Abstract
Estrogens act in the adult brain to modulate cognition, enhancing performance on some learning tests and impairing performance on others. Our previous research has revealed an impairing effect of chronic 17β-estradiol treatment in young and aged rats on a prefrontally-mediated working memory task, delayed spatial alternation (DSA). Little is known about the mechanisms of these impairing effects. The current study examined the effects of selective estrogen receptor (ER) α or ERβ activation on DSA performance in middle-aged female rats. Ovariectomized 12 month old Long Evans (LE) rats were treated by subcutaneous injection with the ERα agonist propyl pyrazole triol (PPT) or the ERβ agonist diarylpropionitrile (DPN) at 0.02, 0.08, or 0.20 mg/kg/day, or with oil vehicle and tested on an operant variable delay DSA task. A 17β-estradiol group (10% in cholesterol) was included as a positive control group. We replicated our previous finding of a 17β-estradiol-induced deficit on DSA performance and this effect was paralleled by low dose (0.02 mg/kg/day) DPN treatment. Higher doses of DPN failed to produce a significant change in performance. The highest dose of PPT (0.20 mg/kg/day) also impaired performance, but this effect was subtle and limited to the longest delay during the final block of testing. These data confirm our earlier findings that chronic 17β-estradiol treatment has an impairing effect on the DSA task, and suggest that ERβ activation may underlie the deficit.
Keywords: estrogen receptor agonists, cognition, aging, DSA, working memory
Introduction
A large number of studies in rodent models have revealed that estradiol modulates performance on several different types of cognitive tasks (see Dohanich et al, 2009; Frick, 2009; Korol, 2004; Luine, 2008). In particular, 17β-estradiol has been shown to improve the performance of young ovariectomized female rats on tasks that engage the hippocampus (Daniel et al., 1997; Daniel and Dohanich, 2001; Davis et al., 2005; Korol and Kolo, 2002; Zurkovsky et al., 2006, 2007) and to prevent age-related decline in performance on hippocampally-mediated tasks (Daniel et al., 2006; Foster et al, 2003; Markham et al., 2002; Rodgers et al., 2010; Talboom et al., 2008; Walf et al., 2009). Conversely, 17β-estradiol impairs the performance of young and aged ovariectomized female rats on tasks engaging prefrontal cortical and striatal systems (Davis et al., 2005; Korol and Kolo, 2002; Wang et al., 2008, 2009; Zurkovsky et al., 2007). Together, these findings suggest that the direction of the effects of 17β-estradiol on cognition may be dependent upon the specific tasks that are employed, the brain regions activated during completion of the task, or both (see Korol, 2004).
The cognitive effects of 17β-estradiol are diverse, with the majority of activity likely modulated through the activation of nuclear estrogen receptors (ERs), including the two classical ERs, ERα and ERβ (see McEwen and Alves, 1999; McEwen, 2002). Importantly, activity through other non-classical ERs including GPR30 and/or nongenomic actions may also contribute to 17β-estradiol’s effects on learning and behavior (see McEwen and Alves, 1999; McEwen, 2002; Prokai and Simpkins, 2007; Prossnitz et al., 2008; Toran-Allerand, 2005; Zurkovsky et al., 2006). ERα and ERβ are widely distributed throughout the mammalian brain, with differential distribution patterns in brain regions that play important roles in learning and memory (Kritzer, 2002; Mitra et al., 2003; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001). For example, both ERs are expressed in the hippocampus and cortex, but expression of ERα mRNA and protein is quite low in the cortex in comparison to ERβ mRNA and protein (Khan et al., 2005; Mitra et al., 2003; Shughrue et al., 1997). In contrast, ERα is expressed at a higher level in the hippocampus (Chung et al., 2007; Khan et al., 2005; Mitra et al., 2003; Shughrue et al., 1997), although ERβ is still more abundant than ERα (Weiser et al., 2008). Nonclassical ER’s, such as GPR30, are also expressed differentially throughout the mammalian brain (Brailoiu et al., 2007). Given that the distribution of both classical and nonclassical ERs varies markedly across key brain regions involved in learning and memory, the differences in performance on various cognitive tasks following 17β-estradiol treatment may be mediated by differences in the distribution of ERs in these brain regions.
Two recently synthesized compounds, propyl pyrazole triol (PPT) and diarylpropionitrile (DPN), are selective agonists for ERα and ERβ, respectively (Meyers et al., 2001; Stauffer et al., 2000). Recent studies using these two compounds reveal selective roles for ERα and ERβ in a variety of behavioral tasks. For example, DPN treatment has been shown to improve performance on a reference memory version of the water maze task, and also to reduce anxiety and fear behaviors in a variety of other tasks in rats (Lund et al., 2005; Rhodes and Frye, 2006; Weiser et al., 2009), suggesting ERβ activation is critical for these effects. ERα activation can also alter behavior as PPT treatment improves performance on an object placement task in rats (Frye et al., 2007). Studies have also have found enhanced object recognition and improved performance on a delayed matching-to-position T-maze task following either DPN or PPT treatment (Hammond et al., 2009; Walf et al., 2006). Given that 17β-estradiol has been shown to enhance performance on these tasks as well as other similar tasks (see Dohanich et al, 2009; Frick, 2009; Korol, 2004; Luine, 2008), the mnemonic enhancing effects of selective ER agonists on these tasks is not surprising.
Little is known about the roles of ERα and ERβ on working memory tasks that specifically engage the prefrontal cortex and not the hippocampus. Although maze-based tasks and object recognition/placement tasks have been shown to engage the prefrontal cortex to some extent, each also has a strong hippocampal component (D’Hooge and De Deyn, 2001; Ennaceur et al., 1997; Floresco et al., 1997; Goldman-Rakic, 1995; Jones, 2002; Warburton and Brown, 2010), particularly when longer inter-trial delays are used (Jones, 2002; Lee and Kesnar, 2003). These maze-based tasks often use inter-trial delays in the range of minutes to hours (Dudchenko, 2004; Hodges, 1996; Jones, 2002). Conversely, operant based working memory tasks, including delayed matching-to-sample and delayed alternation tasks have been shown to selectively engage the prefrontal cortex (Izaki et al., 2008; Maruki et al., 2001; Sloan et al., 2006). Performance on operant working memory tasks with brief (< 15 sec) inter-trial delays is impaired by prefrontal cortex inactivation or lesioning, while hippocampal disruption is largely without effect on these tasks (Maruki et al., 2001; Sloan et al., 2006). In contrast, when longer inter-trial delays are used, selective inactivation or lesioning of the hippocampus does produce impairments (Izaki et al., 2008; Maruki et al., 2001). Thus, operant based spatial working memory tasks with brief delays provide a means to selectively engage the prefrontal cortex with little or no hippocampal contribution.
We recently completed a set of studies showing that chronic 17β-estradiol treatment impaired the performance of ovariectomized young (3- and 6- month), middle-aged (12-month), and old (18-month) female rats on an operant delayed spatial alternation (DSA) task (Wang et al., 2008, 2009). The 17β-estradiol-treated rats performed worse than cholesterol treated controls at inter-trial delays of 3-, 6-, and 9-seconds, suggestive of a modulatory effect of 17β-estradiol on prefrontal cortex function (Izaki et al., 2008; Maruki et al., 2001; Sloan et al., 2006). Importantly, these deficits were conserved across age, with the performance of young, middle-aged and old rats all showing impairments with chronic 17β-estradiol treatment.
The current study was conducted to determine if the detrimental effects of 17β-estradiol treatment on the operant DSA task were selectively mediated by ERα or ERβ activation. Although the 17β-estradiol induced impairment was found across several ages, the current study focused on 12-month old ovariectomized rats because the deficit in DSA performance was most striking at that age. Thus, the goal of this study was to determine if the 17β-estradiol induced deficit in operant DSA performance was differentially modulated by ERα or ERβ activation. As the prefrontal cortex is rich in ERβ and has little ERα, we hypothesized that DPN, the ERβ agonist, would mimic the detrimental effects of 17β-estradiol on the operant DSA task, whereas PPT, the ERα agonist would not.
Methods
Animals and Exposure
One hundred twenty-eight female Long-Evans rats were obtained from Harlan (Indianapolis, IN) in two cohorts spaced 3 months apart and were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were housed in a temperature and humidity controlled room (22°C, 40–55% humidity) on a 12-hour reverse light-dark cycle (lights off at 8:30 am). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health, 2002) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Each cohort consisted of 64 middle-aged (12-month old) retired breeders. There were eight experimental groups: PPT at 0.02, 0.08, or 0.20 mg/kg/day, DPN at 0.02, 0.08, or 0.20 mg/kg/day, an oil vehicle control group (Sesame oil, Sigma, St. Louis, MO), and a 17β-estradiol group (10% in cholesterol), included as a positive control group. As previous studies have found daily treatment with PPT at doses above 0.20 mg/kg to significantly reduce food intake and body weight (Harris et al., 2002; Roesch, 2006; Santollo et al., 2007; Wegorzenska et al., 2008), a maximum dose of 0.20 mg/kg was selected to avoid interference with motivation to complete the task. Others have reported behavioral effects with doses of DPN below 0.20 mg/kg (Walf et al., 2008a, 2008b).
Both PPT and DPN were suspended in sesame oil, and delivered via daily s.c. injections one-half hour after behavioral testing. As the 17β-estradiol group served as a positive control, we chose to replicate our previous route of delivery via Silastic implant (Wang et al., 2008, 2009). 17β-estradiol implanted animals were also treated with an injection of oil vehicle daily, thereby exposing them to the same daily injection procedure as the other treatment groups. In addition, all other rats were implanted with a blank Silastic capsule on the day of ovariectomy surgery. The Silastic capsule was 1 cm in length (1.5 mm i.d., 1.96 mm o.d.) and was plugged with silicone and dried overnight before packing with either a 10% 17β-estradiol/cholesterol mixture (Sigma, St. Louis, MO) or left blank, after which the other end was plugged with silicone. Capsules were soaked in sterile saline at 37°C overnight before insertion during surgery. Previous research in this lab (Wang et al., 2008, 2009) has shown that the 17β-estradiol implants produce stable serum estradiol concentrations over the 8 weeks of behavioral testing.
Rats were pair-housed in standard polycarbonate cages (45×24×20cm) with corncob bedding. Upon arrival, estrous cycles were monitored for two weeks prior to ovariectomy surgery via daily vaginal lavage to determine reproductive status prior to surgery, and again post-surgery to confirm ovariectomy. Sterile saline was flushed into the vaginal opening with an eye dropper and the collected fluid was place on a microscope slide. Each slide was then stained with hematoxylin and eosin and determination of the phase of the estrous cycle was made using a light microscope (Olympus BH-2). Slides were scored according to the four-stage cycle detailed in Yener et al. (2007). Following ovariectomy, all rats were maintained on an AIN-93G soy-free diet (Harlan-Teklad, Madison, WI) to avoid exposure to dietary estrogens via the feed. Water was available ad libitum. No change in food intake or body weight was observed following the change in diet. Beginning one week after surgery, rats were weighed daily and food restricted to maintain 85% of their free fed body weights. During behavioral testing, rats were fed one hour after the daily test session was completed. Testing began two weeks following ovariectomy and occurred once daily, six days/week during the dark phase of the light cycle.
Operant Testing
Behavioral testing was conducted in standard automated operant chambers (Med Associates Inc., St. Albans, VT) housed in sound-attenuated wooden boxes. All of the test chambers had the same features and dimensions: 21.6 cm high with a 29.2 cm × 24.8 cm stainless-steel grid floor that rested just above a tray filled with corncob bedding. Soy-free food pellets (45-mg Formula P, P.J. Noyes Inc., Lancaster, NH) were dispensed through a pellet dispenser centered 2.5 cm above the floor on the operant panel. A pair of retractable response levers and a pair of stimulus cue lamps, one above each lever, were positioned symmetrically on both sides of the pellet dispenser. The levers were 5.7 cm from midline and 7.0 cm above the floor and the cue lights were located 5.7 cm above the levers. Each chamber also contained a Sonalert tone generator, a white noise generator, and a house light located on the back wall. Experimental contingencies were programmed using the Med-State behavioral programming language (Med-Associates, Vermont).
Response shaping and lever press training
Rats were trained to press the response levers by using an autoshaping program that has been used extensively, both by our group and by others for lever press training (e.g. Newland et al., 1986; Verma et al., 1996; Widholm et al., 2001, 2003). Autoshaping test sessions terminated after 60 minutes elapsed or 100 reinforcers were delivered, whichever occurred first. Criterion for this condition was set at 100 lever presses within a single session. Following autoshaping, the rats were exposed to a continuous reinforcement schedule in which the lever associated with reinforcement alternated following delivery of every fifth reinforcer. The purpose of this schedule was to strengthen the recently acquired lever press response and to prevent the rats from developing a lever or side preference. This cycle of alternating levers terminated after 100 reinforcers were received or 60 minutes had elapsed. A performance criterion of 100 reinforcers for two consecutive sessions was established for this condition.
Training Phases
After lever-press training, the rats were trained on two alternation tasks that have produced consistent and replicable behavior across a variety of treatment paradigms (e.g. Gendle et al., 2004; Roegge et al., 2005; Widholm et al., 2004). The sequence began with cued alternation (CA) training in which a cue light indicated the correct lever on each trial. Each correct, cued lever press was reinforced, and levers were retracted and extended between trials. No delay was imposed between trials in the initial training phase (the time between retraction and extension of the levers was <0.15 seconds). Rats were trained to a criterion of one session above chance defined as >60% correct presses. Next, a non-cued alternation (NCA) task was presented where the cue light no longer indicated the correct lever, as both cue lights were illuminated when the levers were extended. Correct responses again consisted of alternating right and left lever presses, with the levers retracting and extending between presses. Each rat was tested for 10 sessions on the NCA task.
Delayed spatial alternation (DSA) testing
The testing phase was a DSA task in which variable delays of 0, 3, 6, 9, or 18 seconds were imposed randomly between trials (see Roegge et al., 2005; Wang et al., 2008, 2009; Widholm et al, 2004). There were 40 trials at each delay and a total of 200 trials per session. Delays were randomly balanced within each session and any specific delay was not presented on more than three consecutive trials. Each animal was tested for 25 sessions.
Serum and uterine horn collection
Following the completion of behavioral testing, all animals were given an overdose of CO2 and trunk blood was collected from the estradiol and vehicle control groups for determination of estradiol content. The uterine horn was then dissected from the peritoneal cavity of all treatment groups by separating the uterine horns from the underlying tissue, and excising the uterine body. Uterine horns were weighed immediately following removal.
Estradiol radioiummoassay (RIA)
To confirm estradiol serum levels in the positive control group, RIA analyses were conducted on serum collected from the estradiol treated group and the vehicle treated control group using the DSL ultra sensitive 125I-estradiol RIA (DSL-4800, Diagnostics Systems Laboratories, Webster, TX). The kit was run according to manufacturer’s instructions and has a lowest detection limit of 2.2 pg/mL, with intra- and interassay coeffcients of variance of less than 10%.
Statistical Analyses
The behavioral data were analyzed via repeated measures ANOVA using SPSS for Windows, Version 15.0. Treatment and cohort were included in the analyses as between subject factors and significance was set at p<0.05. When appropriate, Tukey post hoc tests were run for pair-wise comparisons. The Tukey post hoc analyses included all treatment groups, but to simplify presentation of the findings from the post hoc comparisons between groups, the results and the corresponding figures are organized by treatment (estradiol, DPN, PPT). Tukey is a conservative post hoc test that protects for experimentwise error when multiple comparisons are conducted, thus some differences that may appear to be significant on the graphs did not reach statistical significance in the Tukey comparisons. Many of these had p values between 0.05 and 0.10.
For CA, cumulative errors across all sessions and sessions to criterion served as the measures of learning, and were analyzed using between-subjects ANOVA for treatment and cohort. For NCA, the overall proportion correct across the ten sessions served as the primary measure of learning, and was analyzed using an 8 (treatment) × 2 (cohort) × 10 (session) mixed ANOVA where session was a repeated measures factor. Latency to respond following either a correct or an incorrect response during NCA testing were also analyzed using an 8 (treatment) × 2 (cohort) × 10 (session) mixed ANOVA where session was a repeated measures factor.
For DSA, the proportion correct across the 25 test sessions was first averaged across blocks of five test sessions to produce five 5-session test blocks. Proportion correct at each delay across the 25 test sessions was then analyzed using a mixed 8 (treatment) × 2 (cohort) × 5 (block) × 5 (delay) repeated measures ANOVA with block (1 – 5) and delay (0, 3, 6, 9, 18 sec) serving as repeated measures factors. Error pattern analyses were used to assess the rats’ tendency to repeat a correct or incorrect response. A win-stay error was defined as an incorrect response on the same lever that had been correct on the previous trial. A lose-stay error was defined as an incorrect lever press on the same lever that had been incorrect on the previous trial. Win-stay and lose-stay errors were analyzed separately using a mixed 8 (treatment) × 2 (cohort) × 5 (block) repeated measures ANOVA with block (1 – 5) serving as a repeated measures factor. Latency to respond following both a correct and incorrect response during DSA testing was also analyzed using an 8 (treatment) × 2 (cohort) × 5 (block) mixed ANOVA where block was a repeated measures factor.
Uterine horn weights were analyzed using between-subjects ANOVA with treatment serving as the between subjects factor. RIA results were analyzed via an independent samples t-test, with the estradiol treated and vehicle treated control groups serving as the between subjects factors.
Results
Cycling Data
Cycle status was classified as regular cycle (4–5 day cycle), irregular cycle (6–12 day cycle), or extended estrus (periods of estrus lasting 3 or more days) (Fentie et al., 2004). On average, about 1/3 of all rats were cycling regularly in each treatment group. The remaining rats had either an irregular cycle or an irregular cycle that included periods of extended estrus. All rats stopped cycling post-ovariectomy and remained in a phase resembling diestrus (Hubscher et al., 2005).
Serum Estradiol Levels
As expected, the estradiol treated group had significantly higher serum estradiol levels (Mean ± SEM = 20.82 ± 3.41 pg/ml) than vehicle treated controls (2.55 ± 0.61 pg/ml). This was confirmed by independent samples t-test comparing estradiol serum levels between the estradiol treated and vehicle control groups, t(13)=4.87, p<0.001.
Uterine Horn Weights
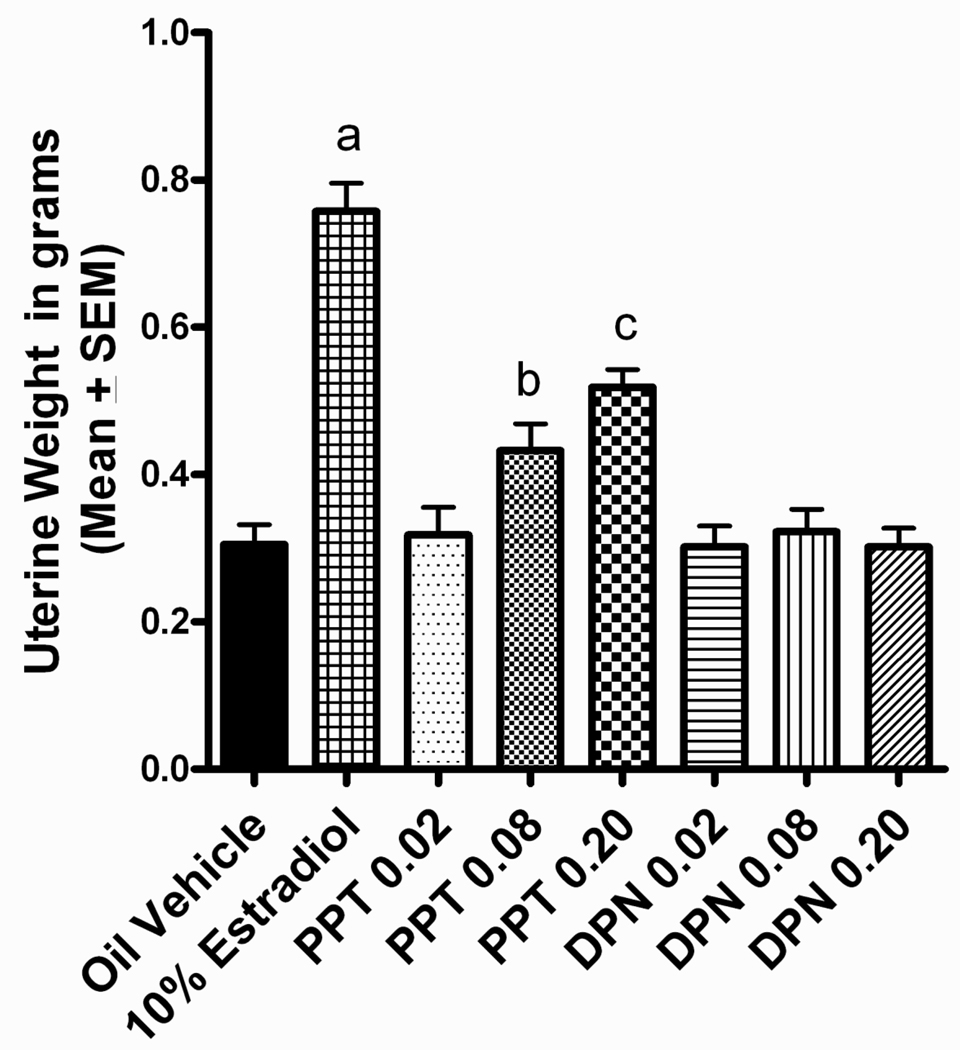
Both estradiol and PPT treatment increased uterine horn weights. This was revealed by a significant main effect of treatment, F(7,110)=29.326, p<0.001. Tukey post hoc analyses found the estradiol treated group to have heavier uterine horn weights than all other dose groups, p<0.05 (Figure 1). In addition, there was a dose-dependent effect of PPT on uterine horn weights, with both the high dose and middle dose PPT groups having heavier uterine horn weights than vehicle controls, p<0.05 (Figure 1). As expected, DPN did not alter uterine horn weight.
Figure 1.
Uterine horn weight in grams. The estradiol treated group had a heavier uterine horn than all other groups (ap<0.05). The middle dose PPT group had a heavier uterine horn weight than the vehicle control, high and low dose DPN group, but a lighter uterine horn than the estradiol treated group (bp<0.05). The high dose PPT group had a heavier uterine horn weight than all but the middle dose PPT group, while having a lighter uterine horn weight than the estradiol treated group (cp<0.05).
Cohort Effects
Because this experiment was conducted in two separate, equally balanced replicates of rats spaced 3 months apart, cohort was included as a factor in all statistical analyses for behavior. Few statistical effects of cohort were found. For DSA proportion correct, a significant 4-way interaction of block x delay x treatment x cohort was uncovered. F(112,1760) = 1.613, p=0.010. However, post hoc analyses revealed that a significant difference between cohorts was present in only one of the eight treatment groups, the high dose DPN group, and only at the 18-second delay during the final two blocks of testing. This effect did not appear to alter the overall treatment effects measured on the DSA task. A block x treatment x cohort effect was uncovered for latency to respond following an incorrect lever press, F(28,440) = 2.751, p=0.008. Although subsequent post hoc analyses failed to uncover any significant treatment by cohort differences, it appeared that the high dose PPT group in the first cohort had longer latencies to respond in the first block of testing. This effect did not carry over to subsequent blocks of testing, and, therefore, is unlikely to have altered the overall treatment effects observed on the DSA task.
Training Phases Prior to the DSA Task
Two training phases, CA and NCA, were run in sequence to train animals to alternate their responses between the two levers before beginning DSA testing. There were no significant treatment-related effects on the primary measures of learning during either of these training phases (p>0.05), although the high dose of PPT did increase the latencies to respond during the NCA phase of training. Specifically, a significant main effect of treatment was observed for latency to respond following a correct lever press, F(7,100)=3.463, p=0.002. Tukey post hoc analysis found that latency to respond was longer in the high dose PPT group than all three DPN dose groups and the estradiol treated group, p<0.05, but not than the other two PPT groups or the vehicle control group.
Delayed Spatial Alternation
Proportion Correct
The treatments did influence proportion correct during DSA testing. Repeated measures ANOVA uncovered a significant block x delay x treatment interaction, F(112,1760)=1.565, p=0.015. In addition, the block x treatment, F(28,1760)=2.173, p=0.006 and delay x treatment interactions, F(28,1760)=2.628, p<0.001, were also significant. The significant vehicle and estradiol-treated post hoc differences are indicated on Figures 2 and 3; however, for ease of comparison the data for the estradiol group are repeated on the figures depicting the DPN and PPT results (Figures 4–8).
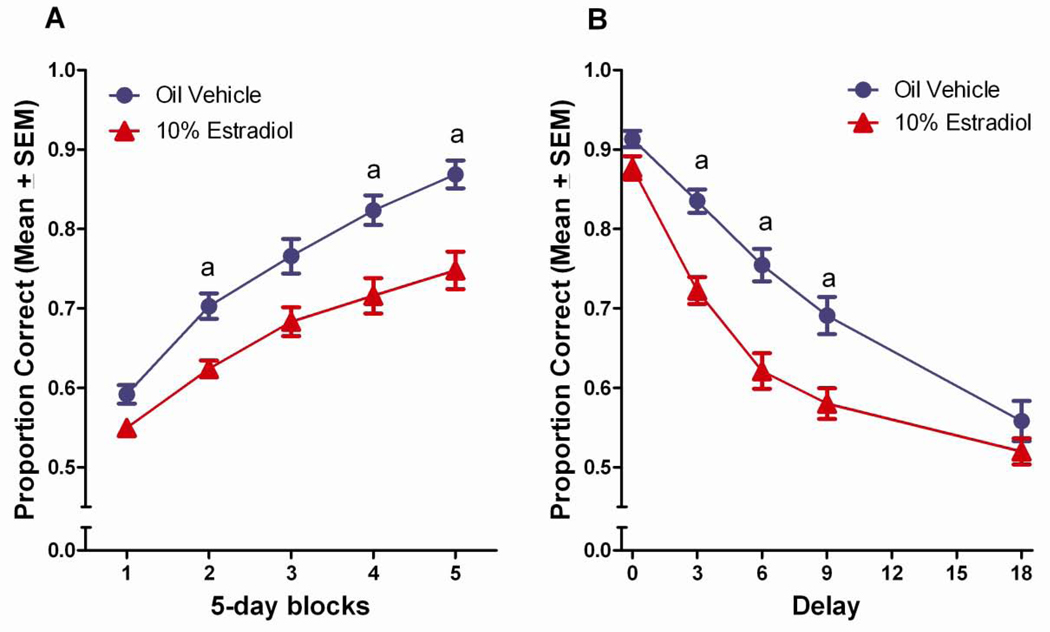
Figure 2.
(A) Proportion correct on the DSA task across 5-day blocks of testing for the vehicle control and estradiol-treated groups. aEstradiol treated<vehicle treated, (p<0.05). (B) Proportion correct on the DSA across 5 delays for the vehicle control and estradiol-treated groups. aEstradiol treated<vehicle treated, (p<0.05).
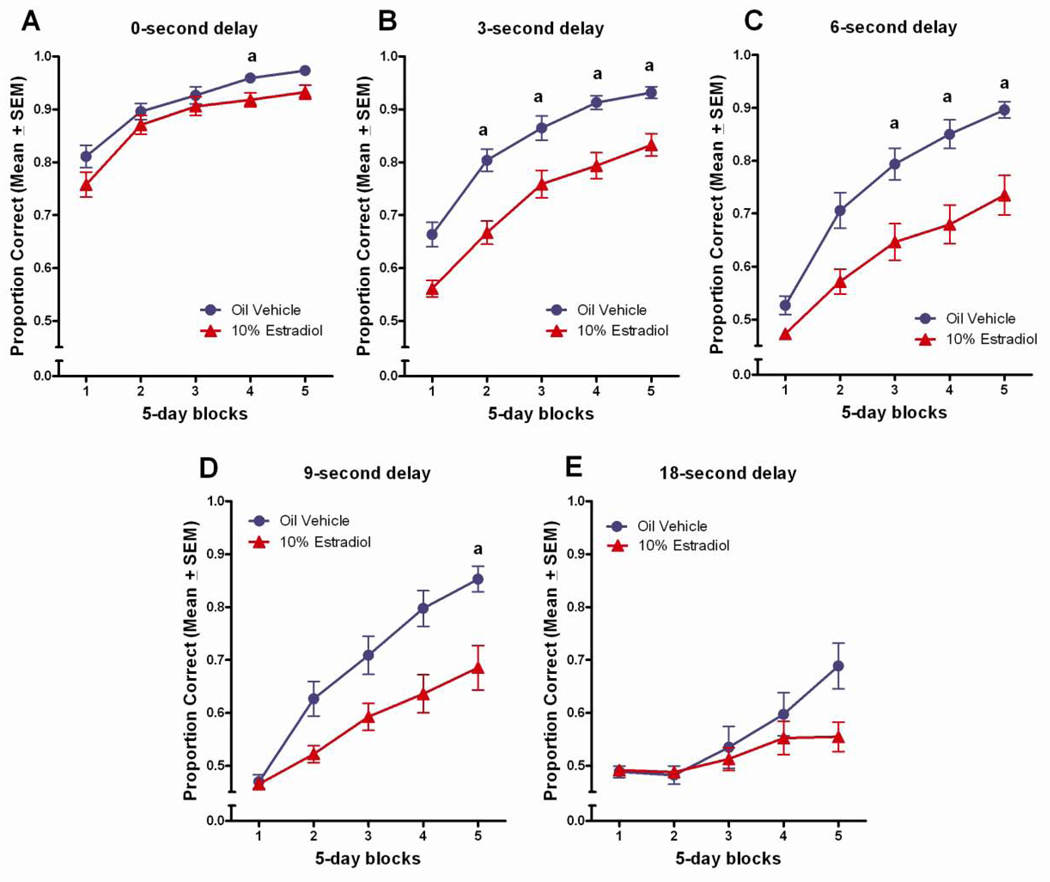
Figure 3.
Proportion correct across 5-days blocks of testing, by delay for the vehicle control and estradiol-treated groups. aEstradiol treated<vehicle treated, (p<0.05).
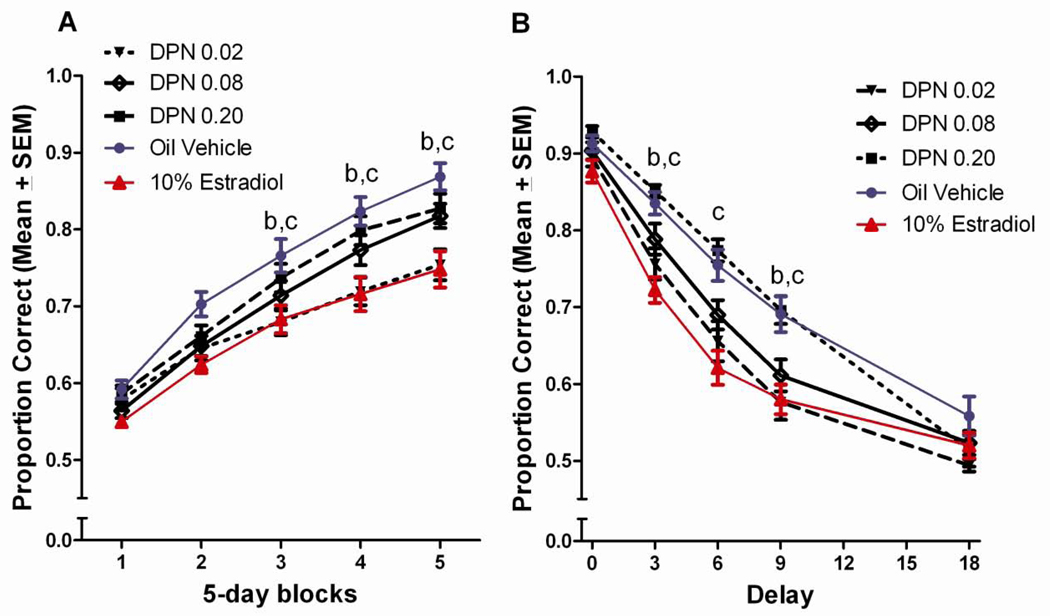
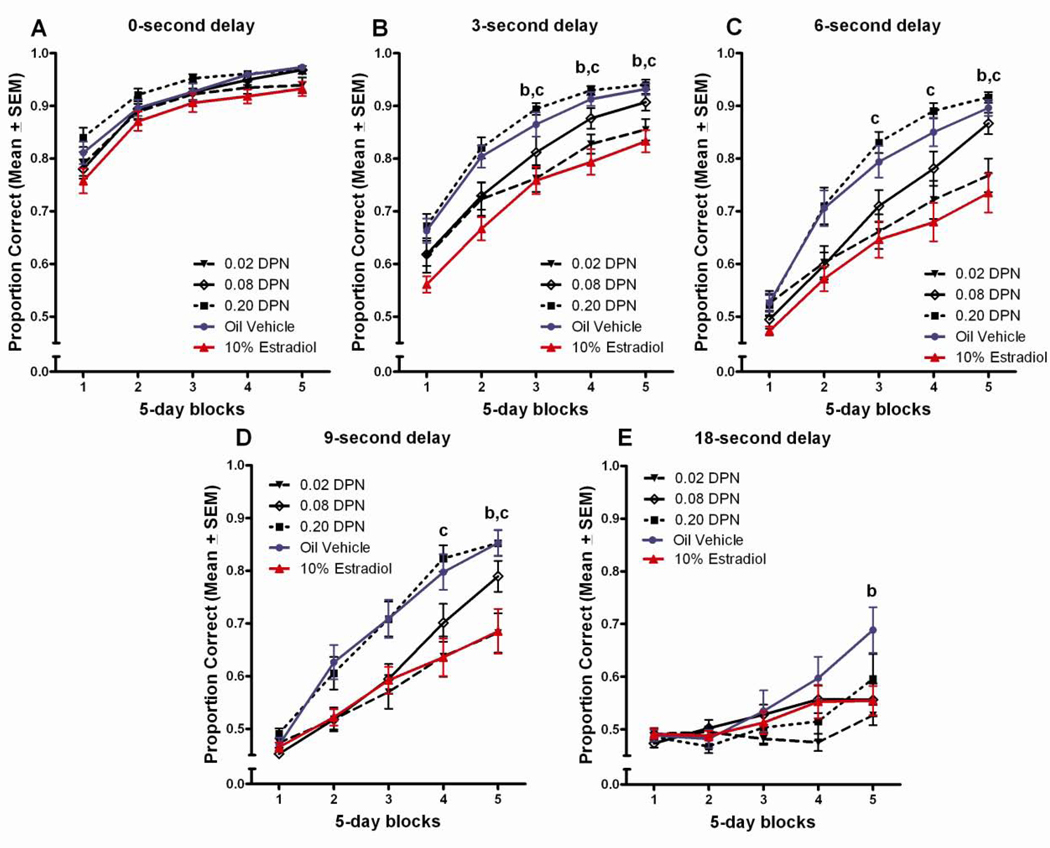
Figure 4.
(A) Proportion correct on the DSA task across 5-day blocks of testing for DPN treated groups. bLow dose DPN<vehicle treated, cLow dose DPN<high dose DPN, (p<0.05). (B) Proportion correct on the DSA across 5 delays for DPN treated groups. bLow dose DPN<vehicle treated, cLow dose DPN<high dose DPN.
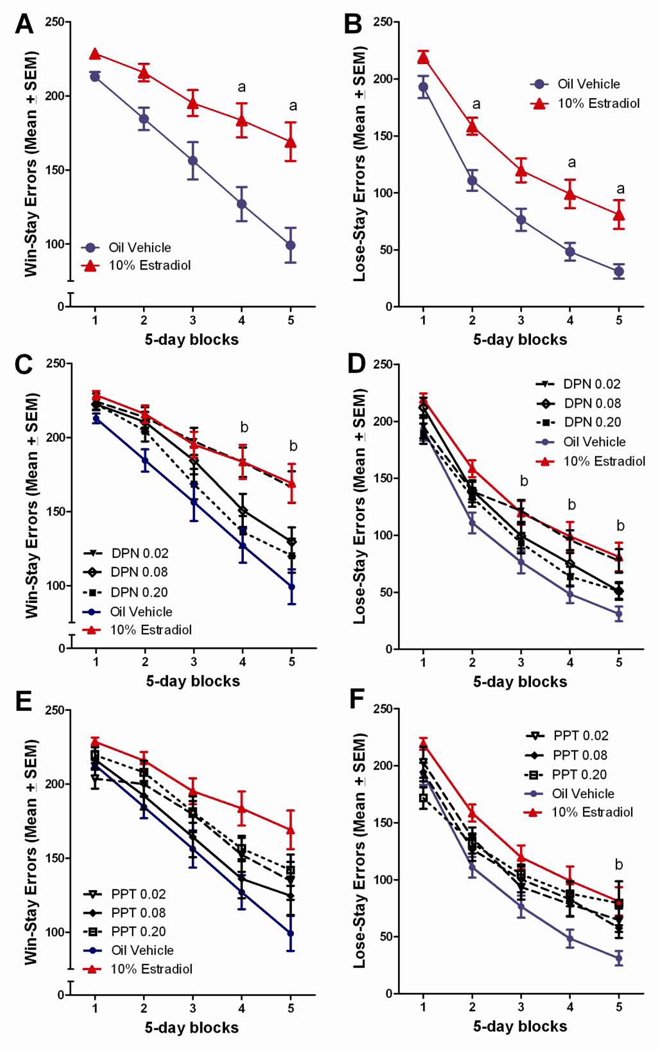
Figure 8.
(A) Total win-stay errors committed on the DSA task across 5-day blocks of testing for the vehicle control and estradiol-treated groups. aEstradiol treated>vehicle treated, (p<0.05). (B) Total lose-stay errors committed on the DSA task across 5-day blocks of testing for the vehicle control and estradiol-treated groups. aEstradiol treated>vehicle treated, (p<0.05). (C) Total win-stay errors committed on the DSA task across 5-day blocks of testing for the DPN treated groups. bLow dose DPN>vehicle treated, (p<0.05). (D) Total lose-stay errors committed on the DSA task across 5-day blocks of testing for the DPN treated groups. bLow dose DPN>vehicle treated, (p<0.05). (E) Total win-stay errors committed on the DSA task across 5-day blocks of testing for the PPT treated groups. (F) Total lose-stay errors committed on the DSA task across 5-day blocks of testing for the PPT treated groups. bHigh dose PPT>vehicle treated, (p<0.05).
Estradiol
Similar to our published findings, estradiol treatment impaired performance on the DSA task relative to vehicle treated controls (Wang et al., 2008, 2009). Tukey post hoc comparisons revealed that the estradiol treated rats performed significantly worse than vehicle controls did during the 2nd, 4th and 5th blocks of testing, p<0.05 (Figure 2a). Tukey post hoc comparisons following up on the delay x treatment effect found the estradiol treated animals to perform worse than the vehicle control group at the 3, 6, and 9 second delays, p<0.05, also consistent with our published findings (Figure 2b).
The estradiol induced deficit was also evident in the Tukey post hoc comparisons following up on the significant three-way block x delay x treatment interaction. At the 0 and 18 second delays, few group differences were noted, whereas at the 3-second delay, the estradiol treated group performed worse than the vehicle control group in blocks 2–5, p<0.05 (Figure 3b). At the 6-second delay, the estradiol treated group performed worse than the vehicle treated control group in blocks 3–5, p<0.05 (Figure 3c), and at the 9-second delay, the estradiol treated group performed worse than the vehicle control group in block 5, p<0.05 (Figure 3d).
DPN
There was a dose-effect function for DPN, although this effect was the reverse of typical dose functions, with only the lowest dose DPN group showing an impairment relative to the vehicle control group. This effect was similar in magnitude to that observed with 10% estradiol. The Tukey post hoc comparisons found the low dose DPN group to perform worse than both the vehicle treated control group and the high dose DPN group during the last three blocks of testing, p<0.05 (Figure 4a). Tukey post hoc comparisons following up on the delay x treatment interaction found the low dose DPN group to perform worse than the vehicle control group during the 3 and 9 second delays, and worse than the high dose DPN group at the 3, 6, and 9 second delays, p<0.05 (Figure 4b).
The impaired performance of the low dose DPN group was also evident in Tukey comparisons following up on the significant block x delay x treatment interaction. The low dose DPN group performed worse than the vehicle control group during testing blocks 3–5 at the 3-second delay (Figure 5b), and during the 5th block of testing at the 6 (Figure 5c), 9- (Figure 5d), and 18-second delays (Figure 5e), p<0.05. Additionally, the low dose DPN group performed significantly worse than the high dose DPN group during the 3rd–5th blocks of testing at the 3 (Figure 5b) and 6-second delays (Figure 5c), and during the 4th and 5th blocks of testing at the 9-second delay (Figure 5d), p<0.05.
Figure 5.
Proportion correct across 5-days blocks of testing, by delay for DPN treated groups. bLow dose DPN<vehicle treated, cLow dose DPN<high dose DPN, (p<0.05).
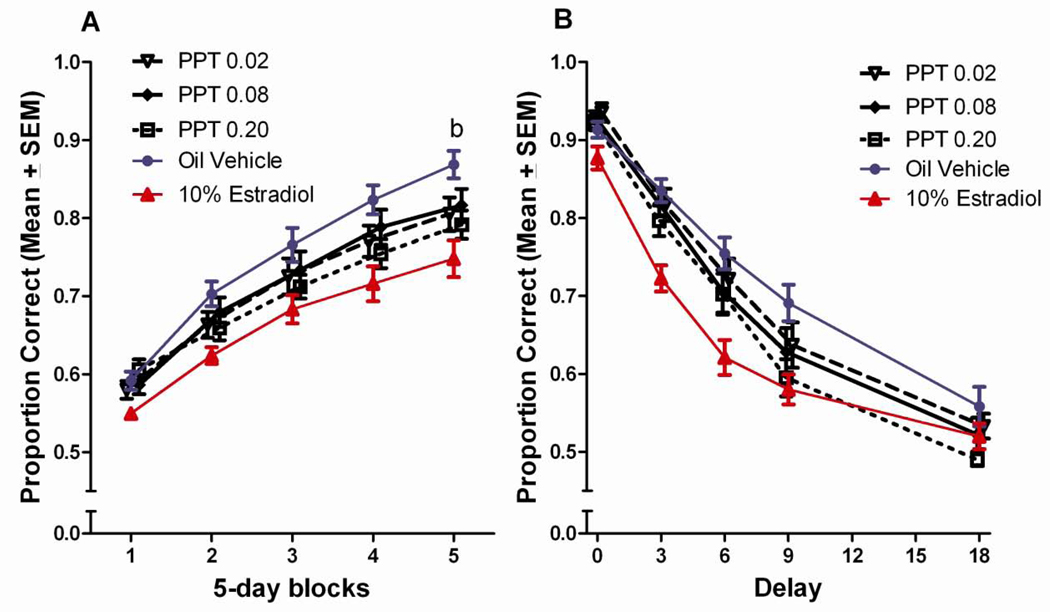
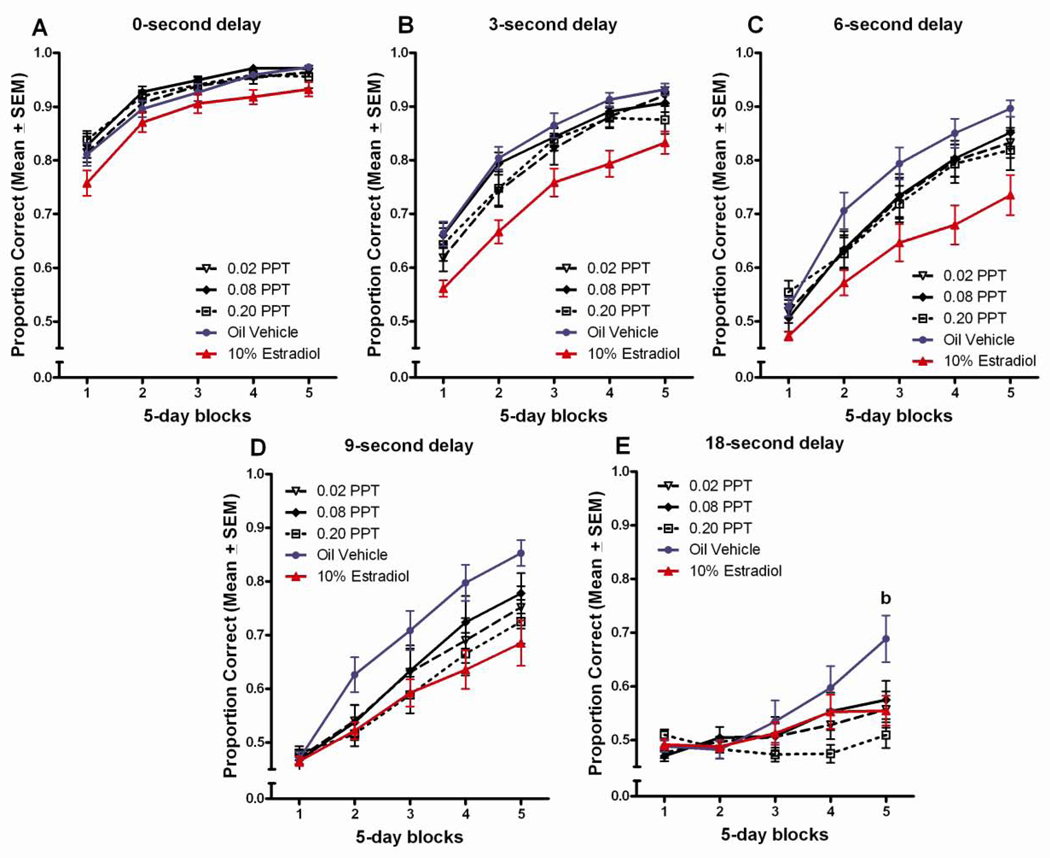
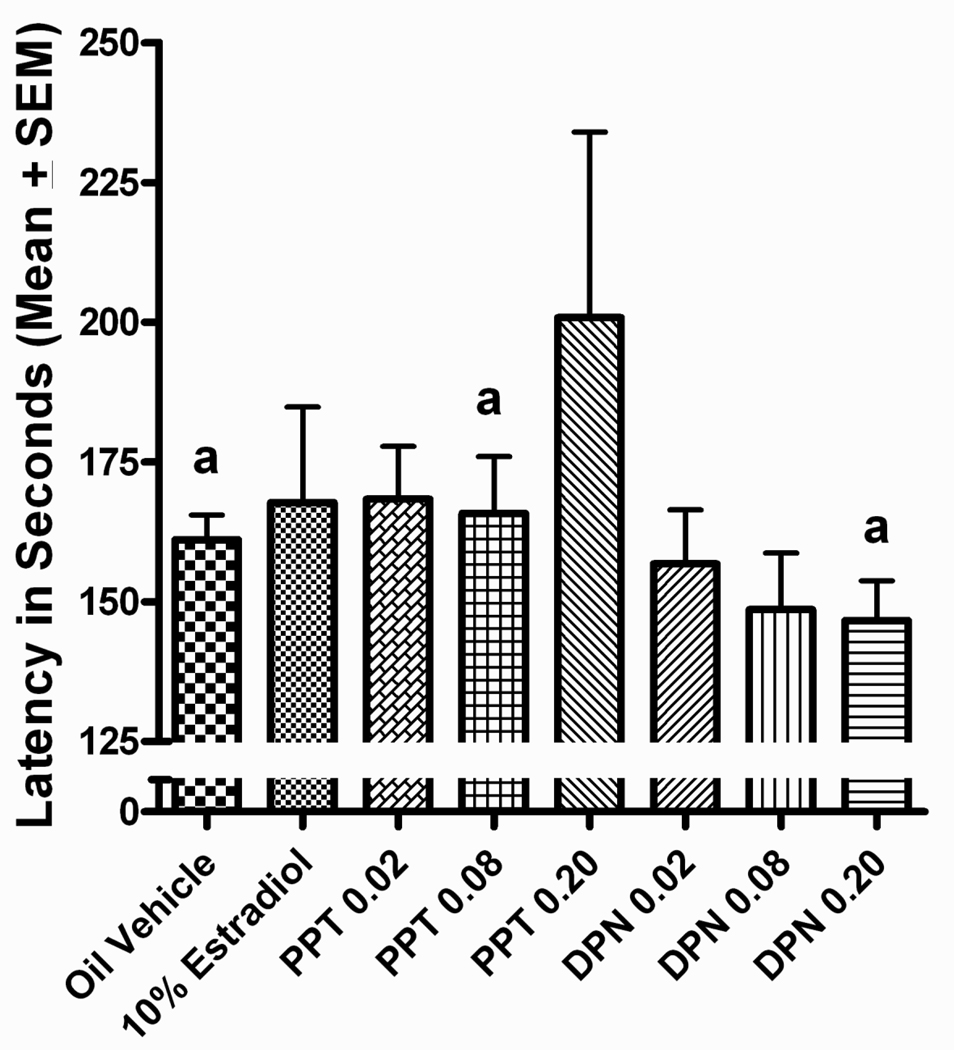
PPT
The effects of PPT on DSA performance were limited relative to those observed with DPN and estradiol and revealed no clear pattern of deficits or improvements relative to vehicle controls. The only evidence of a deficit relative to vehicle controls was in the last block of testing were the high dose PPT group performed worse than the vehicle control group, p<0.05 (Figure 6a). Tukey post hoc comparisons following-up on the delay x treatment interaction did not reveal any significant differences between the PPT groups and the vehicle control group at any of the delays (Figure 6b).
Figure 6.
(A) Proportion correct on the DSA task across 5-day blocks of testing for the PPT treated groups. bHigh dose PPT<vehicle treated, (p<0.05). (B) Proportion correct on the DSA across 5 delays for PPT treated groups.
Similarly, few effects of PPT treatment were revealed in post hoc comparisons following up on the significant block x delay x treatment interaction. The only evidence of a deficit relative to vehicle-treated controls was at the 18 second delay in the last block of testing, where the highest dose PPT group performed worse than the vehicle control group, p<0.05 (Figure 7e).
Figure 7.
Proportion correct across 5-days blocks of testing, by delay for PPT treated groups. bHigh dose PPT<vehicle treated, (p<0.05).
Errors
Treatment was found to influence error patterns during DSA testing. Repeated measures ANOVA revealed significant block x treatment interactions for both win-stay errors, F(28,440)=2.318, p=0.003, and lose-stay errors, F(28,440)=1.715, p=0.030. Significant main effects of treatment were also present for both win-stay errors, F(7,110)=3.425, p=0.002 and lose-stay errors, F(7,110)=3.033, p=0.006. As for the DSA proportion correct analyses, subsequent Tukey post hoc analyses that were conducted included all treatment groups, but to simplify presentation the findings from these post hoc comparisons are again organized by treatment (estradiol, DPN, PPT).
Estradiol
Estradiol treatment was found to influence error patterns during DSA testing, as previously reported (Wang et al., 2008, 2009). Tukey post hoc analyses found the estradiol treated group to commit significantly more win-stay errors than did the vehicle control group during the 4th and 5th testing blocks, p<0.05 (Figure 8a), and significantly more lose-stay errors than did the vehicle control group during the 2nd, 4th, and 5th blocks of testing, p<0.05 (Figure 8b).
DPN
Similar to estradiol, the low DPN dose also increased both win-stay and lose-stay errors during DSA testing. Tukey post hoc analyses found the low dose DPN group to commit significantly more win-stay errors than the vehicle control group during the last two testing blocks, p<0.05 (Figure 8c), and significantly more lose-stay errors than the vehicle control group during the 3rd–5th blocks of testing, p<0.05 (Figure 8d).
PPT
In line with the relative lack of effects of PPT treatment on proportion correct, PPT had relatively little effect on errors committed during DSA testing. The only significant difference from the vehicle control group occurred during the last block of testing, with the PPT group making more lose-stay errors than controls made, p<0.05 (Figure 8f).
Lever Press Latencies
PPT treatment tended to increase latencies to respond following an incorrect lever press, although this effect only reached statistical significance in the high dose group. This was revealed by a significant main effect of treatment for latency to respond following an incorrect lever press, F(7,110)=2.996, p=0.006. Tukey post hoc analyses found the high dose PPT group to have a longer latency to respond following an incorrect response than the vehicle control group, the high dose DPN group, and the middle dose PPT group, p <0.05 (Figure 9). In addition, significant block x treatment interactions were also observed for latency to respond following either a correct lever press, F(28, 440)=2.097, p=0.033 or an incorrect lever press, F(28,440)=2.194, p=0.034. However, Tukey post hoc comparisons failed to uncover any significant differences between treatment groups and the vehicle control group.
Figure 9.
Lever press latency following an incorrect response on the DSA task. The high dose PPT group had a longer latency than vehicle treated, high dose DPN, and middle dose PPT groups, (ap<0.05).
Discussion
The current study expanded upon our previous finding of a 17β-estradiol induced impairment on performance of an operant working memory task by investigating whether this effect was differentially modulated by selective ERα or ERβ activation. We replicated the performance deficit on the DSA task following 17β-estradiol treatment (Wang et al., 2008, 2009), and we reported new findings, suggesting that ERβ activation may play a more important role in the 17β-estradiol induced deficit in performance on the DSA task. Specifically, the lowest dose of the ERβ agonist DPN that we employed (0.02 mg/kg/day) produced significant performance deficits that were similar in magnitude to those observed with chronic 10% 17β-estradiol implants. As with 17β-estradiol, these impairments were present primarily at the shorter delays (3-, 6-, and 9-second), and are suggestive of prefrontal cortical disruption (Maruki et al., 2001; Sloan et al., 2006).
The low dose DPN treated rats also showed an impairment relative to vehicle controls at the 18-second delay, an effect not seen in 17β-estradiol treated rats in this or previous (Wang et al., 2008, 2009) studies. While this may be a departure from the pattern seen with 17β-estradiol, this difference was only present in the last block of testing and appeared to be driven primarily by the fact that the vehicle control group in the current study performed exceptionally well during the final block of testing (>70% accuracy) compared to controls in our previous studies (Wang et al., 2008, 2009). Finally, the low dose DPN group committed a mix of win- and lose-stay errors, closely paralleling the pattern of errors in 17β-estradiol treated rats.
Conversely, few significant changes were seen following treatment with the ERα agonist PPT. Although some subtle changes in DSA performance were evident following PPT dosing, only high dose PPT treatment produced a significant deficit in DSA performance, and this effect was present only at the 18-second delay during the final block of testing, similar to the effect seen with the low dose DPN treatment at the 18-second delay. Although this may be a real treatment-related effect, it also could be driven by the unusually good performance of the vehicle control group during the last testing block. At this stage, we cannot rule out a subtle effect of PPT on working memory. However, given that ERα activation by PPT is known to reduce food intake and body weight (Harris et al., 2002; Roesch, 2006; Santollo et al., 2007; Wegorzenska et al., 2008), it is possible that the subtle effect of PPT reflects decreased motivation to complete this appetitive task rather than a working memory deficit. The rats in this study were on a restricted food regimen and the PPT rats did consume their entire daily food ration. However, it was observed that the high dose PPT treated rats tended to take longer to consume all of their food than did other treatment groups, although time to consume daily rations was not recorded for experimental purposes. A motivational effect is further suggested by the fact that the high dose PPT treatment group had longer latencies to respond following an incorrect lever press. However, the fact that increased latencies were only observed after incorrect responses could also indicate increased reactivity to the lack of reward, that is, an ‘emotional’ response to committing an error (reviewed in Strupp and Beudin, 2006). Whatever the explanation for the extended latencies to respond, research has shown that the longer the latency to respond following a lever press the less likely it is that there will be a correct response on the following trial (Elsner, 1986; Elsner et al., 1988), as reflected in the performance of the high dose PPT group.
Rats in the 17β-estradiol treated group, which served as a positive control group in this study, were implanted with Silastic capsules containing 17β-estradiol because we have documented clear impairments in DSA performance using this route of exposure (Wang et al., 2008, 2009). In contrast, the PPT and DPN treatments were administered via injection. To control for this difference in route of exposure, all rats in the PPT, DPN and control groups were implanted with blank Silastic capsules at the time of ovariectomy. Similarly, because the stress of daily injections could potentially affect performance (see Foy et al., 2008; Holmes and Wellman, 2009; Lupien and Lepage, 2001), all rats in the 17β-estradiol and control groups received daily injections of vehicle. Although these controls were included, it is important to note that injections of PPT and DPN would be expected to lead to a different pharmacokinetic profile than implanted 17β-estradiol. The 17β-estradiol implants should result in a relatively stable exposure level throughout the study period (Mannino et al., 2005). Conversely, the injections of PPT and DPN would result in a peak of exposure shortly after injection (around 30 minutes), with a steady decline following this initial peak (Harris et al., 2002; Lund et al., 2005). The differential exposure profile following these two methods of delivery could have affected the results of the study, although the deficit we measured following DPN injection was similar in pattern and magnitude to that observed in the 17β-estradiol implant group. The magnitude of the 17β-estradiol induced deficit observed in this study was also very similar to that observed in our previous research (Wang et al., 2008, 2009). Thus, the impairing effect of 17β-estradiol on the DSA task did not seem to be altered by the addition of the daily injections. Similarly, the performance of the vehicle control group did not differ from that of control groups in previous studies, with the exception of somewhat better performance in the last block of test sessions at the 18 second delay. Therefore, it does not appear that daily injections produced a notable change in behavior on this task.
Although we did not directly measure serum levels of PPT or DPN, uterine horn weights were measured. Uterine proliferation is mainly regulated via ERα (Gruber et al., 2002), with previous research showing PPT to increase uterine horn weight, while DPN has not been reported to produce any changes in uterine weight (Harris et al., 2002; LeSaux and DiPaolo, 2005; Lund et al., 2005). As expected, we found a dose-dependent increase in uterine horn weight following PPT treatment, with the middle dose and high dose PPT groups having heavier uterine weights than vehicle controls had. Uterine horn weights following DPN treatment did not differ from control weights. Additionally, serum estradiol levels were tested in the positive control group. In our previous research (Wang et al., 2008, 2009) serum estradiol levels were measured with a Coat-A-Count RIA kit (Siemens Healthcare Diagnostics Inc., #TKE21, Los Angeles, CA) that we recently demonstrated over estimates estradiol levels in rodent serum (Neese et al, submitted; Wang et al., 2009). Based on our previous studies we would have predicted serum estradiol levels around 60 pg/ml in the positive control group and 35 pg/ml in the ovariectomized vehicle control group, but analyses with the more accurate DSL-4800 ultra sensitive estradiol RIA kit (Diagnostics Systems Laboratories, Webster, TX) yielded serum estradiol levels of around 20 pg/ml in the positive control group and around 3 pg/ml in the vehicle controls. These serum levels are well within the physiological range, resembling levels seen during the diestrus period (Overpeck et al., 1978). Although use of a different RIA kit for quantification yielded lower serum estradiol levels, we did accurately replicate our previously published behavioral effect (Wang et al., 2008, 2009), using implants made to the same specifications.
Recent research suggests that selective ERα or ERβ activation underlies certain aspects of cognitive behavior, although the majority of this work has targeted behaviors that engage the hippocampus (Hammond et al., 2009; Lund et al., 2005; Rhodes and Frye, 2006; Walf et al., 2006; Weiser et al., 2009). Of these, only one study reported similar effects following either ERα or ERβ activation (Hammond et al., 2009). In that study, PPT, DPN, 17β-estradiol, and a GPR30 agonist each restored performance in ovariectomized rats on a delayed matching to place T-maze task, suggesting that 17β-estradiol can work through a variety of estrogen receptors to improve performance on a task that engages both the hippocampus and prefrontal cortex. The present work expands this selective ERα or ERβ activation research to a working memory task that relies heavily on prefrontal cortical activation, specifically, a short-delay operant DSA task (Maruki et al., 2001; Sloan et al., 2006). As the prefrontal cortex mainly expresses ERβ (Chung et al., 2007; Khan et al., 2005; Mitra et al., 2003; Shughrue et al., 1997), it is not surprising to find an effect of the ERβ agonist DPN on this task. Interestingly, the effect measured with DPN was found at the lowest dose we tested, with the performance of the highest dose group being nearly identical to that of the vehicle treated control group. Given that the performance of the middle dose DPN group was somewhat impaired by DPN treatment, approaching significance in some blocks of testing, these results are suggestive of a monotonic dose-effect function, with the lowest dose being the most effective. Importantly, as other hormones have been shown to have non monotonic dose-response curves in various behavioral paradigms (Abrari et al., 2009; Boccio et al., 1998; Hu and Becker, 2008; Isaacson et al., 1995; Korol and Gold, 2007; Roozendaal, 2000), future studies should expand the dose range to include both lower and higher doses of DPN in order to confirm whether a linear or possibly a U-shaped dose-response curve in fact exists for DPN on this task.
The operant DSA task is a spatial working memory task that largely taps the prefrontal cortex at the delays tested here (Izaki et al., 2008; Maruki et al., 2001; Sloan et al., 2006). Related tasks that result in prefrontal cortical activation in humans, such as the subject-ordered working memory test (see Chudasama and Robbins, 2006 for a comparative review), have failed to find an effect of estradiol treatment in menopausal women, as treatment with conjugated estrogens did not influence performance (Janowksy et al., 2000). In addition, recent randomized trials using other testing paradigms that tap working memory suggest impaired performance in menopausal women following hormone therapy (Grady et al., 2002; Maki et al., 2007; Resnick et al., 2006). Our recent research found working memory deficits in middle-aged rats following 17β-estradiol treatment (Wang et al., 2009), and the current study suggests that ERβ activation may underlie this deficit.
The animals in this study were tested during the dark portion of a reverse light-dark cycle, whereas studies testing hormonal influences on behavior often test animals during the light phase of the cycle (e.g. Galea et al., 2001; Gresack and Frick, 2006; Korol and Kolo, 2002). Research suggests an interaction between performance on certain behavioral tasks (specifically those including delays) and the time of light cycle when testing occurs (Winocur and Hasher, 1999, 2004). Aged rats maintained on a reverse light-dark cycle performed better on both an operant delayed alternation task and a water maze task when tested in the early part of the dark cycle, while younger rats performed better when tested in the latter portion of the dark cycle, albeit in the water maze only (Winocur and Hasher, 1999, 2004). Due to the complex nature of these age and light/dark cycle interactions, it is difficult to predict how testing during the dark phase of the cycle may have affected the performance of the middle-aged rats used in this study.
Cycle status can influence a range of behaviors in aging rats, including activity levels (Kopp et al., 2006), as well as performance in some cognitive tasks (Markowska, 1999; Savonenko and Markowska, 2003; Warren and Juraska, 2000). Rats begin to undergo a loss of cyclicity around 12–14 months of age, resembling the menopausal transition in humans (Huang et al., 1978; LeFever and McClintock, 1988). Although all of the experimental animals were ovariectomized prior to behavioral testing, we cycled the animals in order to determine cycle status prior to surgery. A range of regular and irregular cycles were found, with these cycle variations being evenly distributed across treatment groups. As such, any potential differences due to differing cycle status should not have affected the outcome of this study.
The underlying effects of estrogen receptor activation and subsequent performance deficits on the DSA task may be mediated by a variety of prefrontal modulatory neurotransmitter systems (see Robbin and Arnsten, 2009). Of particular importance is the dopaminergic system (see Floresco and Magyar, 2006; Williams and Castner, 2006), with alterations in prefrontal dopamine resulting in impairments on delayed response tasks in rodents (Bubser and Schmidt, 1990; Kozlov et al., 2001; Mizoguchi et al., 2009; Vijayraghavan et al., 2007; Zahrt et al., 1997). Importantly, chronic 17β-estradiol treatment has been shown to reduce prefrontal dopamine in the rodent (Dupont et al., 1981; Luine et al., 1998; Pandaranandaka et al., 2006), an effect which may underlie the deficits in performance in both the 17β-estradiol and DPN-treated rats. Interestingly, chronic treatment (28 days) with 17β-estradiol alone or 17β-estradiol together with progesterone increased the fiber density of tyrosine hydroxylase containing fibers in the prefrontal cortex of ovariectomized adult rhesus monkeys (Kritzer and Kohama, 1998; Kritzer et al., 2003), an effect which might then lead to an increase in available dopamine in this brain region. Further, Gibbs et al. (2006) found chronic (2 year) dietary treatment with conjugated equine estrogens (CEE) together with medroxyprogesterone acetate (MPA) to produce subtle effects on brain monoamines and their metabolites. Specifically, CEE with MPA was found to reduce serotonin levels in the forebrain. Although the effects on brain dopamine were limited to a decrease in the hypothalamus, with the method used, dopamine levels were below the level of detectability in the frontal cortex. As the effects of 17β-estradiol treatment on dopamine levels in the adult primate prefrontal cortex are not known at this time, it is difficult to reconcile these findings with the rodent literature.
At this point, the effects of chronic selective ERβ activation on dopamine concentrations in the rodent prefrontal cortex are unknown. However, research does suggest a selective ERβ effect on the dopaminergic system in rodent brain areas outside of the prefrontal cortex. Specifically, short-term treatment (2 weeks) with DPN, but not PPT, upregulated the expression of both the dopamine transporter and the dopamine D2 receptor in the striatum and nucleus accumbens of ovariectomized rats in a manner similar to that seen following 17β-estradiol treatment (LeSaux and DiPaolo, 2006; LeSaux et al., 2006). These results must be taken with caution, as short term hormonal treatment may not have the same result as more chronic treatments. Specifically, acute 17α- and 17β-estradiol treatments have been shown to elevate prefrontal DOPAC, while marginally increasing dopamine (Inagaki et al., 2010). That said, our results are suggestive of a role for selective ERβ regulation of the prefrontal dopaminergic system. That is, the underlying mechanism of action through which both 17β-estradiol and DPN affect performance of the operant DSA task may be altered prefrontal DA function.
In summary, this study assessed the effects of ERα and ERβ agonists on the performance of middle-aged ovariectomized rats on an operant DSA task. Our previous research found chronic 17β-estradiol treatment to impair performance on this task (Wang et al., 2008, 2009), an effect which we replicated here. The current study also extended those results to reveal a similar impairment following ERβ activation, whereas ERα activation had only very subtle effects. The lowest DPN dose (0.02 mg/kg/day) impaired DSA performance to the same extent as 17β-estradiol, whereas the intermediate and high doses (0.08 and 0.20 mg/kg/day) did not differ significantly from vehicle controls, suggesting the possibility of a reverse dose-effect function. The deficits were observed primarily at short delays (3-, 6-, and 9-seconds), implicating prefrontal cortical dysfunction (Maruki et al., 2001; Sloan et al., 2006). Future research should include a broader range of doses in order to determine if the dose-effect function for DPN effects on DSA performance is linear or U-shaped. Subsequent research should also address whether 17β-estradiol and DPN act via the prefrontal dopaminergic system to produce these deficits.
Research Highlights.
17-β estradiol impairs the performance of ovariectomized middle-aged rats on an operant delayed spatial alternation (DSA) test of working memory.
The estrogen receptor β agonist diarylpropionitrile (DPN) also impairs the performance on this task, while the estrogen receptor α agonist propyl pyrazole triol (PPT) had limited effects.
These data confirm 17β-estradiol treatment has an impairing effect on the DSA task, and suggest that ERβ activation may underlie the deficit.
Acknowledgements
The authors would like to thank Dr. Meagan Mann and Dr. Victor Wang for their help in the completion of this work. This research was supported by National Institute on Aging Grant P01 AG024387 (SLS; JAK), National Institute of Diabetes and Digestive Diseases R37 DK015556 (JAK), and National Science Foundation IOB 0520876 (DLK). Steven Neese also received support from National Institute of Environmental Health Sciences Grant T32 ES007326.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol. Learn. Mem. 2009;91:260–265. doi: 10.1016/j.nlm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Kopf SR, Baratti CM. Effects of a single administration of oxytocin or vasopressin and their interactions with two selective receptor antagonists on memory storage in mice. Neurbiol. Learn. Mem. 1998;69:136–146. doi: 10.1006/nlme.1997.3817. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav. Brain. Res. 1990;37(2):157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys, and humans. Biol. Psychol. 2006;73(1):19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Chung WCJ, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Estorgen receptor beta splice variant protein (ERβ2) in the adult female rat forebrain and midbrain regions. J.Comp. Neurol. 2007;505:249–267. doi: 10.1002/cne.21490. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav. 1997;32(3):217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement ehances working memeory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147(1):607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediated the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbair S, Mizumori SJY. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol. Learn. Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Korol DL, Shors TJ. Steroids and Cognition. In: Pfaff D, Arnold A, Rubin R, Fahrbach S, Etgen A, editors. Hormones, Brain and Behavior. 2nd ed. New York: Academic Press; 2009. In press. [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004;28(7):699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dupont A, DiPaolo T, Gagne B, Barden N. Effects of chronic estrogen treatment on dopamine concentrations and turnover in discrete brain nuclei of ovariectomized rats. Neurosci. Lett. 1981;22:69–74. doi: 10.1016/0304-3940(81)90287-1. [DOI] [PubMed] [Google Scholar]
- Elsner J. Testing strategies in behavioral teratology: III. Microanalysis of behavior. Neurobehav. Toxicol. Teratol. 1986;8(5):573–584. [PubMed] [Google Scholar]
- Elsner J, Alder S, Zbinden G. Interaction between ethanol and caffeine in operant behavior of rats. Psychopharmacology. 1988;96:194–205. doi: 10.1007/BF00177560. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulated cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fentie IH, Greenwood MM, Wyss JM, Clark JT. Age-related changes in gonadal hormones in long-evans rats. Endocrine. 2004;25(1):15–22. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without delay. J. Neurosci. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory markers of brain aging. Neurobiol. Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17β-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav. Neurosci. 2008;122(2):301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm. Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav. Brain Res. 2001;126(1–2):115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Prenatal cocaine exposure does not alter working memory in adult rats. Neurotoxicol. Teratol. 2004;26(2):319–329. doi: 10.1016/j.ntt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Edwards D, Lazar N, Nelson D, Talameh J. Effects of long-term hormone treatment and of tibolone on monoamines and monoamine metabolites in the brains of ovariectomised, Cynomologous monkeys. J. Neuroendocrinol. 2006;18(9):643–654. doi: 10.1111/j.1365-2826.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann. N.Y. Acad. Sci. 1995;15(769):71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky H, Hsia J, Hully S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II) J.A.M.A. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115(1):135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschuggel W, Schneeberger C, Huber JC. Production and actions of estrogens. N. Engl. J. Med. 2002;346(5):340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm. Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in the estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology. 2002;143(11):4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res. Cogn. Brain Res. 1996;3(3–4):167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depen. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103(5):1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem. 2005;80(2):79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav. 2010;58(3):415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RL, Varner JA, Baars JM, de Wied D. The effects of pregnenolone sulfate and ethylestrenol on retention of a passive avoidance task. Brain Res. 1995;689:79–84. doi: 10.1016/0006-8993(95)00493-a. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Takita M, Akema T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur. J. Neurosci. 2008;27(11):3029–3034. doi: 10.1111/j.1460-9568.2008.06284.x. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J. Cogn. Neurosci. 2000;12(3):407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr. Mol. Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Khan MM, Hadman M, Wakade C, De Sevilla L, Dhanapani KM, Mahesh VB, Vadlamudi RK, Brann DW. Cloning, expression, and localization of MNAR/PELP1 in rodent brain: Colocalization in estrogen receptor-α- but not in gonadotropin-releasing hormone-positive neurons. Endocrinology. 2005;146(12):5215–5227. doi: 10.1210/en.2005-0276. [DOI] [PubMed] [Google Scholar]
- Kopp C, Ressel V, Wigger E, Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav. Brain Res. 2006;167(1):165–174. doi: 10.1016/j.bbr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav. Neurosci. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol. Learn. Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Hormones and Behavior. In: Kesner R, Martinez J, editors. Neurobiology of Learning and Memory. 2nd ed. New York: Elsevier; 2007. pp. 243–268. [Google Scholar]
- Kozlov AP, Druzin MY, Kurzina NP. The role of D1-dependent dopaminergic mechanisms of the frontal cortex in delayed responding in rats. Neurosci. Behav. Physiol. 2001;31(4):405–411. doi: 10.1023/a:1010488612338. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J. Comp. Neurol. 1998;395(1):1–17. [PubMed] [Google Scholar]
- Kritzer MF. Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cereb. Cortex. 2002;12(2):116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience. 2003;122(3):757–772. doi: 10.1016/s0306-4522(03)00548-7. [DOI] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol. Reprod. 1988;38(4):780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSaux M, DiPaolo T. Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology. 2005;30(3):251–260. doi: 10.1016/j.psyneuen.2004.08.002. [DOI] [PubMed] [Google Scholar]
- LeSaux M, DiPaolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J. Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- LeSaux M, Morrissette M, DiPaolo T. ERbeta mediated the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial task and effects levels of monoaminergic neurotransmitters. Horm. Beh. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J. Neuroendocrinol. 2008;20(6):866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;142(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav. Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J. Pain. 2005;6(12):809–816. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm. Behav. 2002;42(3):284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J. Neurosci. 1999;19(18):8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain Res. 2001;895(1–2):273–276. doi: 10.1016/s0006-8993(01)02084-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J.Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilknison HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Tanaka Y, Maruyama W, Tabira T. Age-related spatial working memory impairment is caused by prefrontal cortical dopaminergic dysfunction in rats. Neuroscience. 2009;162(4):1192–1201. doi: 10.1016/j.neuroscience.2009.05.023. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Rockville, MD: NIH/Office of Laboratory Animal Welfare. 2002
- National Research Council Institute for Laboratory Animals Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, D. C: National Academy Press; 2003. [PubMed] [Google Scholar]
- Neese SL, Koss WL, Lowry NC, Pisani SL, Korol DL, Juraska JM, Schantz SL. Comparison of Commercially Available Radioimmunoassay Kits for Determination of Low Serum Estradiol Concentrations in Rats. submitted. [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Miller RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34(3):231–241. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J. Toxicol. Environ. Health. 1978;4(5–6):785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsons S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol. Beh. 2006;87:828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol. Therapeut. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Ann. Rev. Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The Neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Ann. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Widholm JJ, Engeseth NJ, Wang X, Brosch KO, Seegal RF, Schantz SL. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicol. Teratol. 2005;27(3):485–495. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Roesch DM. Effects of selective receptor agonists on food intake and body weight gain in rats. Physiol. Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol. Learn. Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151(3):1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293(6):R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119(3):821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J.Comp. Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain, Res. 2006;171(1):116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective relationships. J.Med. Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Strupp BJ, Beaudin SA. Assessing the neurobehavioral effects of early toxicant exposure: a perspective from animal research. In: Bellinger D, editor. Human Developmental Neurotoxicology. New York: Taylor & Francis Group; 2006. pp. 415–445. [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann. N.Y. Acad. Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J. Neurosci. 1996;16(1):373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Beh. Neurosci. 2008a;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol. Learn. Mem. 2008b;89(4):513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HK, Ju Y, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav. Neurosci. 2008;122(4):794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm. Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus, and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48(8):2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurbiol. Learn. Mem. 2000;74(3):229–240. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, Handa RJ, Wu J. Postovariectomy weight gain in female rats is reversed by estrogen receptor α agonist, propylpyrazoletriol. Am. J. Obstet. Gynecol. 2008;199(1):67. doi: 10.1016/j.ajog.2007.11.054. e1–67.e5. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Wu J, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Neuroendocrinology. 2009;150(4):1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Toxicol. Appl. Pharmacol. 2001;174(2):188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol. Teratol. 2003;25(4):459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol. Sci. 2004;82(2):577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Aging and time-of-day effects on cognition in rats. Behav. Neurosci. 1999;113(5):991–997. doi: 10.1037//0735-7044.113.5.991. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Age and time-of-day effects on learning and memory in a non-matching-to-sample test. Neurbiol. Aging. 2004;25(8):1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Yener T, Tunc AT, Aslan H, Aytan H, Caliskan AC. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat. Histol. Emryol. 2007;36:75–77. doi: 10.1111/j.1439-0264.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17(21):8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol. Learn. Mem. 2006;86(3):336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144(1):26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]