Abstract
Purpose
Studies suggest that there is a gender difference in the development and outcomes of acute lung injury (ALI). Few studies have directly addressed the association of gender and alveolar fluid clearance (AFC), a process that is critical to ALI resolution.
Materials and Methods
To test the hypothesis that female gender is associated with an increased AFC rate, we measured AFC rates in 150 mechanically ventilated patients with acute pulmonary edema and a pulmonary edema fluid-to-plasma protein ratio (EF/PL) diagnostic of low permeability (EF/PL <0.65, N=69) or high permeability (EF/PL ≥ 0.65, N=81) edema. We measured protein concentration in serial samples of undiluted EF collected within 4 hours of intubation and calculated net rate of AFC. In addition, plasma levels of receptor for advanced glycation end products (RAGE) were measured as a surrogate marker for alveolar epithelial injury.
Results
In patients with ALI, women had higher rates of net AFC at 4 hours compared to men (11.9 % per hour vs. 4.3% per hour, p=0.017) and more females had maximal rates of AFC. There were no differences in circulating levels of RAGE between men and women.
Conclusions
These findings may have significant implications for future ALI studies and potential therapies.
Keywords: Gender, acute respiratory distress syndrome, mortality, receptor for advanced glycation end products, pulmonary edema
Introduction
The effect of gender on outcomes from acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) is uncertain. In patients with ARDS, female gender has been associated with a lower risk of death in both a retrospective study of outcomes in India [1] and one large epidemiologic study. [2] In premature infants who develop respiratory distress syndrome, females have a better survival in the acute setting [3] and a lower incidence of developing long term pulmonary complications [4] compared to male infants. On the other hand, several epidemiologic studies have shown no gender differences in either the risk of developing ARDS [5] or clinical outcomes from ARDS. [6] Also, two studies reported that genetic susceptibility to ARDS is dependent on gender. [7, 8] Gong et al [7] reported that a polymorphism for surfactant protein B was associated with an increased risk of ARDS in women but not men. Sheu et al [8] found that epidermal growth factor haplotypes are associated with an increased risk of ARDS in males but not females.
Outcomes from ARDS are dependent on several factors including age [9] and the number of other organ failures. [10] In addition, physiologic functions have been identified that are important for resolution of lung injury and edema. In order to restore normal gas exchange, the proteinaceous edema fluid that floods the airspace must be removed by vectorial ion and fluid transport, a process that is termed alveolar fluid clearance (AFC). A reduced rate of net AFC has been associated with higher mortality in clinical ALI. [11] AFC is dependent on an intact distal lung epithelial barrier [12] and preserved alveolar epithelial sodium transport function [13]. An increase in expression and activity of the lung epithelial sodium channel (ENaC), a major determinant of the rate of AFC, has been reported in female compared to male rats. [14]
In single center studies, we have previously studied rates of AFC in patients with hydrostatic pulmonary edema [15] and ALI/ARDS [11]. We found that the majority of patients with ALI have impaired alveolar fluid clearance and that male gender was associated with having lower rates of AFC. However, the observation of gender differences in AFC was not fully explored in our prior study of ALI, nor was it defined in hydrostatic edema. Since the publication of the previous studies, we have expanded our patient cohort to include patients from a second institution and patients receiving lung protective ventilation who were enrolled after the publication of the landmark ARDS Network low tidal volume study[16]. We now have an expanded multicenter dataset to study the effects of gender on alveolar fluid clearance. In addition, it is critical to identify any clinical variables that may affect rates of AFC in patients with ALI as there are now new therapies designed to augment alveolar fluid clearance (low tidal volume ventilation and β-adrenergic agonists) that are being studied in patients with ALI.
Based on the available evidence from animal studies and some clinical studies, we hypothesized that net rates of alveolar fluid clearance would be higher in women with ALI compared to men and that this difference would be associated with improved clinical outcomes in women. To test this hypothesis, we compared rates of net AFC in men and women with clinical ALI who were enrolled in a prospective study of alveolar fluid clearance in acute pulmonary edema. [11] For comparison, we also measured the rate of net AFC in men and women with hydrostatic pulmonary edema. To compare the degree of lung epithelial injury between males and females with ALI, we also measured plasma levels of RAGE, a marker of lung epithelial injury; plasma levels of RAGE correlate with the degree of alveolar epithelial injury. [17, 18]
METHODS
Patients
From January 1987 to December 2007, intubated and mechanically ventilated patients at University of California at San Francisco (UCSF) Moffitt Long Hospital, San Francisco General Hospital, and Vanderbilt University Medical Center were enrolled if they had acute pulmonary edema with the acute onset of respiratory failure accompanied by hypoxemia and bilateral infiltrates on chest imaging. Patients were classified as having ALI/ARDS based on the consensus definition of 1. Bilateral infiltrates on chest radiograph; 2. Hypoxemia with paO2/FiO2 less than 300; 3. Absence of left heart failure [19] as well as an edema fluid to plasma protein (EF/PL) ratio ≥ 0.65 [20]. Hydrostatic (cardiogenic) pulmonary edema was defined as bilateral infiltrates and evidence of left heart failure [15] with an EF/PL ratio < 0.65. Serial samples of pulmonary edema fluid were collected within 6 hours of intubation as previously described [12]. Briefly, a standard suction catheter was inserted through the endotracheal tube and advanced slowly until resistance was met. Gentle suction was applied and a few milliliters of undiluted pulmonary edema fluid were aspirated. Samples were placed immediately on ice, centrifuged at 3,000 × g for 10 minutes to remove cellular components, and the supernatant was stored at −70°C until use. A second edema fluid sample was collected within 4 hours for calculation of the rate of alveolar fluid clearance. Simultaneous plasma samples were obtained for calculation of the EF/PL protein ratio. In addition, demographic and clinical data were collected retrospectively on each patient including age, gender, presence of sepsis or pneumonia, ventilator settings, and death. Radiographic and laboratory data were reviewed and the lung injury score (LIS) [21] and the simplified acute physiology score II (SAPS II) [22] was calculated for each patient. Ventilator settings and static lung compliance measurements were recorded at the time of first edema fluid sampling. There were 81 critically ill mechanically ventilated patients with ALI/ARDS and an initial EF/PL ratio ≥ 0.65 and 69 patients with cardiogenic pulmonary edema and an EF/PL ratio <0.65 enrolled. Data from some of the patients has been previously reported. [11] [15] The study was approved with a waiver of consent by the Institutional Review Boards at UCSF and Vanderbilt University.
Calculation of the net rate of alveolar fluid clearance (AFC)
Protein concentration was measured in all edema fluid and plasma samples using the Biuret method as previously described. [15] The rate of net AFC was calculated using the formula (final protein concentration - initial protein concentration)/final protein concentration and expressed as percent clearance per hour (%/hr). For some analyses, the rate of alveolar fluid clearance was categorized as maximal (≥ 14%/h) or submaximal (< 14%/h) based on a previously defined cutoff. [11]
Measurement of plasma RAGE
Plasma concentrations of receptor for advanced glycation end products (RAGE), a marker of alveolar epithelial type I cell injury were measured in plasma by ELISA (R&D systems, Minneapolis, MN) according to manufacturer’s instructions. All measurements were performed in duplicate.
Statistical Analysis
All statistical analysis was done using SPSS for Macintosh version 13. For non-normally distributed data, comparisons between groups were made using the Mann Whitney U test or the Fisher’s exact test where appropriate with a p < 0.05 considered statistically significant. A non-stepwise multivariable linear regression analysis was done to assess the independent effects of gender, age and severity of illness on alveolar fluid clearance rates.
RESULTS
Patient Characteristics
Compared to patients with ALI/ARDS, patients with hydrostatic pulmonary edema were older, had less severe lung injury scores and a lower mortality rate (Table 1). There were no differences in gender or severity of illness between the two groups. The majority of patients (89%) in both groups were enrolled prior to the adoption of low tidal volume ventilation for acute lung injury and were ventilated with ventilator settings (tidal volume 11–12 ml/kg) that were standard of care prior to the widespread adoption of lung protective strategies. When comparing men and women with acute lung injury pulmonary edema, there were no differences in age, severity of illness, presence of pneumonia or sepsis, lung injury score, tidal volume per kilogram, fluid balance in the 24 hours preceding AFC measurement, days of unassisted ventilation, or ICU free days (Table 2).
Table 1.
Demographic and clinical characteristics of patients with hydrostatic pulmonary edema (low permeability pulmonary edema) and acute lung injury (high permeability pulmonary edema).
| Hydrostatic Edema | Acute Lung Injury | P value | |
|---|---|---|---|
| N=69 | N=81 | ||
| Gender (% men) | 52 | 58 | 0.51 |
| Age (years) | 55 (35–69) | 47 (29–63) | 0.06 |
| SAPS II | 42 (32–51) | 44 (34–61) | 0.10 |
| LIS | 2.7 (2.3–3.0) | 3.0 (2.3–3.3) | 0.02 |
| TV (mL/kg) | 11.2 (9.5–12.6) | 11.4 (9.0–13.3) | 0.84 |
| Mortality (%) | 32 | 51 | 0.03 |
Table 2.
Demographic and clinical characteristics of men and women with acute pulmonary edema
| HYDROSTATIC EDEMA |
ACUTE LUNG INJURY |
|||||
|---|---|---|---|---|---|---|
| Men | Women | P value | Men | Women | P value | |
| N=36 | N=33 | N=47 | N=34 | |||
| Age (years) | 59 (35–68) | 54 (34–72) | 0.91 | 50 (32–66) | 46 (29–59) | 0.37 |
| Sepsis (%) | 8 | 6 | 1.00 | 46 | 44 | 1.00 |
| Pneumonia (%) | 16 | 9 | 0.48 | 39 | 28 | 0.34 |
| SAPS II | 42 (32–49) | 42 (32–49) | 0.63 | 49 (35–68) | 44 (32–53) | 0.17 |
| LIS | 2.7 (2.3–3.0) | 2.7 (1.9–3.0) | 0.64 | 3.0 (2.3–3.3) | 2.7 (2.3–3.3) | 0.48 |
| TV (mL/kg) | 10.7 (9.4–12.5) | 11.4 (9.8–12.7) | 0.25 | 11.8 (9.6–13.1) | 11.1 (7.5–13.6) | 0.81 |
| Fluid Bal (L) | −0.5 (−2.4–0.9) | 0.5 (−1.7–2.1) | 0.26 | 1.2 (−0.9–3.6) | 0.7 (−0.5–2.3) | 0.82 |
| Vent free days | 23 (2–27) | 21 (0–26) | 0.13 | 0 (0–23) | 7 (0–22) | 0.66 |
| ICU free days | 19 (0–25) | 0 (0–23) | 0.12 | 0 (0–21) | 0 (0–20) | 0.69 |
Data expressed as median (IQR) except as noted. SAPS II = simplified acute physiology score [22], LIS = lung injury score [21], TV = tidal volume corrected for actual body weight. Vent free days = days alive and free of unassisted ventilation during the 28 days after enrollment. ICU free days = days alive and free of the ICU during the 28 days after enrollment.
Alveolar fluid clearance
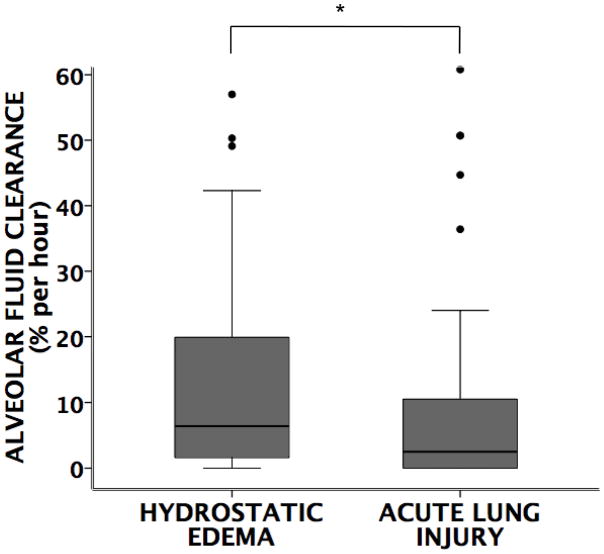
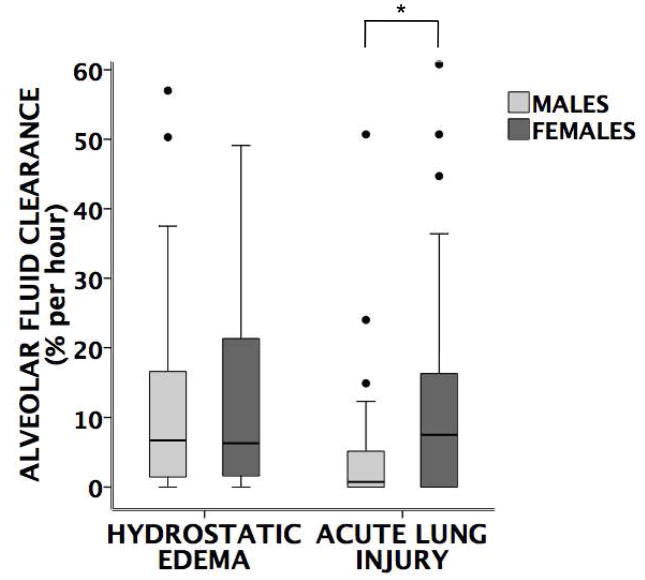
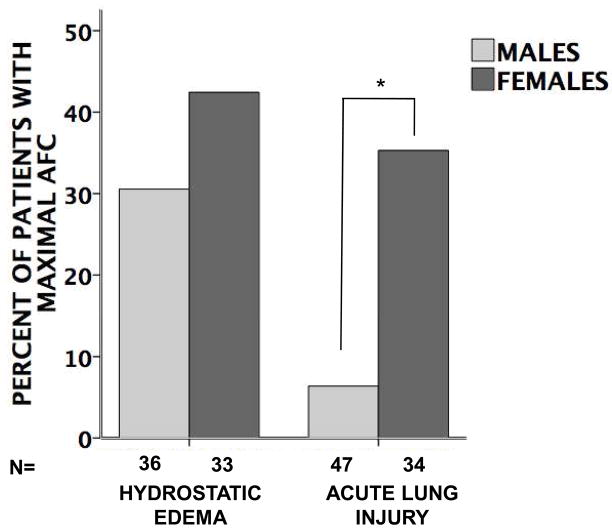
Overall, patients with hydrostatic pulmonary edema had faster rates of AFC compared to those with acute lung injury (Figure 1). In patients with hydrostatic edema, there was no difference in the rates of alveolar fluid clearance in women versus men. However, in patients with acute lung injury, women had significantly faster rates of alveolar fluid clearance compared to men (Figure 2). This difference persisted in a multivariable models corrected for age and severity of illness (Table 3). Furthermore, in acute lung injury edema, a higher proportion of women had maximal rates of alveolar fluid clearance (defined as ≥14% per hour) (Figure 3). There were no differences in rates of AFC in patients receiving inhaled β-adrenergic agonists vs. those not receiving the medication although very few patients (34 total) received inhaled β-adrenergic agonists (data not shown).
Figure 1. Comparison of rates of alveolar fluid clearance.
between patients with hydrostatic (low protein) pulmonary edema (N=69) vs. acute lung injury (high protein) pulmonary edema (N=81). Patients with hydrostatic pulmonary edema have faster rates of AFC compared to those with acute lung injury (*p=0.001 by Mann Whitney U test).
Figure 2. Comparison of rates of alveolar fluid clearance (AFC) by gender.
There is no difference in AFC rates between men (n=36) and women (n=33) with hydrostatic pulmonary edema (p=0.4 by Mann Whitney U test) but women (n=34) with acute lung injury have significantly faster rates of AFC compared to men (n=47) with acute lung injury (*p=0.017 by Mann Whitney U test).
Table 3.
Higher alveolar fluid clearance is independently associated with female gender
| Variable | Unstandardized coefficient (95% CI) | p value |
|---|---|---|
| Gender | 7.78 (2.32–13.23) | 0.006 |
| Age | 0.05 (−0.09–0.19) | 0.470 |
| SAPSII | −0.01 (−0.15–0.13) | 0.904 |
After adjusting for age and severity of illness (SAPSII), female gender remained independently associated with a higher rate of alveolar fluid clearance in patients with acute lung injury. In this linear regression model, the absolute rate of AFC was 7.8% faster in women versus men, 0.05% faster for each additional year of age (p=NS) and 0.01% slower for every increase in SAPS II by 1 (p=NS).
Figure 3. Percent of males and females with maximal (≥ 14% per hour) alveolar fluid clearance (AFC).
There is no difference between genders in patients with hydrostatic pulmonary edema (p=0.33 by Fisher’s exact test) but in patients with acute lung injury, significantly more women have maximal AFC compared to men (*p=0.001 by Fisher’s exact test).
Rates of AFC in low vs. high tidal volume ventilation
One unique aspect of this dataset is that it included patients ventilated with low tidal volume ventilation. For this analysis a tidal volume of 8 ml/kg was chosen as a cut off since only actual body weight and not ideal body weight was available. Although there were no differences in rates of AFC in all patients ventilated with low vs. high tidal volumes, women ventilated at higher tidal volumes had faster rates of AFC compared to men at high tidal volumes (Figure 4), suggesting that women may be less susceptible to the injurious effects of high tidal volume ventilation compared to men.
Figure 4. Boxplot comparing the rate of AFC (ln transformed) in men vs. women.
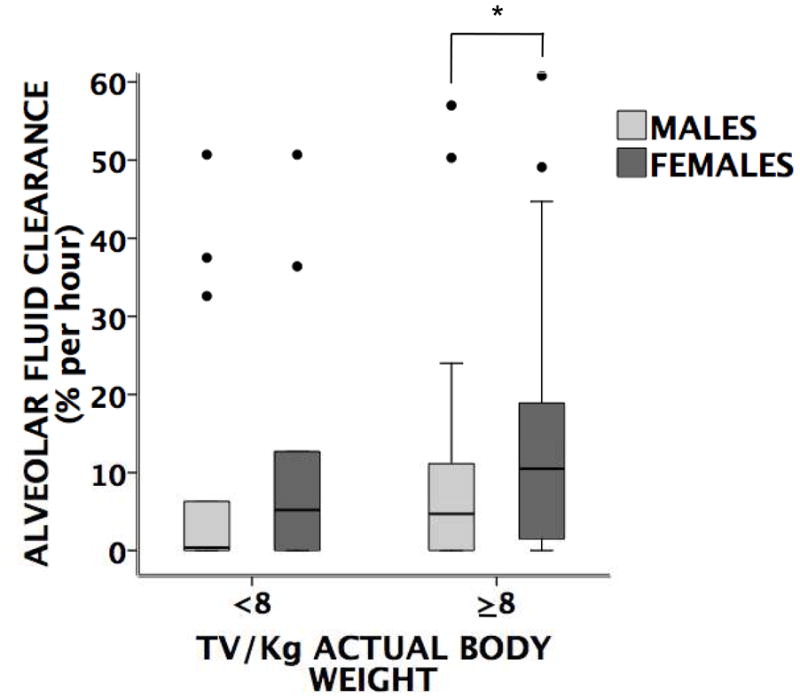
ventilated at either low (< 8 ml/kg actual body weight) or high tidal volumes (≥ 8ml/kg). There was no difference in the rate of AFC between women (N=10) and men (N=14) ventilated with low tidal volumes. However, women (N=53) ventilated with higher tidal volumes had faster rates of AFC compared to men (N=64) ventilated with higher tidal volumes. *p=0.035 by Mann Whitney U test, ● =outliers and extremes. TV = tidal volume, AFC = alveolar fluid clearance
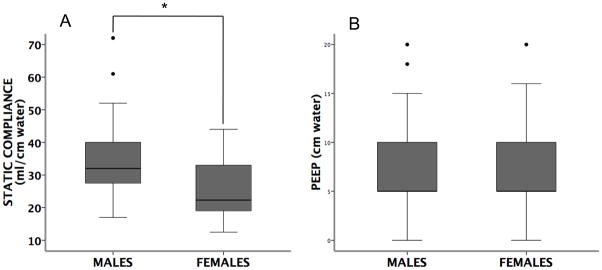
Static lung compliance in men versus women
There were no significant differences for severity of illness or lung injury scores by gender (Table 2). However, static lung compliance was significantly lower in women (Figure 5A). This difference was not explained by differences in PEEP (positive end expiratory pressure); levels of PEEP were the same in men and women (Figure 5B). In addition, there were no differences by gender in oxygenation as measured by either the PaO2/FiO2 ratio or the alveolar-arterial oxygen (A-a) gradient.
Figure 5. Boxplots of static compliance (panel A) and PEEP (panel B).
in males (N=44) vs. females (N=34) with acute lung injury (horizontal bar = median, box = 25–75th percentile, error bars = 10–90th percentile, ● = outliers and extremes). Static compliance was lower in females (p=0.001 by Mann Whitney U test). However, there were no differences in PEEP between males and females (p=0.36 by Mann Whitney U test).
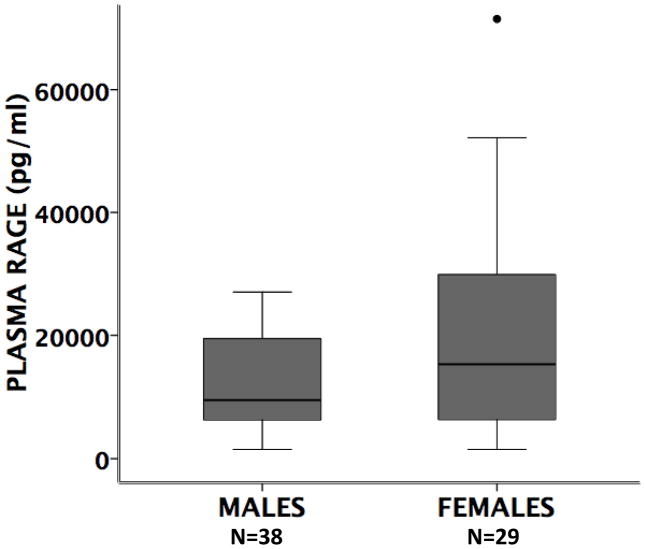
Plasma RAGE levels
Circulating RAGE was measured in plasma samples from acute lung injury patients collected at the same time as edema fluid samples in all patients for whom a sample was available (N=65) (Figure 6). There was no significant difference in plasma RAGE levels in men compared to women.
Figure 6. Boxplot of plasma RAGE levels.
in males vs. females with acute lung injury. (horizontal bar = median, box = 25–75th percentile, error bars = 10–90th percentile, ● = outliers and extremes) P=0.229 by Mann Whitney U.
Mortality in men versus women
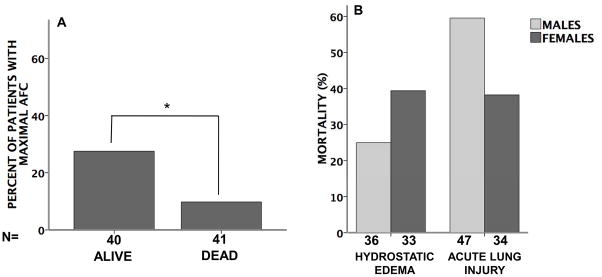
Overall in-hospital mortality was higher in patients with acute lung injury than in patients with hydrostatic edema (51% vs. 32% p=0.031), as expected (Figure 7). Patients with acute lung injury who had preserved maximal rates of AFC (≥ 14% per hour had a lower mortality than those with submaximal rates of AFC (<14% per hour) (Figure 7A). Women had a lower mortality rate compared to men with acute lung injury (38% vs. 60%) but the difference was not significant (p=0.073 by Fisher’s exact test). (Figure 7B).
Figure 7. Hospital mortality.
Panel A shows the percent of patients with maximal AFC (≥14% per hour) in patients who survived 28 days (ALIVE) and those who did not (DEAD). (*p=0.049 by Fisher’s exact test). Panel B shows mortality in men vs. women with either hydrostatic pulmonary edema or acute lung injury. (p=0.30 hydrostatic men vs women and p=0.073 in acute lung injury men vs. women).
DISCUSSSION
In this study of 150 patients with acute pulmonary edema, women with acute lung injury had significantly faster rates of alveolar fluid clearance compared to men. We have previously reported that rates of AFC are impaired in a majority of patients with ARDS [11] and preserved in the majority of patients with hydrostatic pulmonary edema [15]. This study confirms our previous findings that patients with preserved maximal AFC rates have lower hospital mortality compared to those with submaximal rates of AFC. However, the effect of gender on AFC was not well defined in these prior studies. This novel observation may be important for several reasons. First, it provides a possible mechanistic explanation for the previous observation that women with acute lung injury have improved outcomes compared to men. [1, 2] Second, it identifies gender differences in AFC in response to acute lung injury, but not acute hydrostatic pulmonary edema, that could have important implications for treatment of acute lung injury. Finally, since plasma levels of the lung epithelial injury marker RAGE were similar in men and women, this finding suggests that differences in the degree of injury to the alveolar epithelium in women may not be the mechanism for increased alveolar fluid clearance in these patients.
Alveolar fluid clearance is critical for the resolution of lung edema, whether it is caused by increased hydrostatic pressure as in congestive heart failure or by increased alveolar capillary membrane permeability in the setting of acute lung injury.[23] In patients with an uninjured lung (hydrostatic edema), there were no differences between rates of alveolar fluid clearance between men and women. However, in the setting of lung injury where there is extensive inflammation and injury to the alveolar epithelial barrier, women had significantly faster rates of alveolar fluid clearance compared to males. Furthermore, a higher proportion of women with lung injury have preserved maximal (≥ 14% per hour) rates of alveolar fluid clearance. Why might this gender-based difference in the rate of alveolar fluid transport occur in patients with acute lung injury? One possible explanation is that women with acute lung injury have less injury to the alveolar epithelium compared to males. Because it was not possible to sample lung tissue, we utilized a plasma biomarker of lung epithelial injury, the receptor for advanced glycation end products (RAGE). [17, 18, 24] There were no differences in plasma RAGE levels between males and females, suggesting that the intrinsic capacity for a higher rate of alveolar fluid clearance may be greater in women. One caveat to this hypothesis is that a single plasma RAGE level may not accurately reflect subtle changes in alveolar epithelial integrity that may have a major impact on overall alveolar fluid clearance. Interestingly, when comparing rates of AFC in patients receiving low vs. high tidal volume, women ventilated with injurious high tidal volumes (>8 ml/kg) actually had faster rates of AFC compared to men ventilated at high tidal volumes. This suggests that women may be less susceptible to the injurious effects of high tidal volume ventilation.
Animal studies have shown that the expression of the epithelial sodium channel (ENaC) in the lung is higher in females compared to males. [14] Since ENaC is critical for the resolution of alveolar edema and AFC, it is possible that women with acute lung injury have enhanced ENaC expression on the alveolar epithelium compared to men. Another possibility is that women were predisposed to better outcomes, but this seems unlikely since there were no differences in age, severity of illness (SAPSII) or the degree of lung injury as measured by the lung injury score. Further, the difference in AFC rates between men and women persisted in a multivariable model corrected for age and SAPSII. Additionally, there were no differences in total fluid balance in the 24 hours prior to measurement of AFC. Finally, the lower static compliance in women compared to men with ALI in this cohort argues that the women might even have had more severe lung injury with more pulmonary edema than males. Taken together, these results suggest that the differences in AFC and between men and women cannot be explained solely by age, severity of illness, differences in fluid balance, or the magnitude of lung injury. Why, then, did the improved rates of AFC in women not correlate with improved clinical outcomes? Although there was lower mortality in acute lung injury patients with preserved maximal alveolar fluid clerance the was no statistically significant difference in mortality between men and women even though women had faster rates of AFC. However, there was a trend towards lower mortality in women (38% versus 60%) with a p value of 0.073, suggesting that there may be a clinically important difference between mea and women but the study was not powered to detect a difference in mortality.
Although this is the first study to compare rates of AFC in men versus women with hydrostatic pulmonary edema and acute lung injury, there are some limitations to this study. First, the majority of the samples were collected at a time when patients with lung injury were ventilated with higher tidal volumes (10–12 ml/kg) than are recommended currently (4–6 ml/kg). [16] Recent work has shown that in animal models of lung injury, the level of ventilatory pressure delivered affected the rates of AFC with very low and very high pressures reducing AFC rates compared to intermediate pressures. [25] Although studies suggest that higher tidal volumes may affect AFC, this was not true in our study. There were no differences in overall rates of AFC comparing all patients with low vs. high tidal volume ventilation. Furthermore, women ventilated with high tidal volumes had faster AFC rates compared to men suggesting that men and women may respond differently to high tidal volume ventilation. However, less than 20% of our patients were ventilated with lower tidal volumes and these measurements need to be repeated in a larger group of patients. Second, measuring AFC is dependent on collecting serial edema fluid samples from a patient. Since this is not possible in all patients with pulmonary edema, there may be a selection bias in favor of patients who have more pulmonary edema or impaired AFC. Given that AFC measurements are predictors of mortality in ARDS [11], this would be unlikely to account for the differences in gender seen in the current study. In addition, there is the possibility of sampling error using the blind suction catheter method for aspiration of pulmonary edema fluid. However, since both ARDS and hydrostatic edema are diffuse and bilateral it is unlikely that sampling of edema fluid from a subsegment would introduce significant error. Furthermore, using the same method we have previously shown that the EF/PL protein ratio is a good discriminator between ALI and hydrostatic edema [20] and that AFC as measured by this method is an important predictor of clinical outcomes [11]. Finally, it is not possible to measure the function of ENaC without lung tissue, thus it was not possible to study ENaC function as a potential mechanism for the faster AFC rates in women. The similar levels of plasma RAGE levels in the females and males suggest that the differences seen may not result from differences in the degree of alveolar epithelial injury.
Conclusions
Overall these findings are novel and of potential clinical importance in designing future studies in patients with acute lung injury. Clearly there are many factors that affect rates of alveolar fluid clearance in patients with acute lung injury. In this study, we have identified gender as one of those factors. This study was modest in size (n=150 patients) and thus was underpowered to test for a statistically significant difference in important clinical endpoints, such as severity of lung injury, ventilator free days, and mortality. Nevertheless, we have found that women and men may have different physiologic responses to lung injury and may respond differently to treatments for acute lung injury. These findings need to be confirmed in a larger, prospective study. Our findings demonstrate that gender differences need to be considered when planning future studies in the pathogenesis and treatment of acute lung injury.
Acknowledgments
The authors would like to thank Nancy Wickersham for her invaluable help processing samples and assisting with protein measurements.
NIH/NICHD 5 K12 HD 043483-05 and HL090785-01A1 to JAB, UCSF Pediatric Training Grant 5 T32 HD049303 to TO, NHLBI HL51856 to MAM, NIH HL081332 and NIH HL088263 to LBW
List of abbreviations
- ALI/ARDS
acute lung injury/acute respiratory distress syndrome
- AFC
alveolar fluid clearance
- ENaC
lung epithelial sodium channel
- EF
edema fluid
- PL
plasma
- EF/PL
edema fluid to plasma protein ratio
- RAGE
receptor for advance glycation end products
- PEEP
positive end expiratory pressure
- A-a gradient
alveolar-arterial oxygen gradient
- SAPS II
simplified acute physiology score II
- LIS
lung injury score
- TV
tidal volume
Footnotes
Competing interests
The authors declare that they have no competing interests
Authors’ contributions
JAB was responsible for experimental, data analysis and interpretation, and manuscript preparation, TO assisted with data analysis and manuscript preparation, MAM collected the UCSF patient samples, was responsible for the protein measurements, and assisted with data interpretation and manuscript preparation, LBW conceived of the study, was responsible for collecting the Vanderbilt samples and making the protein measurements, experimental design, data interpretation, and manuscript preparation. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006;130(3):724–729. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- 2.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996) Crit Care Med. 2002;30(8):1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Perelman RH, Palta M, Kirby R, Farrell PM. Discordance between male and female deaths due to the respiratory distress syndrome. Pediatrics. 1986;78(2):238–244. [PubMed] [Google Scholar]
- 4.Choi CW, Kim BI, Park JD, Koh YY, Choi JH, Choi JY. Risk factors for the different types of chronic lung diseases of prematurity according to the preceding respiratory distress syndrome. Pediatr Int. 2005;47 (4):417–423. doi: 10.1111/j.1442-200x.2005.02081.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 6.Cooke CR, Kahn JM, Caldwell E, Okamoto VN, Heckbert SR, Hudson LD, Rubenfeld GD. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med. 2008;36 (5):1412–1420. doi: 10.1097/CCM.0b013e318170a375. [DOI] [PubMed] [Google Scholar]
- 7.Gong MN, Wei Z, Xu LL, Miller DP, Thompson BT, Christiani DC. Polymorphism in the surfactant protein-B gene, gender, and the risk of direct pulmonary injury and ARDS. Chest. 2004;125(1):203–211. doi: 10.1378/chest.125.1.203. [DOI] [PubMed] [Google Scholar]
- 8.Sheu CC, Zhai R, Su L, Tejera P, Gong MN, Thompson BT, Chen F, Christiani DC. Sex-specific association of epidermal growth factor gene polymorphisms with acute respiratory distress syndrome. Eur Respir J. 2009;33(3):543–550. doi: 10.1183/09031936.00091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, Lanken PN, Localio AR, Christie JD. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med. 2009;37(6):1913–1920. doi: 10.1097/CCM.0b013e3181a009b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 12.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142(6 Pt 1):1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 13.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82(3):569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 14.Sweezey N, Tchepichev S, Gagnon S, Fertuck K, O’Brodovich H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol. 1998;274(2 Pt 1):C379–386. doi: 10.1152/ajpcell.1998.274.2.C379. [DOI] [PubMed] [Google Scholar]
- 15.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87(4):1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 16.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA. Plasma Receptor for Advanced Glycation End-Products And Clinical Outcomes in Acute Lung Injury. Thorax. 2008 doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L1–5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 20.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the etiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2009 doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 23.Matthay MA, Flori HR, Conner ER, Ware LB. Alveolar epithelial fluid transport: basic mechanisms and clinical relevance. Proc Assoc Am Physicians. 1998;110(6):496–505. [PubMed] [Google Scholar]
- 24.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest. 2009;135(2):269–275. doi: 10.1378/chest.08-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu EN, Traylor ZP, Davis IC. Effect of ventilation pressure on alveolar fluid clearance and beta-agonist responses in mice. Am J Physiol Lung Cell Mol Physiol. 2009 doi: 10.1152/ajplung.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]