with modest, achievable dietary salt restriction [42••]. Interestingly, the antihypertensive effects of dietary salt restriction are relatively modest (∼5 mm Hg reduction in systolic blood pressure and ∼2.7 mm Hg reduction in diastolic blood pressure) according to a recent meta-analysis of studies using sodium restriction for blood pressure reduction [43].
The argument most often cited for the substantial reported reductions in cardiovascular risk in spite of the relatively modest antihypertensive effect of sodium restriction is an epidemiologic one: modest improvements in a risk factor profile due to an intervention in a large population will lead to significant reductions in events by virtue of the size of the population the intervention is affecting. There is evidence, however, that reductions in salt intake may reduce cardiovascular morbidity and mortality by reversing endothelial dysfunction induced by high salt intake. In animal models, the hypertensive effect of salt loading appears mechanistically linked to increased oxidative stress and reduced NO bioavailability [44, 45]. Further, the hypertensive effect of salt loading is exacerbated by inhibition of NO [46].
Overall, the epidemiologic and mechanistic data suggest two potential hypotheses for the beneficial effects of sodium restriction, despite its modest overall antihypertensive effects: 1) direct sodium restriction improves endothelial function in humans with hypertension; and 2) interventions that improve NO bioavailability and reduce systemic oxidative stress may also be able to counteract the effects of endothelial dysfunction induced by a high-salt diet and reduce overall cardiovascular risk. These hypotheses are the subject of two recent human studies [47, 48•]. In a cross-sectional study of patients with prehypertension and stage I hypertension, patients with a self-reported intake of sodium of less than 100 mmol/day had significantly higher brachial FMD than those with an intake between 100 and 200 mmol/day; there was a reasonably strong negative correlation of FMD with self-reported sodium intake [47]. In another recent study, 147 hypertensive individuals were randomized to 1, 3, or 6 servings of fresh fruits and vegetables per day for 12 weeks following a 4-week run-in period of less than 1 serving per day [48•]. Systolic blood pressure decreased by about 4–5 mm Hg in the groups receiving 3 and 6 servings (not a statistically significant difference, probably because the study was underpowered to determine blood pressure changes). Endothelium-dependent forearm blood flow significantly increased by 6.2% for each 1-serving increase of fresh fruits and vegetables. Dietary sodium content was not reported in this study, but it is likely that those randomized to more servings of fruits and vegetable also had a concomitant reduction in salt intake through caloric replacement of foods likely to be higher in salt content. A portion of the effect may also be secondary to high polyphenol content in the fruits and vegetables consumed, which can improve NO bioavailability through mechanisms beyond pure antioxidant activity [49, 50].
Pharmacologic Antihypertensive Therapy and Modulation of Endothelial Function
There are clear differences between classes of antihypertensive agents with respect to their effects on the vascular endothelium. Certain antihypertensive agents, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, aldosterone antagonists, and nebivolol (a novel beta-blocker, unique in its medication class, that can stimulate NO production), reduce blood pressure and improve endothelial function [51–55]. Pharmacologic lowering of blood pressure using other medications, including some calcium channel blockers and thiazide diuretics [55, 56], has not shown consistent, concomitant improvements in endothelial function. The differences in effect may be due to differences in the populations studied, the level of blood pressure reduction achieved, and medication doses used, but it is likely that they are also related to differences in the mechanisms of actions of these agents. Theoretically, antihypertensive agents that concomitantly improve endothelial function would seem to be superior in reducing cardiovascular risk [6].
Significant debate continues, however, about whether the choice of pharmacologic agent has any relevance to clinical outcomes. The publication of The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) in 2002 and the subsequent promulgation of the antihypertension therapeutic guidelines in 2003 support the use of a “diuretic first” strategy for the treatment of hypertension, on the basis that diuretics are inexpensive and that other agents with purported pleiotropic benefits did not appear to have added benefit in the treatment of uncomplicated hypertension [57, 58]. ALLHAT randomized hypertensive individuals at least 55 years of age who had at least one other risk factor to chlorthalidone, amlodipine, or lisinopril. With a mean follow-up of 4.9 years, there were no differences between the three groups in the primary outcome of combined fatal heart disease and nonfatal myocardial infarction. Chlorthalidone bested amlodipine with respect to incident heart failure, and lisinopril with respect to combined cardiovascular events, stroke, and heart failure.
However, ALLHAT far from establishes the primacy of a pharmacologic strategy that concentrates primarily on blood pressure reduction over agent choice. The participants in ALLHAT were, on average, nearly 67 years old; 90% had been on therapy prior to study enrollment; and the duration of their hypertension was not reported. Further, half of the participants enrolled already had clinically relevant atherosclerotic disease. Finally, blood pressure reductions were greater in the chlorthalidone arm than in either of the other study arms. Generalization of these data to younger populations with shorter disease durations and less vascular damage at the time of diagnosis is dubious, and it remains unknown whether a difference in cardiovascular outcomes would be seen when comparing different classes of pharmacologic antihypertensive agents if blood pressure lowering were equivalent.
Large studies have also challenged therapeutic strategies using pharmacologic agents with no known significant endothelial benefits as first-line therapy. The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study demonstrated the superiority of angiotensin II receptor blocker–based therapy (losartan) versus atenolol-based therapy for hypertensive individuals ages 55–80 despite similar reductions in blood pressure [59]. An equivalent number of patients in each study arm also required diuretic therapy for optimal antihypertensive effect. An open-label randomized trial of enalapril versus hydrochlorothiazide in hypertensive individuals ages 65– 84 years showed the superiority of enalapril-based therapy, particularly in men [60]. The recent Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial demonstrated the superiority of the combination of ACE inhibition and amlodipine versus ACE inhibition and diuretic therapy in hypertensive patients at high risk for cardiovascular events. [61] These data question the primacy of thiazide therapy in higher-risk patients and suggest that the addition of previously reported antioxidant effects of amlodipine to ACE inhibition may enhance the overall beneficial effects of ACE inhibition on vascular homeostasis [62, 63]. Taken together, these data suggest that a “diuretic first” strategy may not be ideal for all hypertensive patients.
Given the potential benefits of improving endothelial function in hypertensive patients, on the basis of both blood pressure reduction and the mechanisms of action that may improve endothelial function, recent investigations continue to evaluate whether antihypertensive agents with favorable effects on endothelial function may have added benefits. In particular, studies of renin inhibition and mineralocorticoid receptor inhibition have generated growing interest in light of their potential to augment the favorable effects of lowering blood pressure with favorable effects on endothelial homeostasis.
Renin Inhibition
Aliskiren, an inhibitor of renin that blocks conversion of angiotensinogen to angiotensin I, was initially approved for the treatment of hypertension in 2007. Emerging literature over the past 2 years suggests that renin inhibition exerts favorable effects on vascular endothelial function and overall vascular homeostasis, in addition to lowering blood pressure. Studies in hyperlipidemic and eNOS-deficient mouse models demonstrate that aliskiren therapy improves NO bioavailability (in a non-eNOS knockout model) and inhibits adverse vascular remodeling [64, 65]. Further, its beneficial effects appear to be potentiated by the addition of downstream angiotensin II receptor type 1 blockade [64, 65]. Limited data as to its effect on human vascular endothelial function have been published. In one recent study, aliskiren improved endothelial function as measured by FMD and reduced vascular stiffness in a small group of nonhypertensive patients with type 1 diabetes [66]. However, the effect of renin inhibition on vascular endothelial function in humans with hypertension currently remains unknown.
Mineralocorticoid Inhibition
Publication of the Randomized Aldactone Evaluation Study (RALES), demonstrating the benefits of mineralocorticoid receptor inhibition following myocardial infarction complicated by heart failure, rekindled interest in the use of this class of agents [67]. Aldosterone’s genomic and non-genomic alterations exert rapid and significant effects on the vasculature [68]. In animal models of hypertension, aldosterone inhibition reduces oxidative stress through inhibition of NADPH oxidase while augmenting glucose-6-phosphate dehydrogenase activity and reverses endothelial dysfunction in hypertensive models [69–73].
In humans, increased plasma aldosterone levels are associated with an increased risk of incident hypertension, even without elevation outside the range of normal [74, 75]. Aldosterone infusion has been shown to acutely impair endothelial function in some studies [76], but not all [77], whereas aldosterone antagonism has been shown to reverse endothelial dysfunction in humans with hypertension [78]. Hypertensive patients with elevated plasma aldosterone: renin ratios show evidence of adverse vascular remodeling and greater microvascular dysfunction compared with hypertensives with more normal ratios [79–81]. This finding suggests that hypertensive patients with a high plasma aldosterone:renin ratio may derive the most benefit from mineralocorticoid receptor inhibition.
Several recent studies support a beneficial effect of aldosterone inhibition on vascular homeostasis. Selective overexpression of mineralocorticoid receptors on the vascular endothelium leads to an exaggerated constrictor response to either angiotensin II or endothelin-1 infusion [82]. Aldosterone inhibition blocks angiotensin II induction of intimal hyperplasia and medial hypertrophy in large, elastic conduit arteries [83]. In humans with primary aldosteronism, tumor removal results in improved vascular endothelial function with limited correlation to the postoperative reduction in blood pressure, suggesting the potential for a beneficial effect of aldosterone blockade on endothelial function independent of blood pressure lowering [84].
Conclusions
Evidence of an association between hypertension and endothelial dysfunction is convincing, but a wide gulf remains to be bridged with respect to understanding the pathophysiological connections between these two entities. Though it appears clear that hypertension is a state of excessive oxidative stress, local vascular inflammation, and systemic inflammation, our understanding of the underlying mechanisms and interactions of these alterations in vascular homeostasis remains incomplete. The argument about which comes first, hypertension or endothelial dysfunction, seems simplistic. Current data suggest that hypertension and endothelial dysfunction reinforce each other. The etiologies of hypertension are diverse, and the severity and relative contribution of endothelial dysfunction to the pathophysiology of hypertension in an individual likely relates that individual’s unique underlying causes. As our understanding of the mechanisms of hypertension increases, especially with added genomic and proteomic information, our understanding of the interrelationship between these two entities is likely to become clearer.
With respect to antihypertensive therapy, dietary sodium reduction not only reduces blood pressure but also reverses hypertension-associated endothelial dysfunction. Consequently, public health initiatives to reduce sodium intake would be likely to reap significant cardiovascular benefits. Emerging antihypertensive therapies appear to have added benefits with respect to endothelial function, but whether these benefits translate into improved outcomes remains unknown. Answers to these questions gained through future investigations may give us the knowledge to develop and implement novel interventions and strategies to reduce the morbidity and mortality of hypertension.
Acknowledgments
Dr. Dharmashankar is supported by a Ruth L. Kirschstein NIH T32 training grant (HL007792-15). Dr. Widlansky’s work is supported by K23HL089326, AHA Grant-in-Aid 10GRNT3880044, and a grant from the Greater Milwaukee Foundation.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Gokce N, Keaney JF, Jr, Vita JA. Endotheliopathies: Clinical manifestations of endothelial dysfunction. In: Loscalzo J, Shafer AI, editors. Thrombosis and Hemorrhage. Baltimore: Williams & Wilkins; 1998. pp. 901–924. [Google Scholar]
- 5.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 6.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 7. Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074.The authors measured endothelial function by brachial artery reactivity immediately following the diagnosis of cardiovascular disease in 251 patients and remeasured endothelial function 6 months after initiation of intensive therapy. Those with persistently impaired brachial artery reactivity had a significantly higher 3-year event rate (26%) than those with improved reactivity on therapy (10%). Persistent impairment of endothelial dysfunction was an independent predictor of cardiovascular risk
- 8.McMackin CJ, Vita JA. Update on nitric oxide—dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 11.Ignarro LJ, Edwards JC, Gruetter DY, et al. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980;110:275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- 12.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 13.Cox DA, Vita JA, Treasure CB, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 16.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28:e20–e32. doi: 10.1111/j.1755-5922.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widder JD, Fraccarollo D, Galuppo P, et al. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vecchione C, Carnevale D, Di PA, et al. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betaPIX/Rac-1 pathway. Hypertension. 2009;54:1028–1034. doi: 10.1161/HYPERTENSIONAHA.109.136572. [DOI] [PubMed] [Google Scholar]
- 20.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng ZH, Zhang ZH, Luo BH, et al. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens. 2009;31:355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- 24.Marchesi C, Ebrahimian T, Angulo O, et al. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 25.Magen E, Feldman A, Cohen Z, et al. Potential link between C3a, C3b and endothelial progenitor cells in resistant hypertension. Am J Med Sci. 2010;339:415–419. doi: 10.1097/MAJ.0b013e3181d7d496. [DOI] [PubMed] [Google Scholar]
- 26.Didion SP, Kinzenbaw DA, Schrader LI, et al. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 28.Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99:e74–e83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 29.Giannotti G, Doerries C, Mocharla PS, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 30.Hage FG, Oparil S, Xing D, et al. C-reactive protein-mediated vascular injury requires complement. Arterioscler Thromb Vasc Biol. 2010;30:1189–1195. doi: 10.1161/ATVBAHA.110.205377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 32.Treasure CB, Manoukian SV, Klein JL, et al. Epicardial coronary artery responses to acetylcholine are impaired in hypertensive patients. Circ Res. 1992;71:776–781. doi: 10.1161/01.res.71.4.776. [DOI] [PubMed] [Google Scholar]
- 33.Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 34.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 35.Panza JA, Garcia CE, Kilcoyne CM, et al. Impaired endothelium-dependent vasodilation in patients with essential hypertension: evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 36.Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- 37.Rossi R, Chiurlia E, Nuzzo A, et al. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004;44:1636–1640. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Jurva JW, Phillips SA, Syed AQ, et al. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J Am Coll Cardiol. 2006;48(3):588–589. doi: 10.1016/j.jacc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Juonala M, Viikari JS, Ronnemaa T, et al. Elevated blood pressure in adolescent boys predicts endothelial dysfunction: the Cardiovascular Risk in Young Finns Study. Hypertension. 2006;48:424–430. doi: 10.1161/01.HYP.0000237666.78217.47. [DOI] [PubMed] [Google Scholar]
- 40. Shimbo D, Muntner P, Mann D, et al. Endothelial dysfunction and the risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1210–1216. doi: 10.1161/HYPERTENSIONAHA.109.143123.The investigators measured endothelial function by brachial artery reactivity in 3500 ethnically diverse, nonhypertensive individuals in the Multiethnic Study of Atherosclerosis (MESA). Brachial reactivity was not found to be an independent predictor of the future development of hypertension after adjustment for other risk factors. This is the largest and most diverse study to address the question of directionality of the relationship of hypertension and endothelial dysfunction, and the data support the concept of endothelial dysfunction as an effect of hypertension rather than a cause of it
- 41.Quyyumi AA, Patel RS. Endothelial dysfunction and hypertension: cause or effect? Hypertension. 2010;55:1092–1094. doi: 10.1161/HYPERTENSIONAHA.109.148957. [DOI] [PubMed] [Google Scholar]
- 42. Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355.The authors employed the Coronary Heart Disease Policy Model to determine the potential achievable reductions in cardiovascular risk from reducing dietary salt by up to 3 grams per day. The authors report that such a reduction would lead to significant reductions in stroke, myocardial infarction, and cardiovascular death
- 43.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD004937. CD004937. [DOI] [PubMed] [Google Scholar]
- 44.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension. 2005;46:1026–1031. doi: 10.1161/01.HYP.0000174989.39003.58. [DOI] [PubMed] [Google Scholar]
- 45.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension. 2006;47:568–572. doi: 10.1161/01.HYP.0000200027.34925.93. [DOI] [PubMed] [Google Scholar]
- 46.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34(9):946–952. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 47.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3:347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCall DO, McGartland CP, McKinley MC, et al. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation. 2009;119:2153–2160. doi: 10.1161/CIRCULATIONAHA.108.831297.Individuals with hypertension were randomized to either 1, 3, or 6 servings per day of fresh fruits and vegetables after a 1-month run-in period with less than 1 serving per day. Endothelial function was assessed by forearm blood flow using venous plethysmography at the beginning and end of the 12-week intervention period. The authors found a 6.2% increase in forearm blood flow for each single-portion increase in fresh fruits and vegetables, demonstrating that increasing fruit and vegetable consumption can improve a surrogate marker of cardiovascular risk
- 49.Anter E, Thomas SR, Schulz E, et al. Activation of eNOS by the p38 MAP kinase in response to black tea polyphenols. J Biol Chem. 2004;45:46637–46643. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 50.Widlansky ME, Duffy SJ, Hamburg NM, et al. Effects of black tea consumption on plasma catechins, markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic Biol Med. 2004;38:499–506. doi: 10.1016/j.freeradbiomed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 52.Prasad A, Husain S, Quyyumi AA. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: a potential role of bradykinin. J Am Coll Cardiol. 1999;33:796–804. doi: 10.1016/s0735-1097(98)00611-1. [DOI] [PubMed] [Google Scholar]
- 53.Higashi Y, Sasaki S, Nakagawa K, et al. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35:284–291. doi: 10.1016/s0735-1097(99)00561-6. [DOI] [PubMed] [Google Scholar]
- 54.Prasad A, Tupas-Habib T, Schenke WH, et al. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- 55.Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double-blind, crossover study. Circulation. 2001;104:511–514. doi: 10.1161/hc3001.094207. [DOI] [PubMed] [Google Scholar]
- 56.Ghiadoni L, Huang Y, Magagna A, et al. Effect of acute blood pressure reduction on endothelial function in the brachial artery of patients with essential hypertension. J Hypertens. 2001;19(3 Pt 2):547–551. doi: 10.1097/00004872-200103001-00005. [DOI] [PubMed] [Google Scholar]
- 57.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 58.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 59.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 60.Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 61.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 62.Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–1286. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 63.Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens. 2004;17:167–171. doi: 10.1016/j.amjhyper.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Imanishi T, Tsujioka H, Ikejima H, et al. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto E, Kataoka K, Dong YF, et al. Aliskiren enhances the protective effects of valsartan against cardiovascular and renal injury in endothelial nitric oxide synthase-deficient mice. Hypertension. 2009;54:633–638. doi: 10.1161/HYPERTENSIONAHA.109.133884. [DOI] [PubMed] [Google Scholar]
- 66.Cherney DZ, Lai V, Scholey JW, et al. Effect of direct renin inhibition on renal hemodynamic function, arterial stiffness, and endothelial function in humans with uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2010;33:361–365. doi: 10.2337/dc09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 68.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 69.Sanz-Rosa D, Oubiña MP, Cediel E, et al. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxid Redox Signal. 2005;7:1294–1301. doi: 10.1089/ars.2005.7.1294. [DOI] [PubMed] [Google Scholar]
- 70.Leopold JA, Cap A, Scribner AW, et al. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001;15:1771–1773. doi: 10.1096/fj.00-0893fje. [DOI] [PubMed] [Google Scholar]
- 71.Leopold JA, Zhang YY, Scribner AW, et al. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 72.Leopold JA, Dam A, Maron BA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virdis A, Neves MF, Amiri F, et al. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 74.Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 75.Pratt JH. A not-so-modest proposal that a “modest” increase in aldosterone causes hypertension and more. Hypertension. 2008;51:39–40. doi: 10.1161/HYPERTENSIONAHA.107.100024. [DOI] [PubMed] [Google Scholar]
- 76.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002;103:425–431. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt BM, Oehmer S, Delles C, et al. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42:156–160. doi: 10.1161/01.HYP.0000083298.23119.16. [DOI] [PubMed] [Google Scholar]
- 78.Nishizaka MK, Zaman MA, Green SA, et al. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 79.Duffy SJ, Biegelsen ES, Eberhardt RT, et al. Low-renin hypertension with relative aldosterone excess is associated with impaired NO-mediated vasodilation. Hypertension. 2005;46:707–713. doi: 10.1161/01.HYP.0000184231.84465.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahn DF, Duffy SJ, Tomasian D, et al. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 81.Park S, Kim JB, Shim CY, et al. The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J Hypertens. 2007;25:1279–1283. doi: 10.1097/HJH.0b013e3280f31b6e. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen Dinh CA, Griol-Charhbili V, Loufrani L, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 83.Sakurabayashi-Kitade S, Aoka Y, Nagashima H, et al. Aldosterone blockade by spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis. 2009;206:54–60. doi: 10.1016/j.atherosclerosis.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 84.Tsuchiya K, Yoshimoto T, Hirata Y. Endothelial dysfunction is related to aldosterone excess and raised blood pressure. Endocr J. 2009;56:553–559. doi: 10.1507/endocrj.k09e-014. [DOI] [PubMed] [Google Scholar]