Abstract
Transcription and multiple processing steps are required to produce specific 22 nucleotide microRNAs (miRNAs) that can regulate the expression of target genes. In C. elegans, mature lin-4 miRNA accumulates at the end of the first larval stage to repress its direct targets lin-14 and lin-28, allowing the progression of several somatic cell types to later larval fates. In this study, we characterized the expression of endogenous lin-4 and found that temporally regulated independent transcripts, but not constitutive lin-4 containing RNAs derived from an overlapping gene, are processed to mature lin-4 miRNA. Through an RNAi screen, we identified a conserved RNA binding protein gene rbm-28 (R05H10.2), homologous to the human RBM28 and yeast Nop4p proteins, that is important for lin-4 expression in C. elegans. We also demonstrate that rbm-28 genetically interacts with the lin-4 developmental timing pathway and uncover a previously unrecognized role for lin-14 and lin-28 in coordinating organismal growth.
Keywords: lin-4, miRNA, developmental timing, C. elegans, lin-14, lin-28, rbm-28
INTRODUCTION
MicroRNAs (miRNAs) are a large class of short regulatory RNAs that typically direct down-regulation of target genes. Since the discovery of the first miRNA gene, lin-4 and its target, lin-14 in C. elegans (Lee et al., 1993; Wightman et al., 1993), hundreds of miRNAs have been uncovered in many multicellular organisms, often with high levels of conservation across phylogeny (Niwa and Slack, 2007). The biological functions carried out by these small regulators are diverse, but the majority of work to date has characterized their roles in development, including developmental timing (Ambros and Horvitz, 1984; Feinbaum and Ambros, 1999; Lee et al., 1993; Moss et al., 1997; Reinhart et al., 2000; Slack et al., 2000; Wightman et al., 1993), spatial patterning (Yoo and Greenwald, 2005), defining left/right asymmetry in neurons (Chang et al., 2004; Johnston and Hobert, 2003), differentiation of specific tissues including neurons (Wu and Belasco, 2005; Wulczyn et al., 2007) and immune cells (Chen et al., 2004; Rodriguez et al., 2007; Thai et al., 2007), control of hox-gene expression (Stark et al., 2008; Woltering and Durston, 2008; Yekta et al., 2004), defining leaf polarity in plants (Garcia et al., 2006; Kidner and Martienssen, 2004; Nogueira et al., 2007), and fat storage and apoptosis in flies (Brennecke et al., 2003; Xu et al., 2003). In mammals, many miRNAs have been implicated in oncogenic pathways, either as tumor suppressors or as oncogenes (for review see (Visone and Croce, 2009)), roles that are coherent with their ability to promote cell division or differentiation.
The biogenesis of miRNAs is generally understood but complex regulatory mechanisms can be involved in processing some miRNAs (Kim et al., 2009). Transcription by RNA polymerase II generates miRNA primary transcripts, which can be multiple kilobases long. Endonucleolytic cleavage, performed by the Microprocessor complex comprised minimally of Drosha (an RNaseIII nuclease) and Pasha/DGCR8 (an RNA-binding protein), releases a hairpin structure containing the miRNA. This precursor is transported by Exportin 5 from the nucleus to the cytoplasm, where it encounters the RNaseIII nuclease Dicer, which in turn excises the mature miRNA and facilitates its loading into a protein complex, whose identity and role is defined by the presence of Argonaute protein family members. This complex utilizes the miRNA as a sequence-specific guide to mediate degradation or translational control of target mRNAs (Chekulaeva and Filipowicz, 2009). Transcription and each processing step can be subject to regulation and mounting evidence indicates that post-transcriptional regulation plays a significant role in controlling the expression of many miRNAs (Davis and Hata, 2009).
As the founding member of the miRNA family of small RNAs, the biological function of lin-4 has been extensively characterized. It was one of several genes that initially defined the heterochronic class (Ambros, 1989; Ambros and Horvitz, 1984); mutations in these genes affect developmental timing of certain tissues relative to gonad development, which is largely unaffected. For example, loss of function mutations in lin-4 cause reiteration of earlier somatic cell fates at later times, a condition known as retarded development, which is particularly noticeable in the hypodermal and vulval lineages of C. elegans. In contrast, the two most widely studied targets of lin-4, lin-14 and lin-28, cause the opposite effect when inactivated: some early stages are skipped altogether, in essence shifting the entire developmental cascade forward, which causes later developmental events to occur earlier than normal—a condition known as precocious development (Ambros, 1989; Ambros and Horvitz, 1984).
Consistent with its role in promoting the transition from the first (L1) to later larval cell fates, mature lin-4 miRNA accumulates towards the end of L1 (Chendrimada et al., 2007; Feinbaum and Ambros, 1999; Holtz and Pasquinelli, 2009). The FLYWCH transcription factors (flh-1, -2) have been shown to repress transcription of lin-4 during embryogenesis in C. elegans (Ow et al., 2008). However, inactivation of these genes did not result in mis-regulation of LIN-14 or heterochronic phenotypes during post-embryonic development, suggesting that other factors control lin-4 expression in larvae (Ow et al., 2008). Previous studies concluded that temporal accumulation of mature lin-4 during larval development is at the transcriptional level. Reporter strains expressing GFP fused to sequences upstream of lin-4 did not exhibit detectable fluorescence until the mid-L1 stage (Baugh and Sternberg, 2006; Esquela-Kerscher et al., 2005; Martinez et al., 2008; Ow et al., 2008), which is when endogenous mature lin-4 begins to accumulate.
In this study, we investigated the temporal expression of lin-4 by analyzing endogenous transcriptional start sites and primary transcript expression patterns. We find that transcriptional up-regulation of lin-4 primary transcripts is coincident with mature lin-4 accumulation, but it occurs in the context of ubiquitously expressed lin-4-containing transcripts that originate from an overlapping gene. We present evidence that only the independently expressed lin-4 transcripts are utilized for processing to the mature miRNA. Furthermore, the conserved RRM-domain protein RBM-28 (R05H10.2) was identified as a regulator of lin-4 accumulation. RNAi depletion of this factor results in strongly reduced levels of mature lin-4 without a significant effect on primary or precursor levels, suggesting it has a role in stabilizing the mature miRNA. Consistent with insufficient lin-4 levels, rbm-28 RNAi results in retarded heterochronic phenotypes. Finally, our study of this gene in controlling organismal development revealed a previously unknown function for lin-14 and lin-28 in coordinating growth of the entire organism.
MATERIALS AND METHODS
Worm strains and culture conditions
The following C. elegans strains were used: N2 Bristol, lin-4(e912) (DR721), lin-14(n179) (GR1106), lin-14(n179);lin-4(e912) (IH008), lin-28(n719) (GR1115), rrf-3(pk1426) (NL2099), pha-1(e2123) (GE24). pRBM28::GFP (BC14466) expresses GFP from the rbm-28 promoter. Worms were cultured at 20°C, unless otherwise indicated, and synchronized by vigorous shaking in hypochlorite solution (20% bleach, 0.5 M KOH) for six minutes, washing in M9 solution [22 mM KH2PO4, 42 mM Na2HPO4, 85.5 mM NaCl, 1mM MgSO4], and incubation in M9 solution overnight at 20°C. Development was initiated by plating starved L1 hatchlings on plates seeded with bacteria.
RNA interference
RNAi was carried out as described in (Kamath et al., 2003), except the IPTG concentration was increased from 1 mM to 5 mM. L4 stage worms were grown on RNAi plates seeded with bacteria containing vector control plasmids or plasmids expressing double-stranded RNA targeting the indicated gene. Synchronized, starved L1 progeny from these worms were grown on the same RNAi food for the indicated time before collecting for molecular or phenotypic analyses.
Expression constructs and transgenic strains
For the lin-4 promoter fusion construct, the plin4::GFP (pJRB3) plasmid was created by PCR amplification of ~400 bp of sequence 100 nt upstream of mature lin-4. This insert and the Fire plasmid pPD95.75 were digested with Age-I and ligated by standard subcloning techniques. pJRB3 was linearized with Spe-I and injected at 50 ng/ul per plasmid, along with the pha-1 rescuing plasmid pBX (Granato et al., 1994) at 50 ng/ul into pha-1(e2123) mutant animals, and transgenic animals were identified by rescue of pharynx development in F1 animals at 25°C.
The lin-4 sensor (pJRB15) was constructed from Fire Vector pPD136.15 (L4809), which contains the let-858 promoter driving GFP (containing 3x SV40 NLS) followed by the let-858 3′UTR. This vector was digested with Nhe-I and Apa-I to remove the let-858 3′UTR, which was then replaced by the lin-28 3′UTR that was amplified from genomic DNA. The ΔLCE construct (pJRB16) was generated by PCR stitching together two fragments overlapping in the region of the lin-4 site but containing an exact deletion of lin-4 complementary sequences. The ΔLCE lin-28 3′UTR was digested with Nhe-I and Apa-I and ligated into pPD136.15 as described above. For injection, pJRB15 and pJRB16 were digested with Pvu-I to linearize them, and were coinjected at 25 ng/ul along with the pha-1 rescuing plasmid pBX (Granato et al., 1994) at 75 ng/ul. This mixture was injected into the gonads of pha-1(e2123) mutant animals, and transgenic F1 animals were identified by rescue of pharynx development in F1 animals at 25°C.
RNA Analyses
For northern blotting analyses, total RNA from synchronized, staged worm populations was extracted using TRIzol reagent (GIBCO-BRL), and analyzed by PAGE northern blotting (<200 nt) or agarose northern blotting (>200 nt) as previously described (Bracht et al., 2004). Total RNA was separated by electrophoresis in 11% denaturing polyacrylamide gels or 1% agarose gels and transferred to nylon membranes (Zeta-Probe GT, Biorad, Hercules, CA). Probe templates are listed in Supplementary Table 1. Starfire labeled oligo probes (IDT) or single-stranded DNA probes labeled with the Prime-It Kit (Stratagene) were hybridized to the blots in prehypridization solution [5X SSC, 7% SDS, 0.02 M sodium phosphate, and 1X Denhardt’s solution] for approximately 12 hours at 50°C. Blots were washed twice in Wash Solution 1 [3X SSC, 5% SDS, 0.025 M sodium phosphate, and 10X Denhardt’s solution] and twice in Wash Solution 2 [0.4X SSC and1.4% SDS] at 50°C, before being exposed to a Typhoon Phosphorimager screen or a BioMax MR X-ray film overnight, and analyzed by ImageQuant software.
For RT-PCR analyses, total RNA from synchronized, staged populations was extracted using TRIzol reagent (GIBCO-BRL). All RNAs were subjected to two sequential deoxyribonuclease digestions using RQ1 DNase (Promega). cDNA synthesis was performed by random-primed RT (Invitrogen Superscript II, performed according to manufacturer’s instructions). Control –RT reactions were also performed to verify that RT-PCR products were dependent on reverse transcription of RNA. The resulting RT products were diluted 5-fold and used as templates for PCR with varying primer sets. All PCR reactions were carried out with Promega GoTaq as specified in manufacturer’s instructions but supplemented with MgCl2 to 1.5 mM final concentration. Reactions were carried out at 58°C annealing for 30 seconds, followed by extension at 72°C for 60 seconds per kb. Reaction cycles were adjusted by primer set to prevent saturation. Cycle numbers and primer sequences utilized for PCR are listed in Supplementary Table 1. Ethidium bromide stained agarose gels were scanned on a Typhoon Trio PhosphorImager (GE Healthcare) and band signals were quantified used ImageQuant software. The average and standard error of the mean (s.e.m.) from three or more independent experiments were calculated and a Student’s t-test was used to evaluate significance between samples.
For qRT-PCR analyses, RNA was extracted and cDNA synthesis was completed as described above with random oligos or oligo dT. qPCR was performed with SYBR Green (Applied Biosystems) and 6.25 pmol of each primer (Supplementary Table 1) on an ABI Prism 7000 real time PCR machine.
Rapid amplification of cDNA ends (RACE) methods
To identify the 5′ and 3′ ends of endogenous lin-4 primary transcripts, the Invitrogen GeneRACER kit was used as previously described (Bracht et al., 2004). Primers used for RACE analysis are listed in Supplementary Table 1. To clone potential Drosha cleavage products, a modified 5′RACE protocol (GeneRACER, Invitrogen) was adapted by the elimination of the initial phosphatase (CIP) and tobacco acid pyrophosphatase (TAP) treatments. Instead, 4 hour and 16 hour RNA samples were double RQ1-DNAse treated and added to the lyophilized RNA oligo. Ligation and all following steps were as per manufacturer’s instructions. Primers used for Drosha cleavage product analysis are listed in Supplementary Table 1. Products of this reaction were used as substrates for TOPO cloning and sequencing. Cleavage products were analyzed only if they contained the 5′ RNA oligo sequence ligated to lin-4 sequence.
RESULTS
Developmental regulation of lin-4 miRNA biogenesis
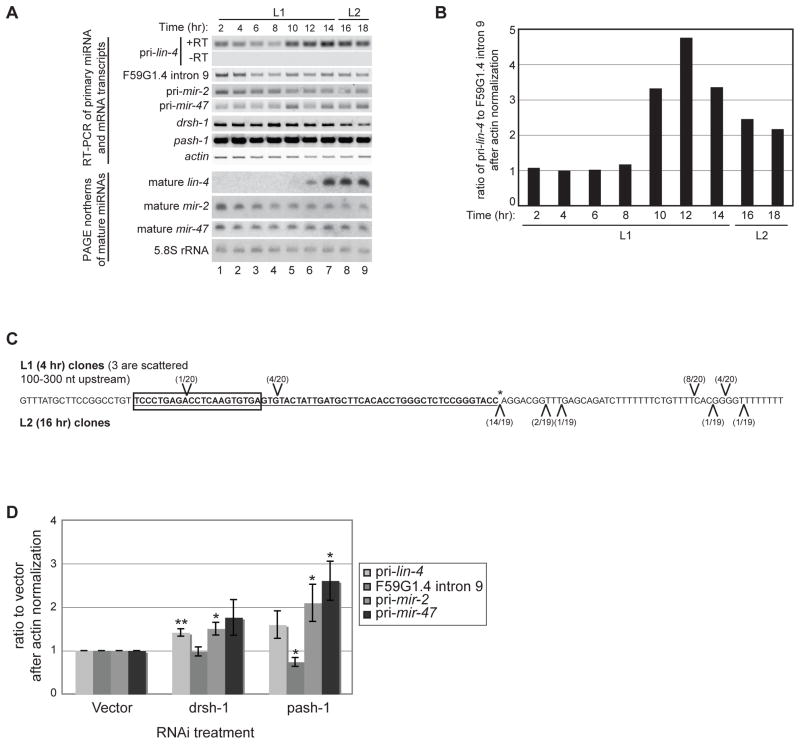
MicroRNAs that are embedded within introns of protein-coding genes are often co-expressed with a common transcript giving rise to both gene products (Baskerville and Bartel, 2005; Kim and Kim, 2007; Saini et al., 2007). Although lin-4 is encoded in the sense direction within the 9th intron of an overlapping gene F59G1.4 (see Fig. 2D below), transcription of the miRNA can be regulated independently because a relatively short (~700 nt), entirely intronic, DNA fragment is sufficient to rescue the lin-4(e912) null mutation (Lee et al., 1993). To analyze the temporal expression of potentially independent lin-4 transcripts relative to the overlapping gene, we collected RNA every 2 hours from 2 to 18 hours of development on food at 20°C from synchronized N2 wild-type worms. We performed random-primed reverse transcription of the RNA samples and used oligos that flank the lin-4 hairpin (pri-lin-4) or that correspond to the intron of the overlapping F59G1.4 gene (F59G1.4 intron 9) and are upstream of the minimal lin-4 rescue construct for PCR analyses. Both primer sets resulted in detectable products at all time points (Fig. 1A). Since the primers used to detect pri-lin-4 transcripts would also amplify sequences from the overlapping gene, we used quantitative RT-PCR (qRT-PCR) to compare the detection of the pri-lin-4 and F59G1.4 intron 9 products (Fig. 1B). In early L1, the ratio of these PCR products is one, indicating that signal from the pri-lin-4 PCR is from the overlapping gene. However, towards the end of the L1 stage, the pri-lin-4 signal increased 3–4 fold, likely resulting from independent expression of lin-4 primary transcripts. The up-regulation in pri-lin-4 transcription coincided with accumulation of mature lin-4 at the end of L1 (Fig. 1A). Taken together, these results suggest that expression of the overlapping F59G1.4 gene is responsible for the early detection of lin-4 containing transcripts; whereas up-regulation of lin-4 primary transcript expression at the end of L1 is supported by transcripts originating from within the overlapping gene.
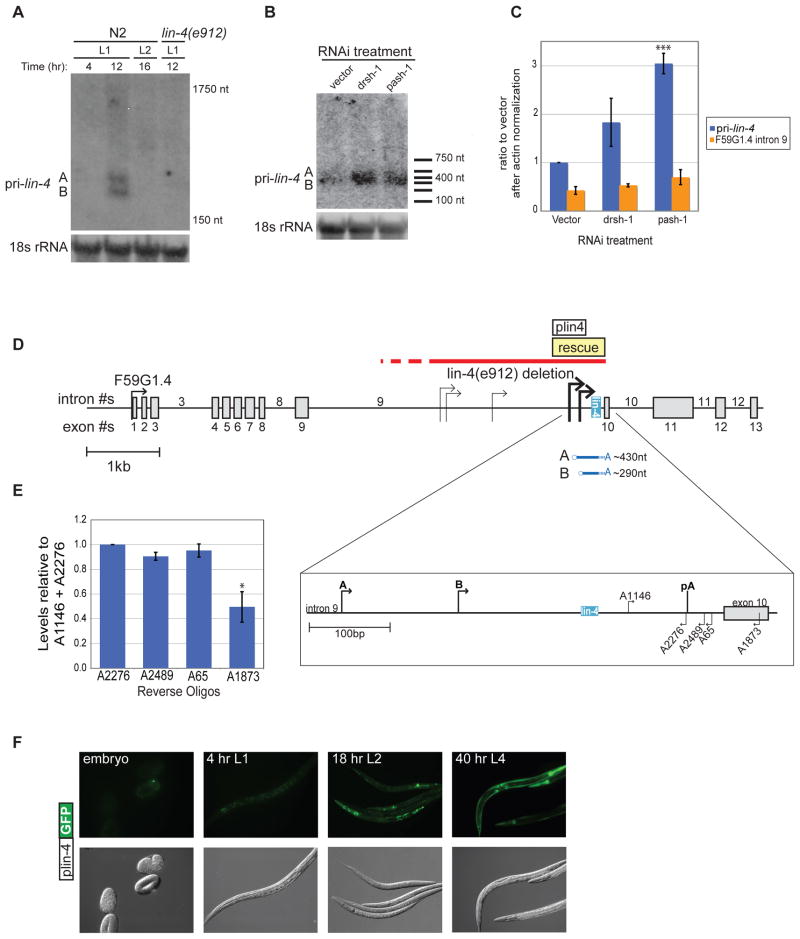
Figure 2.
Identification of primary lin-4 transcripts. (A) Total RNA was isolated from synchronized wild-type N2 or lin-4(e912) mutant worms at the indicated time points. Primary lin-4 transcripts and 18s rRNA were analyzed by agarose northern blotting. The sizes correspond to migration of the 18S and 5.8S rRNAs. Representative blots from three independent experiments are shown. (B) Synchronized rrf-3(pk1426) worms were subjected to vector control, Drosha (drsh-1) or Pasha (pash-1) RNAi and collected at the 12 hr (late L1) time point. RNA from three independent experiments was isolated and used for Northern blotting as described in (A). A representative blot is shown. Sizes based on an RNA marker are indicated. (C) The RNA samples from (B) were also used for qRT-PCR to detect pri-lin-4 and F59G1.4 levels. After normalization to actin mRNA, levels were compared to the vector pri-lin-4 sample. The average and s.e.m. of three independent sets are graphed and were analyzed by Student’s t-tests (***, p<0.0005) (D) Transcription start (arrows) sites for primary lin-4 within intron 9 of the predicted protein-coding gene F59G1.4 were mapped by 5′ RACE methods using RNA from L1 stage wild type worms at 12 hours of development. The indicated sites were detected in two independent experiments. The pri-lin-4 transcripts that correspond to the transcripts detected by northern blotting are depicted below the gene model. Enlargement of this region shows the predicted polyadenylation site (pA) and the primers used to map the 3′ end. The red line indicates the region of the lin-4(e912) null mutation, but the extent of the deletion is unknown (dashed line); the position of a minimal rescue fragment is shown with the yellow box (Lee et al, 1993). The white box indicates the lin-4 promoter region (plin-4) that starts ~500 nt upstream of mature lin-4 and was cloned into a GFP expression construct. (E) Three independent 12 hr RNA samples were used for qRT-PCR with a common forward primer (A1146) and a series of reverse primers. The average and s.e.m. of RNA levels relative to the A1146 + A2276 primer set are graphed and were analyzed by Student’s t-tests (*, p<0.05). (F) Representative images of transgenic worms expressing the plin-4::GFP reporter construct throughout development. Identical results were observed with 4 independent transgenic lines.
Figure 1.
Developmental expression of lin-4 transcripts. (A) Total RNA was isolated from synchronized wild-type N2 worms during the L1 and L2 stages at 20°C. RT-PCR was used to detect the indicated primary, intronic, and protein-coding sequences. PAGE northern analyses were used to detect mature miRNAs or control 5.8S rRNA from the same RNA preparations used for the RT-PCR analyses. (B) Total RNA was isolated from synchronized wild-type N2 worms at the indicated time points. Levels of primary lin-4, F59G1.4 intron 9, and actin mRNA were analyzed by qPCR after reverse transcription with random primers. Representative analyses of the ratio of pri-lin-4 to F59G1.4 intron 9 after actin normalization from three independent experiments are shown. (C) Total RNA from 4 hour L1 (above sequence) and 16 hour L2 (below sequence) wild-type N2 worms was used in 5′ RNA oligo ligation reactions to map potential Drosha cleavage products. The oligo ligation positions identified by cloning and sequencing are indicated relative to pre-lin-4 (bold and underlined), mature lin-4 (boxed) and the expected 3′ Drosha cleavage site (asterisk). (D) Synchronized rrf-3(pk1426) worms were subjected to vector control, Drosha (drsh-1) or Pasha (pash-1) RNAi and collected at the L2 stage. Four independent samples from each RNAi treatment were analyzed by RT-PCR to detect miRNA primary, F59G1.4 intronic or actin RNA levels, as indicated. The average and s.e.m. of RNA levels in drsh-1 and pash-1 relative to control RNAi are graphed and were analyzed by Student’s t-tests (*, p<0.05; **, p<0.005).
The presence of lin-4 containing transcripts in the form of intronic F59G1.4 sequences present throughout L1 and early L2 raised the question of whether they are utilized for miRNA processing differently than the independent lin-4 transcripts. The constitutive presence of lin-4 containing transcripts, but not mature lin-4 miRNA, in early L1 could result from failure of Drosha and its RNA binding protein partner Pasha to recognize or process these transcripts early in worm development. In general, these biogenesis factors appear to be present and active throughout the time course analyzed in Fig. 1A. We found steady state mRNA levels of drsh-1 and pash-1 to be relatively unchanged through the first 18 hours of worm development at 20°C (Fig. 1A). Moreover, the constitutive expression of primary and mature mir-2 and mir-47 RNAs (Fig. 1A) argues that the microprocessor complex is active but does not act on the early-accumulating lin-4 containing transcripts. In order to directly test for activity of the microprocessor on early versus late-accumulating lin-4 primary transcripts, we adopted a ligation and cloning approach to detect endogenous Drosha cleavage products. As expected, 80% of the cloned cleavage products from L2 stage worms mapped precisely to the predicted drsh-1 cleavage site (Fig. 1C; canonical cleavage site is marked with an asterisk). However, at early L1 none of the cloned products corresponded to canonical drsh-1 cleavage sites (Fig. 1C). To further investigate whether there is differential processing of lin-4 containing transcripts from within the intron of F59G1.4 versus from independent transcripts, we performed RNAi depletion against the miRNA processing factors Drosha (drsh-1) and Pasha (pash-1) and collected L2 stage RNA samples. Compared to vector control, RNAi against these processing factors resulted in accumulation of lin-4, mir-2, and mir-47 primary transcripts, but not the F59G1.4 intronic RNA (Fig. 1D). Taken together, these data indicate that independent transcription of lin-4 towards the end of L1 is important for producing transcripts that undergo processing to mature lin-4.
Characterization of endogenous lin-4 primary transcripts
To identify the independent lin-4 primary transcripts that accumulate towards the end of L1, we collected RNA from wild-type worms at the peak of pri-lin-4 expression (12 hrs) based on the qRT-PCR analyses, as well as at early L1 (4 hrs) and early L2 (16 hrs). By northern blotting, we detected two specific transcripts that migrated similarly to a ~300 nt marker (Fig. 2A). The two transcripts, designated A and B, were detected in the 12 hr, but not 4 or 16 hr wildtype samples, nor in a 12 hr sample from the lin-4(e912) deletion mutant (Fig. 2A). Furthermore, these transcripts accumulated upon drsh-1 or pash-1 RNAi conditions (Fig. 2B). RNAi of these microprocessor factors resulted in a ~2–3 fold increase in pri-lin-4 levels but had no affect on the expression of intron 9 of the overlapping gene (Fig. 2C). Thus, these lin-4 transcripts are specific, temporally regulated and substrates for miRNA processing.
To characterize the lin-4 primary transcripts, we performed 5′ and 3′ RACE (rapid amplification of cDNA ends) using RNA from the late L1 12 hr time point. Five potential 5′ start sites were reproducible in two independent cloning experiments (Fig. 2D). Two of these sites reside within the lin-4 rescue sequence and, when combined with our 3′ end analyses, correspond to the sizes of the lin-4 primary transcripts detected by northern blotting (Fig. 2A, B). Although we were unable to map the 3′ end using RACE methods, two lines of evidence suggest that the lin-4 primary transcripts end shortly after a canonical polyadenylation sequence (AAUAAA) located 129 nt downstream from the mature lin-4 sequence. Northern blotting using a probe that starts in exon 10 and extends to the 3′ end of exon 11 of the unspliced overlapping gene detected an in vitro synthesized control RNA but not the ~300nt pri-lin-4 transcripts (data not shown). Additionally, using a series of reverse primers near the polyadenylation site for qRT-PCR, we observed a significant decrease in pri-lin-4 detection about 50 nt downstream from this site (Fig. 2E). Taken together, these results show that the lin-4 gene expresses transcripts from two different start sites that terminate shortly after a canonical polyadenylation site to produce transcripts of ~430 and ~290 nucleotides (Fig. 2D). Notably, both start sites and the polyadenylation site are all included in the original minimal lin-4 rescue construct (Lee et al., 1993).
In order to test whether sequences associated with the mapped start sites are sufficient to drive transgene expression, we created a promoter-GFP construct that includes the two sites within the rescue region (Fig. 2D). Consistent with a previous report (Esquela-Kerscher et al., 2005), we found that constructs that contained only the rescue-fragment promoter (plin-4) expressed GFP in pharynx, neurons, and seam cells coincident with when mature lin-4 is detected at the end of the first larval stage (Fig. 2F). We also compared this expression pattern to a previously published reporter strain that includes about 2kb of sequence upstream of the rescue fragment (Martinez et al., 2008; Ow et al., 2008). No differences in temporal or spatial expression patterns were detected in strains expressing our plin-4 reporter and the longer lin-4 promoter constructs (data not shown).
Regulation of lin-4 expression by RBM-28
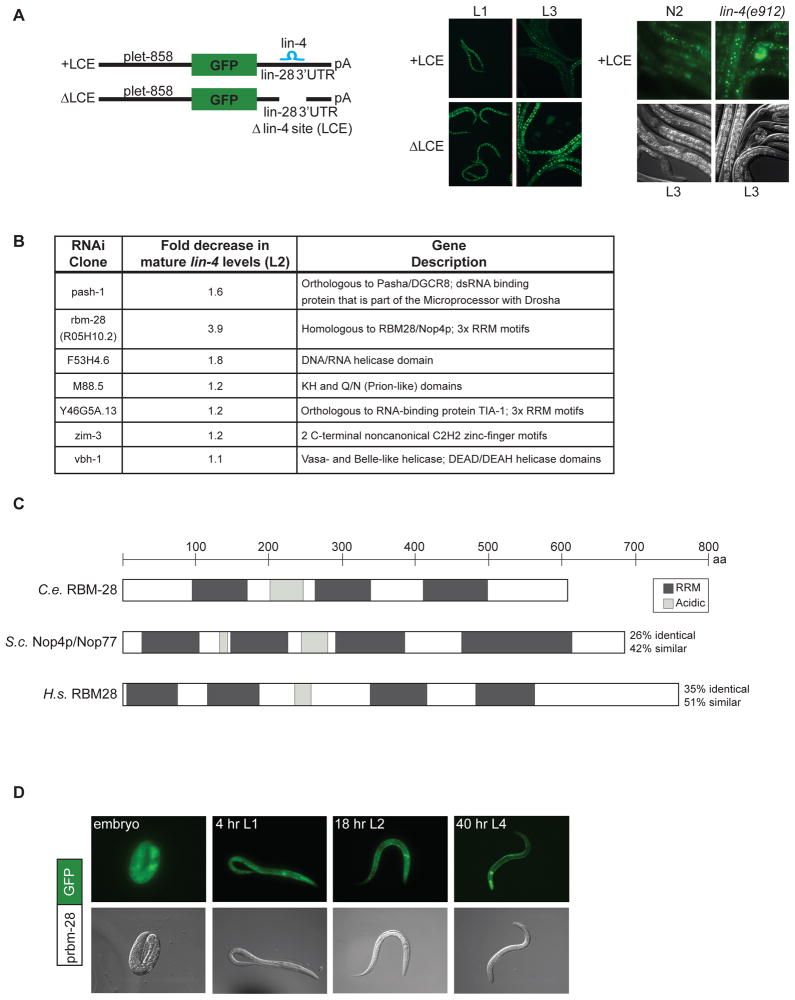
To identify factors involved in the transcription, processing or stability of lin-4, we performed a candidate-based screen for potential regulators of lin-4 biogenesis. Candidate regulators of lin-4 expression were chosen on the basis of two criteria: 1) genes annotated as nucleic-acid binding by Gene Ontology (GO), or 2) genes implicated as miRNA pathway effectors through genetic or proteomic methods (Gregory et al., 2004; Parry et al., 2007; Shiohama et al., 2007). Since insufficient lin-4 miRNA causes obvious developmental abnormalities, we eliminated nucleic acid binding candidates that lacked evidence for phenotypic effects in previously reported whole-genome RNAi screens (Kamath et al., 2003; Sonnichsen et al., 2005), assuming either that they are not regulators of the lin-4 pathway or that they are not sufficiently inactivated by feeding RNAi. This narrowed the candidate list from 1100 to 216. The addition of the miRNA pathway effectors from the literature brought the total candidates tested to 249 (Supplementary Table 2). For this RNAi screen, we created a GFP-based lin-4 sensor, using the 3′UTR of its established target lin-28 (Moss et al., 1997). Transgenic worms ubiquitously expressing GFP fused to the lin-28 3′UTR containing an intact lin-4 complementary element (+LCE) exhibited decreased fluorescence from L1 to L3 in a lin-4 dependent manner, and deletion of this site (ΔLCE) prevented down-regulation of GFP (Fig 3A). To identify genes that regulate expression of functional lin-4 miRNA, we screened for RNAi candidates that resulted in premature (negative regulators) or failed (positive regulators) down-regulation of GFP fused to the +LCE 3′UTR. The 24 RNAi clones that passed this initial screen were then tested for effects on GFP expression from the control LCE reporter. Only 6 clones passed this secondary screen (Fig. 3B), indicating that many candidates affected the +LCE reporter independently of lin-4.
Figure 3.
A candidate-based RNAi screen for factors involved in lin-4 biogenesis. (A) A visual sensor for lin-4 activity was generated by fusing the promoter of let-858, which drives ubiquitous expression, to GFP appended to the lin-28 3′UTR with an intact (+LCE) or deleted (ΔLCE) lin-4 complementary element. Representative micrographs show down-regulation of the +LCE GFP reporter in L3 compared to L1 worms and persistent expression in lin-4(e912) mutants or in transgenics that lack the lin-4 site (ΔLCE). (B) The pash-1 positive control and 6 candidates that passed the visual RNAi screen were analyzed for mature lin-4 levels during L2 by northern blotting. The average decrease in lin-4 compared to vector control RNAi conditions from three independent experiments is listed along with descriptions of the genes. (C) RBM-28 is homologous to the human RBM28 protein (Damianov et al., 2006) and the yeast Nop4 protein (Sun and Woolford, 1997). All proteins contain multiple RNA recognition motifs (RRMs) (dark grey) and one or more acidic regions (light grey). (D) Worms expressing GFP driven by the rbm-28 promoter were analyzed throughout development. Representative images are shown.
To distinguish if mis-regulation of the lin-4 sensor by the six candidates could be attributed to defective lin-4 miRNA biogenesis versus function, we performed northern blot analysis of the expression of mature lin-4 in L2 stage worms undergoing RNAi against each of these genes. The positive control pash-1 (RNAi) treatment resulted in a ~1.6-fold decrease in mature lin-4 levels (Fig. 3B). Strikingly, inactivation of rbm-28 caused a nearly 4-fold decrease in mature lin-4 (Fig. 3B). RNAi depletion of the other five genes resulted in minor or variable effects on endogenous mature lin-4 levels (Fig. 3B), and they were not pursued further.
The C. elegans R05H10.2 gene, renamed rbm-28, encodes an RNA binding protein with homology to the human RBM28 and S. cerevisiae Nop4/Nop77 proteins (Damianov et al., 2006; Sun and Woolford, 1994; Sun and Woolford, 1997). These proteins contain acidic regions and multiple RNA recognition motifs (RRMs) (Fig. 3C). To examine the spatial and temporal expression pattern of RBM-28, we utilized a transgenic worm strain expressing GFP fused to the promoter of this gene. We detected constitutive GFP expression in all somatic tissues in embryos and during larval development (Fig. 3D). Thus, RBM-28 is potentially present and in the same tissues as lin-4 to regulate its expression. However, the up-regulation of independent lin-4 transcription and accumulation of mature lin-4 at the end of L1 does not seem to be associated with developmentally regulated expression of RBM-28.
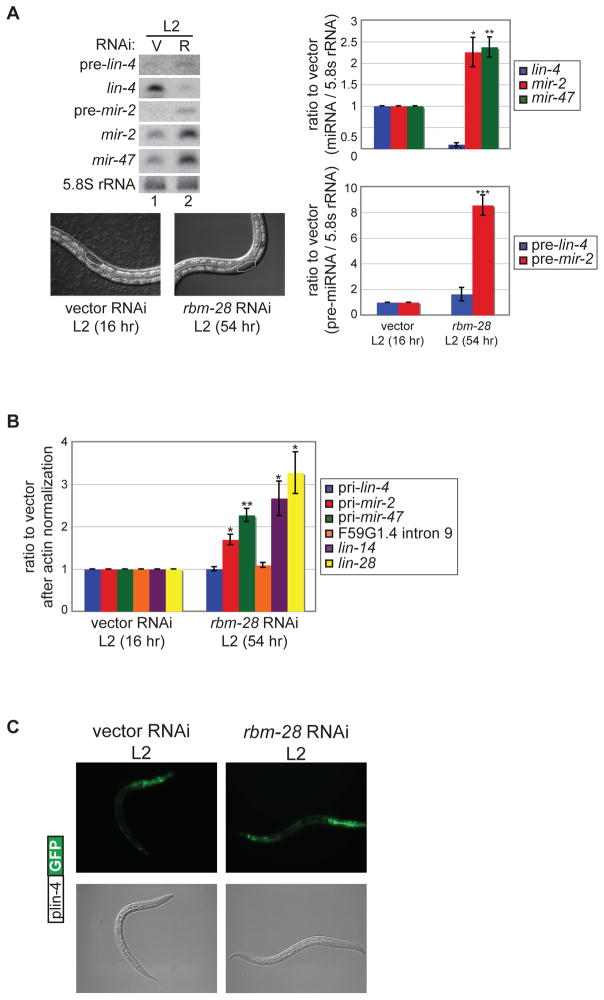
Further analysis of rbm-28 revealed that it has different effects on the biogenesis of lin-4 compared to two other miRNAs. Since rbm-28 RNAi results in delayed growth (see below), we analyzed the expression of lin-4 and other miRNAs in synchronized worms collected at the L2 stage based on gonad development. While depletion of rbm-28 resulted in ~4-fold less mature lin-4 miRNA, steady state levels of the constitutive mir-2 and mir-47 miRNAs were elevated ~2-fold (Fig. 4A). The increase in mir-2 correlated with increased levels of precursor and primary transcripts; mir-47 primary transcripts were also elevated in rbm-28 compared to vector control RNAi but precursor was undetectable under both conditions (Fig. 4A, B). We observed no significant change in levels of lin-4 precursor or primary RNA in rbm-28 compared to vector control RNAi conditions (Fig. 4A, B). Additionally, we found that the plin-4:GFP transcriptional reporter was also expressed similarly in control versus rbm-28 RNAi conditions (Fig 4C). These results indicate that rbm-28 has a role in stabilization of mature lin-4 since transcription and primary and precursor lin-4 levels were unaffected by RNAi depletion of this factor. In contrast, the effect of rbm-28 inactivation on mir-2 and mir-47 is consistent with an increase in transcription or stabilization of primary transcripts leading to greater levels of mature miRNAs.
Figure 4.
Characterization of the role of rbm-28 in miRNA biogenesis. (A) Synchronized rrf-3(pk1426) worms were cultured at 20°C on vector control (V) or rbm-28 (R) RNAi and collected at the L2 stage based on size and gonad development (16 hours for vector and 54 hours for rbm-28). Micrographs are shown of representative worms under the RNAi conditions with the L2 stage gonad shape circled. RNA from the vector and rbm-28 RNAi treated L2 worms was used in PAGE northern blot experiments to detect precursor and mature lin-4, mir-2 and mir-47 miRNAs, and control 5.8S rRNA. The average and s.e.m. of three independent experiments comparing vector to rbm-28 RNAi are shown and were analyzed by Student’s t-tests (*, p<0.05; **, p<0.005; ***, p<0.0005). (B) RNA from L2 stage worms grown on vector and rbm-28 RNAi was subjected to RT-PCR to detect the indicated primary miRNA, intronic F59G1.4 and protein-coding RNAs. The average and s.e.m. of three independent experiments comparing rbm-28 to vector RNAi are shown and were analyzed by Student’s t-tests (*, p<0.05; **, p<0.005). (C) Transgenic worms expressing the plin-4::GFP reporter were grown on vector or rbm-28 RNAi, and analyzed for GFP expression during the early L2 stage based on size and gonad development (16 hours for vector and 42 hours for rbm-28). Representative images are shown.
The diminished expression of mature lin-4 under rbm-28 RNAi conditions is associated with mis-regulation of its target genes. Up-regulation of lin-4 miRNA at the end of the first larval stage promotes mRNA degradation of two characterized direct targets, lin-14 and lin-28 (Bagga et al., 2005). We detected ~2-fold higher levels of lin-14 and lin-28 mRNAs in L2 worms undergoing RNAi against rbm-28 (Fig. 4B), prompting us to examine the interplay between rbm-28 and lin-4 target genes.
Interaction of rbm-28 with the lin-4 developmental timing pathway
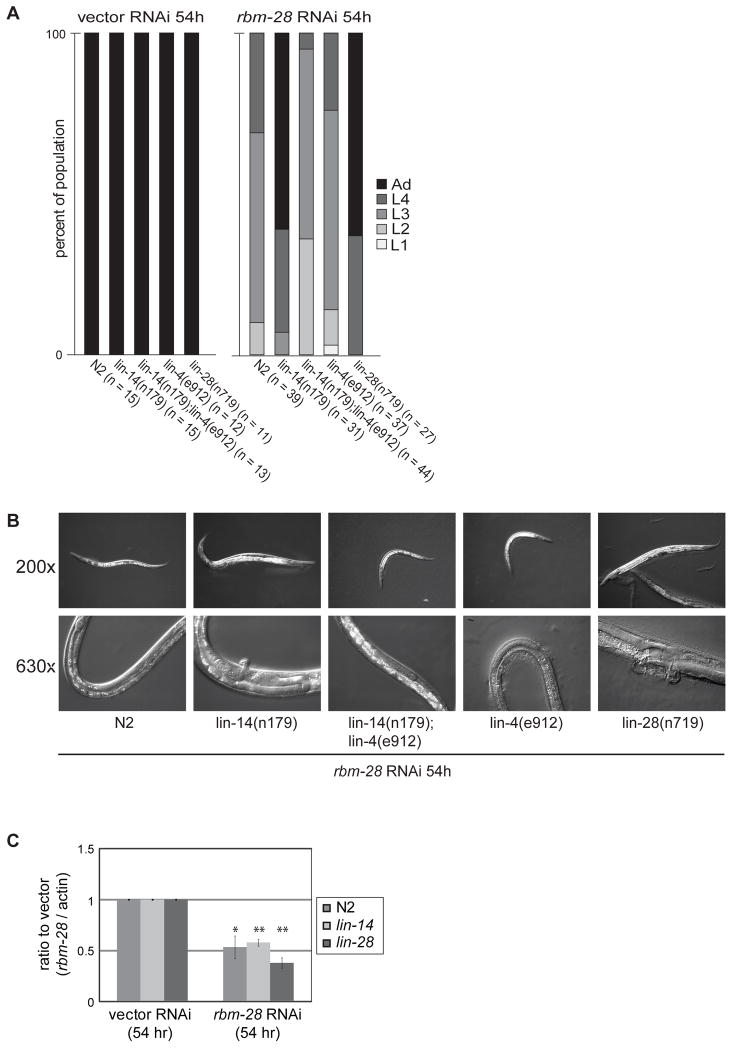
lin-4 and its target genes, lin-14 and lin-28, regulate developmental timing events in some somatic tissues relative to gonad development (Ambros, 1989; Ambros and Horvitz, 1984). Insufficient lin-4 leads seam-cell lineages to reiterate early stage-specific developmental events at later times and, conversely, mutations in lin-14 and lin-28 cause later events to occur precociously (Ambros, 1989; Ambros and Horvitz, 1984). Two distinct phenotypes of worms carrying the lin-4(e912) null mutation are the failure to develop a vulva and the absence of a series of cuticular lateral ridges, known as alae, which are normally produced at the L4 to adult transition. Given the decreased levels of mature lin-4 and defective regulation of its targets (Fig. 4), we asked if depletion of rbm-28 also resulted in lin-4 loss of function phenotypes. In adult stage worms, rbm-28 RNAi resulted in complete failure to develop a vulva in 33% of the animals (Table 1), and nearly all that did form vulvas displayed protruding vulva defects (Table 1). In addition, only 30% of worms depleted of rbm-28 produced adult cuticles with complete alae, displaying instead incomplete alae (60%) or no alae at all (10%) (Table 1). These partially penetrant phenotypes are consistent with the low but detectable levels of mature lin-4 that result from rbm-28 depletion by RNAi (Fig. 4A).
Table 1.
Depletion of rbm-28 results in retarded heterochronic phenotypes. N2 worms cultured at 25°C on vector control or rbm-28 (RNAi) were scored for vulva development (Vul = vulvaless, p-Vul = protruding vulva) and alae formation (incomplete refers to non-contiguous alae ridges due to variable seam cell differentiation). These phenotypes were scored when worms reached adulthood as assessed by gonad development (48 hours for vector and 94 hours for rbm-28)
| Adult Phenotypes: | Vul | p-Vul | Normal | No alae | Incomplete alae | Full alae |
|---|---|---|---|---|---|---|
| vector (n=20) | 0% | 0% | 100% | 0% | 0% | 100% |
| rbm-28 (n=30) | 33% | 60% | 7% | 10% | 60% | 30% |
Worms with loss of function mutations in lin-14 and lin-28 exhibit precocious alae one or two stages earlier than in wild-type animals (Ambros, 1989). The lin-14(n179ts) allele causes partial loss of function phenotypes with precocious alae formation in worms cultured at 25°C but not 15°C (Ambros and Horvitz, 1987). The early expression of alae in lin-14(n179ts) worms is fully suppressed by loss of lin-4 miRNA (Table 2). Previous studies have shown that the precocious alae phenotype of lin-28(n719) mutants can also be suppressed by loss of lin-4 activity, which may be due to increased lin-14 expression (Ambros, 1989). Likewise, we found that depletion of rbm-28 in lin-14(n179ts) or lin-28(n719) worms resulted in strong suppression of the precocious alae phenotypes associated with these mutants (Table 2). Taken together, rbm-28 RNAi results in diminished expression of mature lin-4 miRNA, mis-regulation of its targets and phenotypes consistent with a role for this factor in the lin-4 developmental timing pathway.
Table 2.
Suppression of the precocious alae phenotype of lin-14 and lin-28 mutants by rbm-28 (RNAi). The indicated worm strains were cultured at 25°C on vector control (white rows) or rbm-28 (RNAi) (gray rows) and scored for alae formation (incomplete refers to non-contiguous alae ridges due to variable seam cell differentiation) at the L4 stage based on gonad development.
| Strain scored at L4 stage based on size and gonad development | No alae | Incomplete alae | Full alae |
|---|---|---|---|
| N2 (n=17) | 100% | 0% | 0% |
| N2; rbm-28 RNAi (n=11) | 100% | 0% | 0% |
| lin-14(n179) (n=13) | 15% | 54% | 31% |
| lin-14(n179); rbm-28 RNAi (n=13) | 86% | 7% | 7% |
| lin-14(n179);lin-4(e912) (n=16) | 100% | 0% | 0% |
| lin-14(n179);lin-4(e912); rbm-28 RNAi (n=10) | 90% | 10% | 0% |
| lin-4(e912) (n=15) | 100% | 0% | 0% |
| lin-4(e912); rbm-28 RNAi (n=8) | 100% | 0% | 0% |
| lin-28(n719) (n=18) | 28% | 22% | 50% |
| lin-28(n719); rbm-28 RNAi (n=12) | 100% | 0% | 0% |
Integration of Developmental Timing and Organismal Growth by rbm-28
In addition to the developmental timing abnormalities in worms depleted of rbm-28, we also observed overall delayed growth of the RNAi treated animals. This phenotype is distinct from the heterochronic defects observed in lin-4, lin-28 or lin-14 mutants, which repeat or skip stage specific somatic cellular events without exhibiting substantial irregularities in organismal growth or germline development (Ambros, 1989; Ambros and Horvitz, 1984). Under control RNAi conditions, WT (N2), lin-14(n179ts), lin-14(n179ts);lin-4(e912), lin-4(e912) and lin-28(n719) strains reached adult size with fully formed gonads by 54 hours of development at 25°C (Fig. 5A). At this same time point in N2 worms undergoing rbm-28 RNAi, the majority of the population had only reached the L3 stage as judged by size and gonad development (Fig. 5A and B). Strikingly, over half of the lin-14(n179ts) and lin-28(n719) mutant populations undergoing rbm-28 RNAi were scored as adults by the 54 hour time point (Fig. 5A). However, rbm-28 RNAi in lin-4(e912) or lin-14(n179ts);lin-4(e912) strains resulted in stalled development (Fig. 5A, B), indicating that increased lin-14 activity contributes to the overall growth delay in worms depleted of rbm-28. The effectiveness of rbm-28 RNAi, as judged by mRNA down-regulation, was comparable in N2 and the lin-14 and lin-28 mutants (Fig. 5C). Thus, the suppression of delayed growth caused by rbm-28 RNAi in these strains can be attributed to decreased lin-14 or lin-28 activity.
Figure 5.
Interaction of rbm-28 with heterochronic pathway genes. (A) Population composition of the indicated worm strains cultured on vector versus rbm-28 RNAi for 54 hours at 25°C was determined based on size and gonad development. The number of worms counted for each strain and RNAi condition are indicated. (B) Representative images of worms grown on rbm-28 RNAi bacteria for 54 hours are shown. (C) Total RNA was isolated from N2, lin-14(n179), and lin-28(n719) worms after growth on vector or rbm-28 RNAi for 54 hours at 25°C. rbm-28 mRNA levels and actin mRNA levels were analyzed by qPCR after reverse transcription with random primers. The average and s.e.m. from three independent experiments are shown and were analyzed by Student’s t-tests (*, p<0.05; **, p<0.005).
DISCUSSION
Here we characterize the expression of endogenous lin-4 miRNA transcripts and describe a new factor involved in the lin-4 pathway in C. elegans. Although transcripts containing lin-4 are expressed from the overlapping F59G1.4 gene early in L1, we present evidence that they are not subject to Drosha processing. Instead we define independent lin-4 primary transcripts expressed towards the end of L1, coinciding with the accumulation of mature lin-4 miRNA. We also identified a new factor, the RNA binding protein R05H10.2, renamed RBM-28, involved in lin-4 biogenesis and organismal growth and development pathways (Fig. 6). Inactivation of rbm-28 not only results in lin-4-like heterochronic defects but it also causes delayed growth of the entire organism, including the gonad, which normally is not affected by mutations in most heterochronic pathway genes. Interestingly, loss of lin-14 or lin-28 activity suppressed the delayed growth phenotype of rbm-28 RNAi, indicating that these classic heterochronic genes also coordinate overall organismal development.
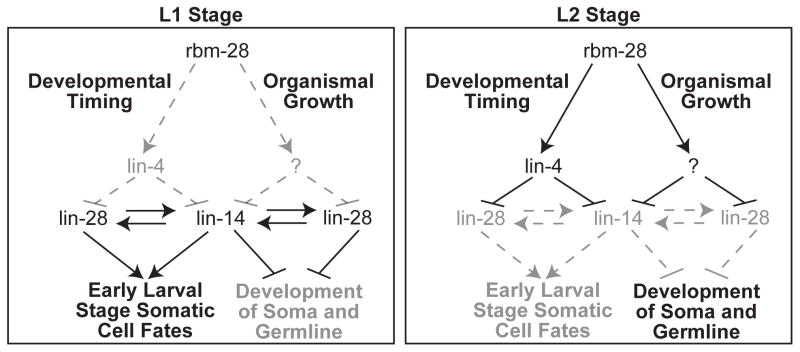
Figure 6.
A model for the role of rbm-28 in the heterochronic and organismal growth pathways. In early L1, lin-4 miRNA is not present, allowing for the expression of LIN-14 and LIN-28 proteins, which promote early larval cell fates and repress organismal growth. By the L2 stage, lin-4 is expressed and stabilized by rbm-28, resulting in the down-regulation of lin-14 and lin-28 activity, which allows later larval stages and overall growth to proceed. This model is based on the findings that loss of rbm-28 or lin-4 results in retarded development of somatic tissues, such as the vulva and hypodermal seam cells. These phenotypes can be suppressed by down-regulation of the lin-4 target genes lin-14 and lin-28, which positively regulate each other and promote early larval cell fates. Depletion of rbm-28 also results in overall delayed growth, including the gonad. This phenotype is not observed in lin-4 mutants, but the rbm-28 RNAi induced growth delay is suppressed in lin-14 and lin-28 mutants, revealing new roles for lin-14 and lin-28 in coordinating overall organismal growth and development. In the organismal growth arm of the pathway, rbm-28 could directly repress lin-14 and lin-28 or their downstream targets to promote development. Although not sufficient, lin-4 activity also contributes to the organismal growth pathway when rbm-28 is depleted because deletion of lin-4 reestablishes delayed growth in the lin-14(n179) mutants. Alternatively, rbm-28 could positively regulate an upstream factor (indicated by the question mark), possibly a miRNA, that inhibits expression of lin-14 and lin-28, which would be consistent with evidence that these genes are regulated by factors besides lin-4 (Holtz and Pasquinelli, 2009; Morita and Han, 2006; Reinhart et al., 2000). Lines do not necessarily indicate direct regulatory relationships.
Temporal regulation of lin-4 expression in C. elegans has been previously ascribed to transcriptional control, based largely upon promoter-GFP reporter assays (Baugh and Sternberg, 2006; Esquela-Kerscher et al., 2005; Martinez et al., 2008; Ow et al., 2008). This conclusion is consistent with the work presented here where we detect up-regulation of endogenous lin-4 primary transcripts when the mature form appears. Additionally, GFP reporters based on the minimal lin-4 rescue construct or more extended sequence restrict GFP expression until the end of L1, which is when mature lin-4 accumulates (this study and (Baugh and Sternberg, 2006; Esquela-Kerscher et al., 2005; Martinez et al., 2008; Ow et al., 2008)). We show that two transcriptional start sites reside within the promoter sequences included in all of these constructs. Utilization of these start sites and a predicted polyadenylation signal 129 nt downstream from mature lin-4 would produce the ~430 and ~290 nt transcripts observed by northern blotting for lin-4 primary transcripts in late L1 stage worms. Since these transcripts are no longer detectable by northern blotting in the L2 stage and our qRT-PCR analyses indicate that independent lin-4 expression peaks at the end of L1, a transient pulse of transcription may be responsible for production of lin-4 during this window of development.
The lin-4-containing RNAs that originate from the overlapping F59G1.4 gene apparently do not contribute to the production of mature lin-4 miRNA. These transcripts were insensitive to Drosha/Pasha RNAi and processed cleavage products were undetectable in early L1 prior to independent lin-4 transcription. It is unclear why lin-4 containing RNAs expressed from the same general locus are differentially subjected to processing. One possibility is that in the context of F59G1.4 intron 9, splicing or other RNA binding factors prevent the lin-4 hairpin from being efficiently recognized by the microprocessor. In contrast, as part of the independent transcripts we identified, the lin-4 precursor is no longer within intronic sequence, which may make it more accessible to Drosha activity.
We used a candidate-based screen to search for trans-acting factors involved in the regulation of lin-4 biogenesis. We predicted that such proteins could include transcription factors that mediate developmentally regulated expression of primary transcripts competent for Drosha processing as well as factors that function at the post-transcriptional level to affect the processing or stability of lin-4 miRNA. Thus, we focused on genes annotated as having nucleic acid binding potential and homologs of proteins previously implicated in miRNA biogenesis by genetic or proteomic methods. Of 249 candidates tested, only one significantly and reproducibly affected lin-4 biogenesis. RNAi of rbm-28 resulted in ~4-fold decrease in mature lin-4 with no obvious effect on precursor or primary transcript levels, suggesting that this gene positively regulates the stabilization of lin-4. Presently, it is unclear if rbm-28 directly regulates lin-4 accumulation. The triple RRMs (RNA recognition motifs) in this protein could mediate interactions with lin-4 RNA to regulate its stability. The closest human homolog, RBM28, associates with spliceosomal RNAs and is nucleolar localized (Damianov et al., 2006). A loss of function mutation in RBM28 has been linked to a syndrome characterized by alopecia, progressive neurological defects, and endocrinopathy (ANE syndrome) (Nousbeck et al., 2008). Cells from ANE patients have abnormal rough endoplasmic reticulum and reduced ribosome density, but the primary effect of the RBM28 mutation in these cells has not yet been determined. The closest yeast homolog, Nop4p/Nop77, is also nucleolar and functions in ribosomal RNA processing and large subunit biogenesis (Sun and Woolford, 1994; Sun and Woolford, 1997). The cellular localization of RBM-28 is yet to be investigated, but if nucleolar residence is conserved it is unlikely that this factor directly binds and regulates mature lin-4 accumulation, as this form of the miRNA is presumed to be primarily cytoplasmic. Instead, regulation of other non-coding RNAs, such as other miRNAs, snRNAs or rRNAs, could result in indirect effects on lin-4 stability.
There is precedence for general RNA binding proteins functioning in diverse RNA regulatory pathways. A recent study showed that the RRM-domain protein hnRNP A1 is important for Drosha-mediated processing of miR-18a but not other miRNAs expressed from the same primary transcript (Guil and Caceres, 2007; Michlewski et al., 2008). Since hnRNP A1 has been well characterized as a mediator of alternative splicing and mRNA export (Caceres et al., 1994; Mayeda and Krainer, 1992; Pinol-Roma and Dreyfuss, 1992), it was surprising to find that it also plays a very specific role in the biogenesis of particular miRNAs (Michlewski and Caceres, 2010; Michlewski et al., 2008). The KH-type splicing regulatory protein (KSRP) is another recent example of an RNA binding protein with diverse substrates and regulatory mechanisms. In addition to its established roles in alternative splicing and in mediating mRNA decay (Gherzi et al., 2004; Min et al., 1997), KSRP was found to have an independent function in facilitating processing of a subset of miRNAs (Michlewski and Caceres, 2010; Trabucchi et al., 2009). The importance of multifunctional RNA binding proteins in regulating specific sets of miRNAs appears to be an emerging theme in miRNA post-transcriptional regulation.
The phenotypes induced by rbm-28 RNAi could be due to defective metabolism of multiple types of non-coding RNAs, including rRNAs, snRNAs as well as miRNAs. However, the defects in vulva formation and alae production resemble the retarded phenotypes associated with loss of lin-4 miRNA. Moreover, the precocious alae phenotype in lin-14(n179ts) hypomorphic mutants is suppressed by rbm-28 RNAi almost to the same degree as genetic deletion of lin-4. Since lin-14 and lin-28 positively cross-regulate each other (Arasu et al., 1991; Moss et al., 1997; Seggerson et al., 2002), it is possible that suppression of the precocious alae phenotype by rbm-28 RNAi in the putative null mutant lin-28(n719) is through up-regulation of lin-14. These results are consistent with the model that rbm-28 functions in the heterochronic pathway by positively regulating lin-4, which in turn inhibits expression of its targets lin-14 and lin-28, thus allowing the transition to later larval cell fates (Fig. 6). rbm-28 was previously reported to potentially function in the heterochronic pathway, since rbm-28 RNAi in certain transgenic strains resulted in precocious expression of a reporter normally expressed in adults (Hayes and Ruvkun, 2006). Our finding that inactivation of rbm-28 also severely delays overall worm growth in a lin-14/lin-28 dependent pathway, reveals a new role for these canonical heterochronic genes. Since loss of lin-4 alone results in reiterated first larval stage fates but relatively normal development of the germline and animal growth, we predict that an unknown target of rbm-28 also represses lin-14 and lin-28 to permit organismal growth (Fig. 6). Alternatively, rbm-28 could directly inhibit these genes or their downstream targets that prohibit development of somatic and germline tissues. This analysis suggests that lin-14 and lin-28 promote earlier life stages and prevent organismal growth of both soma and germline; it is only when expression of these genes is reduced below a threshold in pathways controlling both tissue types that later life stages can be reached, allowing the organism to grow as an integrated whole. The work presented in this paper reveals a larger context for the heterochronic pathway, situating it within a network of genes, controlled by rbm-28, to coordinate the many tissues of the worm and orchestrate development of the whole organism.
Supplementary Material
Acknowledgments
We thank members of the Pasquinelli lab for critical reading of the manuscript and J. Holtz and C. Chang for technical assistance. We thank V. Ambros for sharing the plin-4::gfp (maIs134) reporter strain, M. Li and M. David for sharing their real time PCR machine, and the Caenorhabditis Genetics Center for worm strains. J. B. was supported in part by the NIH CMG Graduate Student Training Grant (UCSD Biology). P.M.V. was supported by a Ruth L. Kirschstein National Research Service Award (F32GM087004) from NIGMS. This work was funded by grants from the NIH (GM071654), Keck, Emerald, Searle Scholar, V and Gruber Foundations to A. E. P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambros V. A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans. Cell. 1989;57:49–57. doi: 10.1016/0092-8674(89)90171-2. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–16. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. The lin-14 locus of Caenorhabditis elegans controls the time of expression of specific postembryonic developmental events. Genes Dev. 1987;1:398–414. doi: 10.1101/gad.1.4.398. [DOI] [PubMed] [Google Scholar]
- Arasu P, Wightman B, Ruvkun G. Temporal regulation of lin-14 by the antagonistic action of two other heterochronic genes, lin-4 and lin-28. Genes Dev. 1991;5:1825–33. doi: 10.1101/gad.5.10.1825. [DOI] [PubMed] [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–63. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–7. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780–5. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Bracht J, Hunter S, Eachus R, Weeks P, Pasquinelli AE. Trans-splicing and polyadenylation of let-7 microRNA primary transcripts. RNA. 2004;10:1586–94. doi: 10.1261/rna.7122604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Caceres JF, Stamm S, Helfman DM, Krainer AR. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–9. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- Chang S, Johnston RJ, Jr, Frokjaer-Jensen C, Lockery S, Hobert O. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature. 2004;430:785–9. doi: 10.1038/nature02752. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–60. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chendrimada TP, Finn KJ, Ji X, Baillat D, Gregory RI, Liebhaber SA, Pasquinelli AE, Shiekhattar R. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823–8. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- Damianov A, Kann M, Lane WS, Bindereif A. Human RBM28 protein is a specific nucleolar component of the spliceosomal snRNPs. Biol Chem. 2006;387:1455–60. doi: 10.1515/BC.2006.182. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hata A. Regulation of MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Johnson SM, Bai L, Saito K, Partridge J, Reinert KL, Slack FJ. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Dev Dyn. 2005;234:868–77. doi: 10.1002/dvdy.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum R, Ambros V. The timing of lin-4 RNA accumulation controls the timing of postembryonic developmental events in Caenorhabditis elegans. Dev Biol. 1999;210:87–95. doi: 10.1006/dbio.1999.9272. [DOI] [PubMed] [Google Scholar]
- Garcia D, Collier SA, Byrne ME, Martienssen RA. Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol. 2006;16:933–8. doi: 10.1016/j.cub.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14:571–83. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res. 1994;22:1762–3. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–40. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Guil S, Caceres JF. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14:591–6. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- Hayes GD, Ruvkun G. Misexpression of the Caenorhabditis elegans miRNA let-7 is sufficient to drive developmental programs. Cold Spring Harb Symp Quant Biol. 2006;71:21–7. doi: 10.1101/sqb.2006.71.018. [DOI] [PubMed] [Google Scholar]
- Holtz J, Pasquinelli AE. Uncoupling of lin-14 mRNA and protein repression by nutrient deprivation in Caenorhabditis elegans. RNA. 2009;15:400–5. doi: 10.1261/rna.1258309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–9. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA. Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature. 2004;428:81–4. doi: 10.1038/nature02366. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Kim YK, Kim VN. Processing of intronic microRNAs. Embo J. 2007;26:775–83. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Martinez NJ, Ow MC, Reece-Hoyes JS, Barrasa MI, Ambros VR, Walhout AJ. Genome-scale spatiotemporal analysis of Caenorhabditis elegans microRNA promoter activity. Genome Res. 2008;18:2005–15. doi: 10.1101/gr.083055.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–75. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- Michlewski G, Caceres JF. Antagonistic role of hnRNP A1 and KSRP in the regulation of let-7a biogenesis. Nat Struct Mol Biol. 2010;17:1011–8. doi: 10.1038/nsmb.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlewski G, Guil S, Semple CA, Caceres JF. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell. 2008;32:383–93. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Turck CW, Nikolic JM, Black DL. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997;11:1023–36. doi: 10.1101/gad.11.8.1023. [DOI] [PubMed] [Google Scholar]
- Morita K, Han M. Multiple mechanisms are involved in regulating the expression of the developmental timing regulator lin-28 in Caenorhabditis elegans. Embo J. 2006;25:5794–804. doi: 10.1038/sj.emboj.7601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–46. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- Niwa R, Slack FJ. The evolution of animal microRNA function. Curr Opin Genet Dev. 2007;17:145–50. doi: 10.1016/j.gde.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Nogueira FT, Madi S, Chitwood DH, Juarez MT, Timmermans MC. Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev. 2007;21:750–5. doi: 10.1101/gad.1528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousbeck J, Spiegel R, Ishida-Yamamoto A, Indelman M, Shani-Adir A, Adir N, Lipkin E, Bercovici S, Geiger D, van Steensel MA, Steijlen PM, Bergman R, Bindereif A, Choder M, Shalev S, Sprecher E. Alopecia, neurological defects, and endocrinopathy syndrome caused by decreased expression of RBM28, a nucleolar protein associated with ribosome biogenesis. Am J Hum Genet. 2008;82:1114–21. doi: 10.1016/j.ajhg.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow MC, Martinez NJ, Olsen PH, Silverman HS, Barrasa MI, Conradt B, Walhout AJ, Ambros V. The FLYWCH transcription factors FLH-1, FLH-2, and FLH-3 repress embryonic expression of microRNA genes in C. elegans. Genes Dev. 2008;22:2520–34. doi: 10.1101/gad.1678808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DH, Xu J, Ruvkun G. A whole-genome RNAi Screen for C. elegans miRNA pathway genes. Curr Biol. 2007;17:2013–22. doi: 10.1016/j.cub.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–2. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci U S A. 2007;104:17719–24. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggerson K, Tang L, Moss EG. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev Biol. 2002;243:215–25. doi: 10.1006/dbio.2001.0563. [DOI] [PubMed] [Google Scholar]
- Shiohama A, Sasaki T, Noda S, Minoshima S, Shimizu N. Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp Cell Res. 2007;313:4196–207. doi: 10.1016/j.yexcr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;5:659–69. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, Hewitson M, Holz C, Khan M, Lazik S, Martin C, Nitzsche B, Ruer M, Stamford J, Winzi M, Heinkel R, Roder M, Finell J, Hantsch H, Jones SJ, Jones M, Piano F, Gunsalus KC, Oegema K, Gonczy P, Coulson A, Hyman AA, Echeverri CJ. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–9. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- Stark A, Bushati N, Jan CH, Kheradpour P, Hodges E, Brennecke J, Bartel DP, Cohen SM, Kellis M. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes Dev. 2008;22:8–13. doi: 10.1101/gad.1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Woolford JL., Jr The yeast NOP4 gene product is an essential nucleolar protein required for pre-rRNA processing and accumulation of 60S ribosomal subunits. Embo J. 1994;13:3127–35. doi: 10.1002/j.1460-2075.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Woolford JL., Jr The yeast nucleolar protein Nop4p contains four RNA recognition motifs necessary for ribosome biogenesis. J Biol Chem. 1997;272:25345–52. doi: 10.1074/jbc.272.40.25345. [DOI] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–4. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–8. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Durston AJ. MiR-10 represses HoxB1a and HoxB3a in zebrafish. PLoS One. 2008;3:e1396. doi: 10.1371/journal.pone.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol. 2005;25:9198–208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulczyn FG, Smirnova L, Rybak A, Brandt C, Kwidzinski E, Ninnemann O, Strehle M, Seiler A, Schumacher S, Nitsch R. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. Faseb J. 2007;21:415–26. doi: 10.1096/fj.06-6130com. [DOI] [PubMed] [Google Scholar]
- Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–5. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–6. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Greenwald I. LIN-12/Notch activation leads to microRNA-mediated down-regulation of Vav in C. elegans. Science. 2005;310:1330–3. doi: 10.1126/science.1119481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.